Abstract
Purpose
To compare the complication rates associated with hepatic arterial infusion chemotherapy (HAIC) for unresectable hepatocellular carcinoma (HCC) using two different catheter tip locations, the right/left hepatic artery (group 1) and the gastroduodenal artery (group 2).
Methods
Between April 2001 and March 2015, 88 patients (group 1, n = 36; group 2, n = 52) with unresectable HCC, underwent HAIC via a transfemorally placed infusion catheter. The incidence of complications related to catheter placement (including hepatic arterial occlusion, catheter dislocation, non-target embolization and port-catheter system infection) as well as catheter patency and patient survival were evaluated.
Results
The technical success rate was 100%. The overall complication rate was 17% (15/88 patients). The specific complications were as follows: hepatic artery occlusion, n = 1 (group 2, n = 1), gastroduodenal ulcer, n = 6 (group 1, n = 2; group 2, n = 4); catheter dislocation, n = 1 (group 2, n = 1); port-catheter system infection, n = 3 (group 2, n = 3); and bleeding at the puncture site, n = 4 (group 1, n = 1; group 2, n = 3).
Conclusions
The complication rates in groups 1 and 2 did not differ to a statistically significant extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous catheter placement for hepatic artery infusion chemotherapy (HAIC) allows high doses of drugs to be delivered to the liver with few systemic side effects. In patients with advanced hepatocellular carcinoma (HCC), HAIC with a portsystem provides a useful treatment option for patients who are not candidates for surgical resection, transcatheter arterial chemoembolization (TACE) or radiofrequency ablation (RFA). Although Hammad et al. [1] reported the excellent outcomes of living donor liver transplantation for patients with advanced HCC, the ability to perform liver transplantation is limited by the low rate of organ donation in Japan [2].
Arai et al. [3] reported various techniques of percutaneous catheter placement. HAIC via a portsystem is associated with a number of potential clinical complications, including obstruction or stenosis of the hepatic artery, acute gastric or duodenal mucosal lesions, catheter dislocation, infection of the port-catheter system, and bleeding around the portsystem [4–6]. In relation to the placement of a side-hole infusion catheter, there are two approaches: either insertion into the right/left hepatic artery (group 1), or insertion into the gastroduodenal artery (GDA) (group 2) [3, 7]. The group 1 procedure involves the use of fewer coils because coil embolization around the tip of the catheter is not necessary.
The purpose of this retrospective study was to evaluate the complications encountered during hepatic arterial infusion chemotherapy (HAIC) and to compare the rates of the complications in patients in whom the catheter tip was inserted into the right/left hepatic artery with those in whom it was inserted into the gastroduodenal artery.
Methods
Our institutional review board approved this retrospective study and waived the requirement for informed consent (R-0222). The two authors (R. I. and T. S.) reviewed the patients’ medical records retrospectively and evaluated the complications that were related to the port-catheter system, the total time of fluoroscopy, the total number of coils used in the initial procedure, the duration for which the port-catheter system could be used, and the overall survival. The duration for which the port-catheter system could be used was defined as the period from the initial use of the port (for HAIC) until the end of its use. Overall patient survival was defined as the period between the date on which the port-catheter system was initially implanted and death or the last follow-up examination.
Patients
From April 2001 to March 2015, 88 patients underwent the percutaneous implantation of port-catheter systems for the treatment of unresectable HCC. Fiftythree of these 88 patients had previously undergone TACE.
The implantation of the port catheter systems via the femoral artery was successful in all patients. In 36 (41%) patients, the side-hole infusion catheter was inserted into the right/left hepatic artery (group 1). In the remaining 52 (49%) patients, the catheter was inserted into the GDA (group 2). The demographic data related to the procedures are listed in Table 1. There were no significant differences in the demographic data of the two groups.
Arterial evaluation and coil embolization
Before the implantation of a port-catheter system, the anatomy of hepatic arteries and all arterial branches supplying the stomach, duodenum, and pancreas was obtained using computed tomography angiography (CTA) or catheter angiography. We decided, based on the vascular anatomy, whether the tip of the infusion catheter should be inserted into either the right/left hepatic artery (group 1) or the gastroduodenal artery (group 2).
All of the procedures were performed under local anesthesia using 1% lidocaine (Xylocaine; Astra-Zeneca, Osaka, Japan). The femoral artery was accessed percutaneously by direct puncture, and a 4 Fr shepherd hook catheter (Terumo Clinical Supply, Tokyo, Japan) was inserted in the femoral artery to perform superior mesenteric and celiac angiography. Following angiography, coil embolization of the right gastric artery was attempted in all cases to prevent the development of acute gastric mucosal lesions during HAIC. In cases in which embolization of the right gastric artery was unsuccessful, we inserted the tip of the infusion catheter into the right/left hepatic artery, and the side hole of the infusion catheter was placed as far as possible from the origin of the right gastric artery to avoid the occurrence of acute gastritis. In the case of patients with variant hepatic arteries, including a replaced right hepatic artery originating from the superior mesenteric artery or an accessory left hepatic artery originating from the left hepatic artery, the variant arteries were embolized to create a single hepatic arterial inflow. Various pushable and detachable microcoils, such as Hilal (Cook, Bloomigton, IN), Tornado (Cook, Bloomigton, IN), C-stopper (Piolax, Kanagawa, Japan), Trufill DCS Orbit (Codman, Johnson & Johnson), Azur (Terumo, Tokyo, Japan), and Penumbra (Medico’s Hirata, Osaka, Japan), were used to perform embolization.
The placement of the infusion catheter
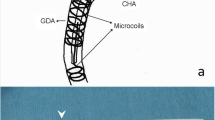
The tip of the infusion catheter was introduced into either the right/left hepatic artery (group 1) or the GDA (group 2). In the patients of group 1, the side of the infusion catheter was placed in the proper hepatic artery, to allow the drugs to infuse the whole liver through the side hole of the infusion catheter. In the patients of group 2, the side hole of catheter was located in the common hepatic artery. As stated above, the decision regarding the location of insertion (right/left hepatic artery vs. the GDA) was made according to the vascular anatomy of the patient. If the infusion catheter could not be inserted into the GDA because the celiac artery descended steeply from the aorta, it was inserted as distally as possible into the right/left hepatic artery with or without coil embolization of the GDA (switch from group 2 to group 1) (Fig. 1). In the patients of group 1, coil embolization of the GDA was not performed when the location of the side hole of the implanted catheter meant that there would be long distance between the bifurcation of the GDA and the PHA. When the side hole of the infusion catheter was expected to be located close to the bifurcation of the GDA and the PHA, coil embolization of the GDA was performed to prevent the anticancer drugs from flowing into the GDA. After the placement of the implanted catheter, contrast medium was injected via the infusion catheter to ensure that no contrast medium was refluxed to the pancreatic and duodenal area. In the patients of group 2, the tip of the catheter was implanted in the GDA with coils around it, and the side hole of the infusion catheter was placed in the common hepatic artery. A polyvinylpyrrolidone-covered catheter with either a tapered tip (outer diameter of the proximal shift, 5 Fr; distal shift, 2.7/3.3/4 Fr) or a non-tapered tip (outer diameter of the proximal and distal shift, 5 Fr) (W spiral catheter, G spiral catheter; Piolax, Kanagawa, Japan) was inserted. If these catheters could not be inserted due to excessive tortuosity, a 2.7 Fr diameter co-axial catheter was inserted over a 5 Fr infusion shepherd hook catheter via the celiac artery (W spiral catheter, coaxial system; Piolax, Kanagawa, Japan). These infusion catheters contain spirallyarranged shaped-memory alloy within the tip. In the case of the 2.7 or 3.3 Fr-diameter catheters, the side hole was created for placement into the PHA or the common hepatic artery. The distance from the side hole to the tip of the catheter was determined based on the length of a micro-guide wire that was drawn from the intrahepatic artery or the PHA to the common hepatic artery. After confirming satisfactory hepatic perfusion and the lack of non-target perfusion with the injection of contrast medium via the infusion catheter, a subcutaneous port (Piolax, Kanagawa, Japan) was connected to the infusion catheter and flushed with heparin.
A 75-year-old man with advanced hepatocellular carcinoma who underwent HAIC. a The infusion catheter was inserted into the right hepatic artery after coil embolization of the posterior-superior pancreaticoduodenal artery and the GDA. b A proper hepatic angiogram performed with the injection of contrast medium via the port-infusion catheter showed no reflux of the contrast media into the pancreaticoduodenal area
Hepatic arterial infusion chemotherapy
The HCC patients underwent chemotherapy with low-dose cisplatin with 5-fluorouracil [8, 9]. One course consisted of daily cisplatin (10 mg/day for 1 h) followed by 5-fluorouracil (250 mg/day for 5 h on days 1–5). Three serial courses of HAIC were scheduled. When progressive disease (PD) was observed after the three courses, HAIC was discontinued and alternative treatments were sought. The catheter was flushed with 100 UI of heparin (10 ml of a solution of 10 UI/mL) before and after HAIC.
Complications
The complications associated with the implantation of a port-catheter system were categorized as hepatic artery occlusion, gastroduodenal ulcer (acute gastric or duodenal mucosal lesions), catheter dislocation, port catheter system infection, and bleeding at the puncture site.
When hepatic artery occlusion was suspected during HAIC, angiography was performed via the port system. In patients with hepatic artery occlusion, the administration of HAIC via the port was abandoned and an alternative treatment was selected. When patients suffered from abdominal pain, upper endoscopy was performed due to the possibility of gastric or duodenal ulcer (acute gastric or duodenal mucosal lesions). When acute gastric or duodenal mucosal lesions were diagnosed during the upper endoscopy, celiac angiography was performed via the contralateral femoral artery with a 4 Fr shepherd hook catheter. If the artery supplying the stomach or duodenum was visible, coil embolization of the artery was performed. When catheter dislocation or migration was confirmed, the infusion catheter was withdrawn and a new catheter in a 2.7 Fr distal shaft was inserted in the right/left hepatic artery via the contralateral femoral artery. When a port-catheter system infection was suspected, the infusion catheter was removed and the patient was treated with antibiotics. If bleeding was observed at the puncture site, the infusion catheter was removed first, and the femoral artery was then surgically repaired.
Statistical analysis
The data were processed and the statistical analyses were performed using a commercially available software program (MedCalc version 12.7.8.0; MedCalc Software, Ostend, Belgium). The incidence of technical complications in relation to the use of the port-catheter system was compared in groups 1 and group 2 using the Chi-square test. Quantitative variables were compared using the Mann-Whitney U test. p values of <0.05 were considered to indicate a statistically significant difference.
Results
The technical success rate was 100% (88/88 patients), and the overall complication rate was 17% (15/88 patients). The devices and the time of fluoroscopy are shown in Table 2. In group 2, the number of coils that were used for the placement of the initial port system was significantly greater (median, 12 coils) than that in group 1 (median, 7 coils; p < 0.001).
The technical complications that led to the cessation of HAIC are shown in Table 3. Hepatic artery obstruction was observed in one (1.1%) of the 88 patients (group 2, n = 1; p = 0.405) at 301 days after the initiation of HAIC. This patient discontinued HAIC due to hepatofugal arterial blood flow to the splenic artery and transcatheter arterial infusion was performed. Thirty-one patients underwent upper gastroduodenal endoscopy due to abdominal pain during HAIC—no gastric or duodenal ulcers were observed in 25 of these patients. In the other 6 patients, a gastroduodenal ulcer was endoscopically confirmed in 6 (6.8% of the 88 patients; group 1, n = 2; group 2, n = 4; p = 0.698) at 8–62 days after initiation of HAIC (mean, 27 days; median, 22 days). The six patients underwent celiac angiography. In three patients, newly appeared duodenal branches were noted and then embolized with microcoils (Fig. 2). In the other three patients, a patent right gastric artery was depicted on hepatic angiography—embolization was then repeated with microcoils. HAIC was continued after the embolization of these vessels. Catheter dislocation occurred in one patient (1.1%; group 2, n = 1) within 95 days after the initiation of HAIC. In the patient, the catheter was exchanged for a 2.7 Fr catheter, the tip of which was placed in the PHA. Port-catheter system infection occurred in 3 (3.4%) of the 88 patients (group 2, n = 3; p = 0.145) at 8–74 days after the initiation of HAIC (mean, 35 days; median, 22 days). In these cases, the infusion catheter was removed and antibiotics were administered. In one of the three patients, a new catheter with a 2.7 Fr distal shaft was inserted into the PHA after two weeks of antibiotic treatment. Bleeding at the puncture site was observed in 4 (4.5%) of the 88 patients (group 1, n = 1; group 2, n = 3; p = 0.51) at 20–136 days after the initiation of HAIC (mean, 60 days; median, 43 days). In two of the four patients, the infusion catheter was withdrawn and the femoral artery was surgically repaired. No significant difference was observed in the rates of technical complications in the two groups.
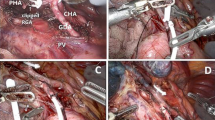
A 40-year-old man underwent HAIC for advanced hepatocellular carcinoma extending into the inferior vena cava. A gastroduodenal ulcer was confirmed by upper gastrointestinal fibroscopy at 62 days after the start of HAIC. a A celiac angiogram showed tumor thrombus in the inferior vena cava (arrow). b The infusion catheter was inserted into the GDA after coil embolization of the RGA, right inferior pancreaticoduodenal artery, posterior-superior pancreaticoduodenal artery and GDA. c A celiac angiogram obtained one day after gastrointestinal fibroscopy showed the duodenal branch arising from the GDA (arrow). d The placement of additional microcoils in the proximal GDA resulted in the complete flow occlusion of the GDA and the duodenal branch
The median period of catheterization for HAIC was 61 days in group 1, and 47 days in group 2 (p = 0.696). At the time of writing, 81 (92%) of the 88 patients have died; 40 (49.4%) of the 81 patients received the best supportive care and 35 (43.2%) underwent transcatheter arterial infusion (TAI) using cisplatin. With regard to the other 6 (7.4%) of the 81 patients, 4 patients received sorafenib and 2 patients were included in a clinical trial. Seven (8%) of the 88 patients remained alive at the end of the follow-up period; 2 patients underwent hepatectomy after the reduction of HCC due to the efficacy of HAIC and 5 patients were continuing HAIC. The median survival time from the date of infusion catheter implantation was 257 days in group 1, and 236 days in group 2 (p = 0.946).
Discussion
The evaluation of the complications associated with a treatment is necessary to understand its feasibility and good long-term durability. The Japan Clinical Oncology Group postoperative complications criteria (JCOG PC criteria) standardized the terms used to define adverse events, and provided detailed grading guidelines based on the Clavien-Dindo classification system [10]. With regards to HAIC using a port-catheter system, HAIC was discontinued when major technical complications occurred. Barnett et al. [4] reviewed 4580 patients who underwent HAIC and evaluated the complications associated with port-catheter systems. They reported that hepatic artery occlusion occurred in 175 (7.8%) of 2256 patients in whom an infusion catheter was inserted into the GDA with an implantable pump. Seki et al. [11] recommended that the infusion catheter be inserted into the GDA to avoid the mechanical stimulation of the vascular wall of the hepatic artery by the catheter tip. In their report, however, an end-hole catheter was inserted to the PHA or CHA without fixation of the catheter tip. Yoshioka et al. [12] described their clinical experience with a side-hole infusion catheter with a spirallyarranged shaped-memory alloy within the tip. In our study, a side-hole infusion catheter with a spiral-shaped tip was inserted into the peripheral hepatic arteries or the GDA in all cases. Hepatic artery occlusion occurred in one patient in whom a side-hole infusion catheter was inserted into the GDA after coil embolization of the replaced RHA. There was no significant difference in the rate of hepatic artery occlusion between our two groups. The use of a variety of catheter sizes might help to reduce the incidence of mechanical stimulation of the vascular wall of the hepatic artery by the catheter tip. Irrespective of the location of the catheter, the risk of hepatic artery occlusion might be low during HAIC, even after a three serial courses of HAIC.
A number of reports have indicated that the embolization of the GDA, the branches of the GDA, and the RGA with microcoils can help to prevent the development of gastroduodenal ulcers (gastric or duodenal mucosal lesions) during HAIC with low-dose cisplatin and 5-fluorouracil [13, 14]. Although we agree with these previous reports, in some cases, it can technically difficult to insert a micro-catheter into the RGA and perform embolization with microcoils due to the narrow diameter and the angles that are involved. Yamagami et al. [15] reported that the left gastric artery was an efficient route for the catheterization of the RGA. In cases in which coilembolization of the RGA could not be performed, we routinely selected to insert the infusion catheter with a side hole distal to the RGA into the right/left hepatic artery. In our study, gastroduodenal ulcers developed in two patients of group 1 and in one patient of group 2 even though sufficient embolization of the RGA and the GDA could be performed. Inaba et al. [13] reported that acute gastric mucosal lesions were endoscopically confirmed in 2.6% (5/192 patients) of patients with complete embolization of the RGA and the GDA—which was similar to our group 2. Despite the fact that all of the extrahepatic vessels were subjected to coil embolization during the placement of the initial port system, a small branch perfusing the pancreaticoduodenal vascular bed might have reopened in some cases. Acute gastric mucosal lesions should be ruled out when patients suffer from upper abdominal pain or nausea, even if the RGA and GDA were completely embolized during the placement of the initial port system. Despite the higher number of coils that were used to induce vessel occlusion in group 2, the rate of acute gastric mucosal lesions in the two groups did not differ to a statistically significant extent.
Frederic et al. [14] reported that the migration of the catheters did not occur in any of the patients (0/14 patients) in group 1 when the infusion catheter was inserted into a segmental hepatic artery, while it occurred in 11% (7/64 patients) of group 2, in which the infusion catheter was fixed into the GDA. In our study, catheter dislocation only occurred in 1 (1.1%) of the 88 patients [1 (1.9%) of 52 patients in group 2]. The rate of catheter system migration in the two groups did not differ to a statistically significant extent. The improvement of the infusion catheter—by the use of spiral catheters—might have reduced the risk of catheter dislocation. In our one patient with catheter system migration, the catheter systems were successfully removed and new catheters were immediately inserted. When the infusion catheter was introduced from the femoral artery, there was no risk of brain infarction due to the release of thrombi from around the tip of the catheter. Yamagami et al. [16] reported the procedure for removal of infusion catheter after insertion into the left subclavian artery. The removal of infusion catheters implanted into the femoral artery was reported to be easier and safer in comparison to catheters inserted through the left subclavian artery.
A previous report stated that infection occurred only in 4 (5.1%) of 77 patients who underwent the insertion of a port-catheter system via the femoral artery [17]. A previous report stated that a port-catheter system inserted via the femoral artery might be susceptible to infection because of its close proximity to the perineal region. It is important to use aseptic technique when accessing the implanted port-catheter system during the femoral approach. All of the patients in our study underwent catheter insertion via the femoral artery, and port-catheter system infection occurred in 3 (3.4%) of 88 patients [0 of the 36 patients in group 1 and 3 (5.8%) of 52 patients in group 2]. Although it was unclear why infections only occurred in group 2, one of the three patients with port infection was immunocompromised. In that patient, the port was difficult to implant inside a skin pocket due to significant weight loss.
Although there are some reports about the therapeutic benefit of HAIC for advanced HCC, all of the reports were based on the original protocol [8, 9]. Ando et al. [8] reported the efficacy of HAIC using low-dose cisplatin and 5-FU for 48 advanced HCC patients with portal vein tumor thrombosis. In their report, the median survival time of 48 patients was 10.2 months (range 1.9–76.9 months) during 1.8–8 (median 4) serial courses of HAIC. They mentioned that additional therapies might be an option for prolonging the survival of patients with residual tumors after HAIC. In our study, alternative treatments might have extended the life of patients who had tumor progression after three serial courses of HAIC.
This study was associated with several limitations. First, the study was retrospective in nature. Second, we did not investigate the possibility of asymptomatic patients with hepatic artery occlusion. Seki et al. [11] noted that a lack of a routine CT/angiographic surveillance might have led to the underestimation of the incidence of angiographic evidence of hepatic artery occlusion.
In conclusion, no significant difference was observed in the complication rates of the two groups in which different catheter tip locations (the right/left hepatic artery and the gastroduodenal artery) were used. Both techniques are technically feasible for HAIC and are associated with low rates of complications. The results suggest that the technique can be selected based on the patient’s vascular anatomy.
References
Hammad A, Kaido T, Ogawa K, Fujimoto Y, Uemura T, Mori A, et al. Liver transplantation for advanced hepatocellular carcinoma in patients with Child-Pugh A and B. Surg Today. 2016;46:248–54.
Soyama A, Eguchi S. The current status and future perspectives of organ donation in Japan: learning from the systems in other countries. Surg Today. 2016;46:387–92.
Arai Y, Takeuchi Y, Inaba Y, Yamaura H, Sato Y, Aramaki T, et al. Percutaneous catheter placement for hepatic arterial infusion chemotherapy. Tech Vasc Interv Radiol. 2007;10:30–7.
Barnett KT, Malafa MP. Complications of hepatic artery infusion: a review of 4580 reported cases. Int J Gastrointest Cancer. 2001;30:147–60.
Tajima T, Yoshimitsu K, Kuroiwa T, Ishibashi T, Irie H, Aibe H, et al. Percutaneous femoral catheter placement for long-term chemotherapy infusions: preliminary technical results. AJR Am J Roentgenol. 2005;184:906–14.
Seki H, Ozaki T, Shiina M. Side-hole catheter placement for hepatic arterial infusion chemotherapy in patients with liver metastases from colorectal cancer: long-term treatment and survival benefit. AJR Am J Roentgenol. 2008;190:111–20.
Yagihashi K, Takizawa K, Ogawa Y, Okamoto K, Yoshimatsu M, Fujikawa A, et al. Clinical application of a new indwelling catheter with a side-hole and spirally arranged shape-memory alloy for hepatic arterial infusion chemotherapy. Cardiovasc Intervent Radiol. 2010;33:1153–8.
Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–95.
Song do S, Bae SH, Song MJ, Lee SW, Kim HY, Lee YJ, et al. Hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2013;19:4679–88.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Seki H, Kimura M, Yoshimura N, Yamamoto S, Ozaki T, Sakai K. Hepatic arterial infusion chemotherapy using percutaneous catheter placement with an implantable port: assessment of factors affecting patency of the hepatic artery. Clin Radiol. 1999;54:221–7.
Yoshioka T, Horikawa N, Takayama K, et al. Implantation of the intra-arterial portcatheter system using W spiral catheter in the peripheral hepatic arteries without fixation for treatment of malignant liver tumors: clinical experience. (in Japanese with English abstract). IVR. 2003; 07;18:282–286.
Inaba Y, Arai Y, Matsueda K, Takeuchi Y, Aramaki T. Right gastric artery embolization to prevent acute gastric mucosal lesions in patients undergoing repeat hepatic arterial infusion chemotherapy. J Vasc Interv Radiol. 2001;12:957–63.
Deschamps F, Rao P, Teriitehau C, Hakime A, Malka D, Boige V, et al. Percutaneous femoral implantation of an arterial port catheter for intraarterial chemotherapy: feasibility and predictive factors of long-term functionality. J Vasc Interv Radiol. 2010;21:1681–8.
Yamagami T, Kato T, Iida S, Hirota T, Nishimura T. Efficacy of the left gastric artery as a route for catheterization of the right gastric artery. AJR Am J Roentgenol. 2005;184:220–4.
Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Withdrawal of port-catheter system for hepatic arterial infusion chemotherapy implanted with fixed catheter tip method. J Vasc Interv Radiol. 2006;17:651–6.
Matsumoto T, Yamagami T, Yoshimatsu R, Morishita H, Kitamura N, Sato O, et al. Hepatic arterial infusion chemotherapy by the fixed-catheter-tip method: retrospective comparison of percutaneous left subclavian and femoral port-catheter system implantation. AJR Am J Roentgenol. 2014;202:211–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with this study.
Rights and permissions
About this article
Cite this article
Imamine, R., Shibata, T., Shinozuka, K. et al. Complications in hepatic arterial infusion chemotherapy: retrospective comparison of catheter tip placement in the right/left hepatic artery vs. the gastroduodenal artery. Surg Today 47, 851–858 (2017). https://doi.org/10.1007/s00595-016-1465-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-016-1465-7