Abstract
Purpose
To assess the available evidence on the prognostic factors for the 5-year survival for patients with distal cholangiocarcinoma (DCC) following surgical resection.
Methods
We performed a comprehensive search of abstracts included in databases where relevant studies were published between January 2000 and August 2015. Risk ratios (RRs), 95 % confidence intervals (95 % CIs), and random-effects model were calculated using RevMan 5.3 software.
Results
A total of 23 observational studies involving 2063 patients with DCC were analyzed. The meta-analysis showed that postoperative adjuvant chemotherapy was not confirmed as a prognostic factor, with similar 5-year survival rates between those receiving and not receiving chemotherapy (RR 0.71; 95 % CI 0.21–2.36; P = 0.57). Perineural invasion (RR 0.51; 95 % CI 0.40–0.64; P < 0.00001), lymph node metastasis (RR 0.51; 95 % CI 0.38–0.70; P < 0.0001), positive resection margin status (RR 2.11; 95 % CI 1.36–3.30; P = 0.001), and not-well-differentiated adenocarcinoma (RR 1.77; 95 % CI 1.39–2.25; P < 0.00001) were associated with shorter survival.
Conclusions
Perineural invasion, lymph node metastasis, resection margin status, and tumor differentiation were the significant prognostic factors for the 5-year survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distal cholangiocarcinoma [DCC] is a relatively uncommon pancreaticobiliary–digestive junction neoplasm, and early diagnosis is challenging. The prognosis and cure rate of DCC has been improved over the past several decades, but mortality remains high. Surgical resection of a periampullary tumor by pancreaticoduodenectomy (PD) is the only chance for curative therapy. Radical resection is the strongest prognostic factor and involves margin-negative resection combined with thorough regional node dissection. In the future, advances in medical treatment and neoadjuvant therapy may improve the success of surgical operation and postoperative survival rates.
Several reports have been published concerning pancreatic and duodenal invasion [1], the surgical margin positivity [2, 9], lymph node metastasis [3–6, 9, 10], perineural invasion [7, 10], grades of tumor differentiation [2, 6, 9], depth of tumor invasion [8], microvascular invasion [11, 12], postoperative adjuvant chemotherapy [3, 12], and overexpression of p53 protein [13] for distal bile duct malignancies. All of these factors have been reported as independent prognostic factors for DCC in multivariate survival analyses.
We, herein, assessed the available evidence on the survival rates of DCC patients following resection with curative intent and analyzed the prognostic factors. We performed an up-to-date meta-analysis of gender, age, perineural invasion, lymph node metastasis, resection margin status, tumor differentiation, and adjuvant chemotherapy, and, when appropriate and possible, established the sources of heterogeneity in the results.
Materials and methods
Data sources
We performed a systematic review of the literature published between January 2000 and August 2015. We performed a comprehensive search of abstracts in the MEDLINE database, OVID database, Springer database, the Science Citation Index, and the Cochrane Library database using the following search terms: “distal cholangiocarcinoma [Title]”, or “distal bile duct [Title]”, or “distal bile duct cancer [Title]”, with limitations to “English language” and “Humans”. Unpublished research was not included.
Inclusion and exclusion criteria
We included patients who underwent surgery for histologically proven distal bile duct cancer, with no limitations on race, sex, or age. The included studies were required to report at least one of the following outcomes: gender, age, perineural invasion, lymph node metastasis, resection margin status, tumor differentiation, and postoperative adjuvant chemotherapy.
Data extraction and assessment of risk of bias
To avoid double-counting, two data extractors compared the retrieved articles for participating institutions and inclusion criteria. To determine the potential risks of bias in the overall results due to the inclusion of studies, a sensitivity analysis and a publication bias analysis were performed on the eligibility criteria. All of the reviewers assessed the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions. If any disagreements occurred, consensus was achieved through discussions.
Statistical analysis
Two independent reviewers extracted the data using a specially developed form and entered it into the freeware program Review Manager (Version 5.3 for Windows, Cochrane Collaboration, Oxford, UK). The risk ratio (RR) for each trial was calculated from the number of evaluable patients, and the RRs with their two-sided 95 % confidence intervals (CIs) were used for dichotomous outcomes as the confirmatory effect size estimate and test criterion. The random-effects model was applied. The hypothesis tests were based on the 95 % CIs, and the P values were used for illustration. During data combination, the heterogeneity was evaluated using the Cochran’s Q test. All of the P values were two-sided, and P < 0.05 was considered statistically significant. The Excel 2007 (Microsoft, Redmond, WA, USA) software program was also used for our research.
Results
Trial and patient characteristics
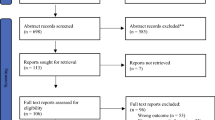
A total of 87 studies were retrieved, and the workflow for identifying relevant trials is shown in Fig. 1. Of these 87 studies, 6 were excluded for not having a clinical trial design. Of the 81 studies of clinical trials potentially suitable for inclusion in the meta-analysis, 58 were excluded for not citing any included outcomes or for having language for other fields. Ultimately, 23 clinical trials with extractable data were included [1, 14, 16–18], all of which were published as full articles. These trials included a total of 2063 patients with DCC who had received surgical resection. The principal characteristics of all of the included studies are shown in Table 1.
Risk of bias
Details regarding the patient characteristics and setting were available in all included trials [1, 3–24] (Table 1). None of the studies were randomized controlled trials. Twenty-three studies provide one or more required outcomes, and at least six of the included trials had the same outcomes. We determined that our meta-analysis was unlikely to be influenced markedly by publication bias, as statistically significant data are more likely to be published than data lacking significance.
Prognostic factors for the 5-year survival
Gender
Ten included studies involving 970 patients reported the influence of gender on 5-year survival. The 5-year survival rates for males and females were 38.8 % (256/659) and 35.0 % (109/311), respectively. Our meta-analysis showed no significant difference in 5-year survival between males and females (RR 0.95; 95 % CI 0.68–1.32; P = 0.76) (Fig. 2). Therefore, we believe that gender is not an influential prognostic factor for the 5-year survival.
Age
Seven included studies involving 357 patients reported the influence of age on 5-year survival. The 5-year survival rates among those ≤65 years old and those >65 years old were 35.6 % (69/194) and 34.4 % (56/163), respectively. Our meta-analysis showed no significant difference in 5-year survival between those ≤65 years old and those >65 years old (RR 1.31; 95 % CI 0.82–2.12; P = 0.26) (Fig. 3). Therefore, we believe that age is not an influential prognostic factor for the 5-year survival.
Perineural invasion
Ten included studies involving 887 patients reported the influence of perineural invasion on 5-year survival. The 5-year survival rates for those with (“YES”) and without (“NO”) perineural invasion were 31.3 % (200/639) and 65.7 % (163/248), respectively. Our meta-analysis showed a significant difference in 5-year survival between those with and without perineural invasion (RR 0.51; 95 % CI 0.40–0.64; P < 0.00001) (Fig. 4). Therefore, we believe that perineural invasion is an influential prognostic factor for the 5-year survival.
Lymph node metastasis
Lymph node metastasis was defined as the presence of malignant cells in single, multiple, or regional lymph nodes. Twelve included studies involving 1203 patients reported the influence of lymph node metastasis on the 5-year survival. The 5-year survival rates for being positive and negative for metastasis were 23.7 % (122/514) and 47.2 % (325/689), respectively. Our meta-analysis showed a significant difference in 5-year survival between those positive and negative for metastasis (RR 0.51; 95 % CI 0.38–0.70; P < 0.0001) (Fig. 5). Therefore, we believe that lymph node metastasis is an influential prognostic factor for the 5-year survival.
Resection margin status
Resection margins were classified as positive when invasive cancer cells were identified histologically at the ductal or radial surgical margins [25]. Nine included studies involving 959 patients reported the influence of resection margin status on the 5-year survival. The 5-year survival rates for having positive and negative margin status were 40.8 % (343/840) and 15.1 % (18/119), respectively. Our meta-analysis showed a significant difference in the 5-year survival between those positive and negative for margin status (RR 2.11; 95 % CI 1.36–3.30; P = 0.001) (Fig. 6). Therefore, we believe that resection margin status is an influential prognostic factor for the 5-year survival.
Tumor differentiation
The predominant pathological grading system for tumor differentiation is classified as well-differentiated, moderately differentiated, or poorly differentiated adenocarcinoma. In our study, the DCC tumors were classified as well- or not well-differentiated. Eight included studies involving 853 patients reported the influence of tumor differentiation on 5-year survival. The 5-year survival rates for those with well- and not well-differentiated tumors were 54.6 % (143/262) and 30.8 % (182/591), respectively. Our meta-analysis showed a significant difference in 5-year survival between those with well- and not well-differentiated tumors (RR 1.77; 95 % CI 1.39–2.25; P < 0.00001) (Fig. 7). Therefore, we believe that tumor differentiation is an influential prognostic factor for the 5-year survival.
Postoperative adjuvant chemotherapy
Postoperative adjuvant chemotherapy used gemcitabine, fluorouracil, oxaliplatin, and paclitaxel [5, 13, 17]. Five included studies involving 328 patients reported the influence of postoperative adjuvant chemotherapy on the 5-year survival. The 5-year survival rates for those with (“YES”) and without (“NO”) postoperative adjuvant chemotherapy were 34.0 % (16/47) and 34.5 % (97/281), respectively. Our meta-analysis showed no significant difference in 5-year survival between those with and without postoperative adjuvant chemotherapy (RR 0.71; 95 % CI 0.21–2.36; P = 0.57) (Fig. 8). Therefore, we believe that postoperative adjuvant chemotherapy is not an influential prognostic factor for the 5-year survival.
Discussion
Cholangiocarcinoma, which is different from gallbladder carcinoma [26], can be classified as intrahepatic, perihilar, or DCC based on the anatomic location, epidemiology, origin, etiology, pathogenesis, and treatment [27, 28]. DCC is a relatively uncommon malignancy with high mortality which is reported to occur more frequently in Japan than in western countries. Andrianello et al. [6] reported that, of the 1490 PDs performed for periampullary disease, only 50 cases were histologically proven DCC (3.3 %). Despite recent technological improvements, early diagnosis of DCC remains difficult. More accurate diagnostic modalities for an early-stage diagnosis are therefore needed.
Several reports have been published concerning significant prognostic factors for DCC, such as surgical margin status [2, 9], lymph node metastasis [3–6, 9, 10], perineural invasion [7, 10], tumor differentiation [2, 6, 9], and postoperative adjuvant chemotherapy [3, 12]. In the present study, we aimed to determine the factors influencing the 5-year survival among DCC patients following surgical resection. Gender, age, and postoperative adjuvant chemotherapy were not confirmed as prognostic factors in our meta-analysis, with roughly similar survival rates reported between males and females (RR 0.95; 95 % CI 0.68–1.32; P = 0.76), those ≤65 years old and >65 years old (RR 1.31; 95 % CI 0.82–2.12; P = 0.26), and those who had and had not received postoperative adjuvant chemotherapy (RR 0.71; 95 % CI 0.21–2.36; P = 0.57). However, perineural invasion, lymph node metastasis, resection margin status, and tumor differentiation were indeed found to be significant prognostic factors following resection of DCC, with survival rates differing significantly between those with and without perineural invasion (RR 0.51; 95 % CI 0.40–0.64; P < 0.00001), those with and without lymph node metastasis (RR 0.51; 95 % CI 0.38–0.70; P < 0.0001), those with negative and positive resection margin status (RR 2.11; 95 % CI 1.36–3.30; P = 0.001), and those with well-differentiated and not well-differentiated tumors (RR 1.77; 95 % CI 1.39–2.25; P < 0.00001).
Lymph node metastasis and perineural invasion were significant prognostic factors in our meta-analysis. Previous studies have similarly reported lymph node metastasis and perineural invasion to be significant prognostic factors for survival, along with margin status and tumor differentiation. DCC recurrence after surgical resection results in poor prognosis and short overall survival times. Positive margin status, perineural invasion, tumor differentiation, and lymph node metastasis were found to be independent prognostic factors for the disease-free survival. Zhou et al. [29] analyzed the prognostic factors for the survival of DCC patients following resection with curative intent and found that R1 resection, lymph node metastasis, perineural invasion, lymphatic invasion, vascular invasion, pancreatic invasion, and pathological tumor stage ≥T3 were associated with lower rates of 5-year survival, concluding that R0 resection resulted in substantially improved survival and represented one of the most important prognostic variables.
Adjuvant chemo- and/or radiation therapy has not yet been standardized, and as postoperative adjuvant chemotherapy, no effective adjuvant therapy has been established at present [30]. Surgical resection associated with adjuvant therapy may provide the most favorable outcome [31]. The present meta-analysis showed that postoperative adjuvant chemotherapy was not a prognostic factor for DCC after surgery.
Conclusion
In conclusion, perineural invasion, lymph node metastasis, resection margin status, and tumor differentiation were the significant prognostic factors for the 5-year survival after resection of DCC, but not gender, age, or postoperative adjuvant chemotherapy. Future efforts should attempt to reduce the rates of surgical complications and improve medical therapy, actions that will promote the overall improvement in perioperative and long-term outcomes for patients with this disorder.
References
Ebata T, Nagino M, Nishio H, Igami T, Yokoyama Y, Nimura Y. Pancreatic and duodenal invasion in distal bile duct cancer: paradox in the tumor classification of the American Joint Committee on Cancer. World J Surg. 2007;31:2008–15.
Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–73.
Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73.
Kiriyama M, Ebata T, Aoba T, Kaneoka Y, Arai T, Shimizu Y, et al. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 2015;102:399–406.
Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Pancreatoduodenectomy for distal cholangiocarcinoma: prognostic impact of lymph node metastasis. World J Surg. 2007;31:337–42.
Andrianello S, Paiella S, Allegrini V, Ramera M, Pulvirenti A, Malleo G, et al. Pancreaticoduodenectomy for distal cholangiocarcinoma: surgical results, prognostic factors, and long-term follow-up. Langenbecks Arch Surg. 2015;400:623–8.
Bortolasi L, Burgart LJ, Tsiotos GG, Luque-De León E, Sarr MG. Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection. Dig Surg. 2000;17:36–41.
Hong SM, Pawlik TM, Cho H, Aggarwal B, Goggins M, Hruban RH, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146:250–7.
Choi SB, Park SW, Kim KS, Choi JS, Lee WJ. The survival outcome and prognostic factors for middle and distal bile duct cancer following surgical resection. J Surg Oncol. 2009;99:335–42.
Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim HJ, et al. Prognostic factors for survival after curative resection of DCC: perineural invasion and lymphovascular invasion. Surg Today. 2014;44:1879–86.
Ercolani G, Dazzi A, Giovinazzo F, Ruzzenente A, Bassi C, Guglielmi A, et al. Intrahepatic, peri-hilar and distal cholangiocarcinoma: three different locations of the same tumor or three different tumors? Eur J Surg Oncol. 2015;41(9):1162–9.
Hernandez J, Cowgill SM, Al-Saadi S, Villadolid D, Ross S, Kraemer E, et al. An aggressive approach to extrahepatic cholangiocarcinomas is warranted: margin status does not impact survival after resection. Ann Surg Oncol. 2008;15:807–14.
Cheng Q, Luo X, Zhang B, Jiang X, Yi B, Wu M. Distal bile duct carcinoma: prognostic factors after curative surgery. A series of 112 cases. Ann Surg Oncol. 2007;14:1212–9.
Sakamoto Y, Kosuge T, Shimada K, Sano T, Ojima H, Yamamoto J, et al. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396–402.
Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, et al. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007;95:207–12.
Shimizu Y, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. The morbidity, mortality, and prognostic factors for ampullary carcinoma and distal cholangiocarcinoma. Hepatogastroenterology. 2008;55:699–703.
Qiao QL, Zhang TP, Guo JC, Zhan HX, Zhao JX, Liu YC, et al. Prognostic factors after pancreatoduodenectomy for distal bile duct cancer. Am Surg. 2011;77:1445–8.
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–8.
Tan X, Xiao K, Liu W, Chang S, Zhang T, Tang H. Prognostic factors of distal cholangiocarcinoma after curative surgery: a series of 84 cases. Hepatogastroenterology. 2013;60:1892–5.
Pomianowska E, Westgaard A, Mathisen Ø, Clausen OP, Gladhaug IP. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol. 2013;20:233–41.
Chung YJ, Choi DW, Choi SH, Heo JS, Kim DH. Prognostic factors following surgical resection of distal bile duct cancer. J Korean Surg Soc. 2013;85:212–8.
Iso Y, Kita J, Kato M, Shimoda M, Kubota K. When hepatic-side ductal margin is positive in N+ cases, additional resection of the bile duct is not necessary to render the negative hepatic-side ductal margin during surgery for extrahepatic distal bile duct carcinoma. Med Sci Monit. 2014;20:471–5.
Bourgouin S, Ewald J, Mancini J, Moutardier V, Delpero JR, Le Treut YP. Predictors of survival in ampullary, bile duct and duodenal cancers following pancreaticoduodenectomy: a 10-year multicentre analysis. J Gastrointest Surg. 2015;19:1247–55.
Miura F, Sano K, Amano H, Toyota N, Wada K, Yoshida M, et al. Evaluation of portal vein invasion of distal cholangiocarcinoma as borderline resectability. J Hepatobiliary Pancreat Sci. 2015;22:294–300.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Yamaguchi J, Kaneoka Y, Maeda A, Takayama Y, Onoe S, Isogai M. Benefit of extended radical surgery for incidental gallbladder carcinoma. Surg Today. 2016;46:453–9.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–29.
Higuchi R, Ota T, Yazawa T, Kajiyama H, Araida T, Furukawa T, et al. Improved surgical outcomes for hilar cholangiocarcinoma: changes in surgical procedures and related outcomes based on 40 years of experience at a single institution. Surg Today. 2016;46:74–83.
Zhou Y, Liu S, Wu L, Wan T. Survival after surgical resection of distal cholangiocarcinoma: a systematic review and meta-analysis of prognostic factors. Asian J Surg. 2015; doi:10.1016/j.asjsur.2015.07.002 (Online 2015 Aug 31).
Furuse J, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, et al. Guidelines for chemotherapy of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:55–62.
Lad N, Kooby DA. Distal cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:265–87.
Acknowledgments
This study was supported by the German Research Council (DFG WE5085/1-1), Scientific Research Fund from Hubei University of Chinese Medicine, Education Project of Medical Talents for Young and Middle-aged in Wuhan City (Grant No. Q2014037), Young Talents Project of Science and Technology Research Program from Hubei Provincial Department of Education (Grant No. Q20132003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with this study.
Rights and permissions
About this article
Cite this article
Wellner, U.F., Shen, Y., Keck, T. et al. The survival outcome and prognostic factors for distal cholangiocarcinoma following surgical resection: a meta-analysis for the 5-year survival. Surg Today 47, 271–279 (2017). https://doi.org/10.1007/s00595-016-1362-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-016-1362-0