Abstract
Purpose
The relationship between the tumor size and organs of recurrence was analyzed to identify a high-risk group for the extrahepatic recurrence of hepatocellular carcinoma (HCC) after resection.
Methods
A total of 544 patients with HCC underwent primary surgical resection for HCC between 2001 and 2010. Of these, 293 patients had a solitary tumor but no macroscopic vascular invasion. The prognostic factors for the overall survival and relapse-free survival were analyzed among these 293 patients. The recurrent organs and frequency of recurrence were also examined.
Results
The analysis of the 293 patients showed that both the overall and relapse-free survival rates of the patients with a large tumor (>7 cm in diameter) were significantly worse than those of the patients with a tumor <7 cm. The incidence of lung metastasis was remarkably high in the group of patients with tumors more than 7 cm (24.0 %), in comparison to those with tumors <7 cm. A multivariate analysis revealed that the tumor size was the only independent risk factor for lung metastasis.
Conclusions
The patients with large HCC tumors more than 7 cm in diameter were at high-risk for a poor prognosis due to a high percentage of lung metastasis, even if there was no macroscopic vascular invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancers. Surgical resection is the mainstay for HCC treatment if the liver function and performance status are sufficient to allow it [1]. Hepatectomy is the only potentially curative treatment, especially for large HCC tumors, because large HCCs are not assumed to be an indication for liver transplantation or radiofrequency ablation (RFA) due to high rates of recurrence [2–4]. However, some reports showed that patients with huge HCCs have poor clinical outcomes because of early recurrence and extrahepatic metastasis [5–7]. Sorafenib is currently the only therapeutic agent available for treating distant metastasis and/or extrahepatic lesions of HCC; it is difficult to obtain a complete response to therapy using sorafenib [8]. Therefore, it is important to predict a poor prognostic group before resection to select the patients who need adjuvant therapy after surgery.
The most important prognostic factors are the tumor size, tumor marker expression and vascular invasion [2, 5, 6]. In particular, macrovascular invasion and multiple tumors are well-known factors associated with the poor prognosis of HCC. However, there have been no reports of the relationship between the tumor size and extrahepatic metastasis after resection. Therefore, to determine whether the tumor size itself is a predictive factor for a poor prognosis, this study analyzed the clinical outcomes of the patients with solitary HCC tumors without macroscopic vascular invasion. Furthermore, the study examined their type of recurrence to clarify the risk factors for extrahepatic metastasis after resection.
Methods
Patients
Five hundred and forty-four patients with HCC underwent surgical hepatic resection at Kyoto University Hospital between February 2001 and October 2010, after excluding the patients who underwent surgery for hepatic recurrence. Written informed consent was obtained from all patients in accordance with the ethics guidelines of Kyoto University Hospital. All patients were evaluated preoperatively using a chest X-ray, abdominal ultrasonography and contrast-enhanced computed tomography (CT) of the chest and abdomen. Additional studies, including magnetic resonance imaging (MRI) and positron emission tomography, were performed as needed. Laboratory blood tests, including those for the hepatitis B surface antigen, antibodies to hepatitis C, serum alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), serum albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase and the prothrombin time, were obtained. The liver functions were assessed by the Child–Pugh classification and by the indocyanine green test. In our institute, preoperative transcatheter arterial chemoembolization (TACE) has not been routinely performed except for cases of ruptured HCCs.
Hepatic resection was usually performed by means of a Mercedes incision. The abdominal cavity was searched during the operation for the extent of local disease, extrahepatic metastases and peritoneal seeding. The liver was mobilized, and intraoperative ultrasound was performed to assess the number and size of the lesions, and to detect other lesions. An intermittent Pringle maneuver, or selective vascular clamping if necessary, was applied to occlude the blood inflow of the liver. Hepatic parenchymal resection was performed using CUSA and a bipolar cautery device equipped with a channel for water dripping [9].
A pathological examination was performed for the tumor and the background liver. The tumor size, tumor number, vascular invasion, serosal invasion, surgical margin invasion and tumor differentiation were determined histopathologically.
This study defined major vascular invasion as tumor invasion to the primary or secondary branches of the portal veins, and/or invasion to the main trunks of the hepatic veins or the inferior vena cava. The micro-vascular invasion was defined as tumor invasion to tertiary or more peripheral branches of the portal and/or hepatic veins.
Follow-up strategy
All patients were followed up by evaluating the serum tumor markers (AFP and DCP) and contrast-enhanced CT or MRI every 3 months. Recurrent tumors were treated by surgical resection, TACE, RFA, percutaneous ethanol injection therapy, hepatic arterial chemotherapies, systemic chemotherapies or best supportive care depending on the number, size and location of the recurrent tumors, as well as the liver function. All recurrent organs and sites were registered during the entire follow-up period.
Statistical analysis
Survival curves were calculated by the Kaplan–Meier method. The survival analyses were performed using the Gehan–Breslow-Wilcoxon test. The recurrence rate was analyzed using a Chi-square test. A P value <0.05 was considered to be statistically significant. A multivariate analysis was performed using a logistic regression analysis, or using Cox proportional hazard models. The Prism and SPSS software packages were used for all statistical analyses.
Results
The pathological examination revealed that 293 of the 544 patients had a solitary tumor and had no major vascular invasion. The clinicopathological features of these 293 patients are summarized in Table 1. All further analyses were based on these 293 patients with a solitary tumor and no major vascular invasion to clarify the impact of the tumor size on the survival and recurrence.
Prognostic factors for survival and recurrence among the 293 patients
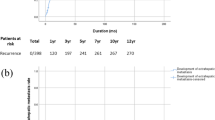
The 5-year overall survival (37.3 %) and 5-year relapse-free survival (30.1 %) rates of the patient with tumors more than 7 cm in diameter were significantly worse than those of the patients with HCC tumors 7 cm or smaller in diameter (64.1 and 32.7 %, respectively; P = 0.008 and P = 0.051). This indicated that the patients with large tumors had a poorer prognosis and higher risk of recurrence (Fig. 1).
The left panel shows the Kaplan–Meier curve for the overall survival, and the right panel shows that for the relapse-free survival between the patients with HCC tumors >7 cm (red line) and those with tumors ≤7 cm (blue line). The Gehan–Breslow-Wilcoxon tests revealed a value of P = 0.008 for the overall survival and P = 0.051 for the relapse-free survival analyses (color figure online)
The outcomes of the univariate analysis of the prognostic factors for the overall survival and relapse-free survival are summarized in Table 2. The prognostic factors for the overall survival were the Child–Pugh score, preoperative AFP value, preoperative DCP value, tumor size, serosal invasion, surgical margin invasion, micro-portal vein invasion and micro-hepatic vein invasion. The prognostic factors for the relapse-free survival were the Child–Pugh score, serosal invasion, surgical margin invasion, tumor size and micro-portal vein invasion. The multivariate analyses using Cox proportional hazard models were performed using these factors, and the results are also summarized in Table 2. Serosal invasion and surgical margin invasion were extracted as independent prognostic factors for relapse-free survival, whereas there were no independent prognostic factors for overall survival among the factors included in the analyses.
Organs affected by HCC recurrence
The 293 patients with a solitary tumor and no major vascular invasion were further divided into three groups: a group of patients with tumors <2 cm in diameter, a group of patients with tumors 2–7 cm in diameter and a group of patients with tumors more than 7 cm in diameter. The rate of lung metastasis was significantly higher in the group of patients with tumors larger than 7 cm (P = 0.0001, Chi square test; Table 3), whereas the rate of lung metastasis remained approximately constant in the patients with tumors <7 cm in diameter (Supplemental fig. 1). However, there were no significant differences among the three groups in the rates of recurrence in other organs, including the liver, bone and lymph nodes.
The univariate and multivariate logistic regression analyses were performed to examine the prognostic factors for lung metastasis of HCC. The tumor size was revealed to be the only independent predictive factor for lung metastasis among all of the factors examined (Table 4).
Discussion
The tumor size is assumed to be one of the most important prognostic factors for HCC [10, 11]. Several reports have shown that patients with huge HCC tumors have poor clinical outcomes due to early intrahepatic recurrence and distant metastasis [12–14]. Large tumors have a tendency to be multinodular and to invade into major vessels. These tumor factors were also risk factors for poor outcomes, thus the 293 patients with solitary HCC tumors but no macroscopic vascular invasion were analyzed in this study to provide clinical risk stratification based on just the size of the tumors. The results showed that large tumors (>7 cm in diameter) were associated with poor clinical outcomes in terms of both the overall survival and relapse-free survival rates, even if they were solitary and did not invade into major hepatic vessels. Large HCC tumors also showed a higher frequency of lung metastasis after hepatectomy in comparison to tumors <7 cm in size. This cutoff value was determined using the ROC analysis, which revealed that the sensitivity was 46.2 % and the specificity was 85.8 % (Supplemental fig. 2). Although a tumor size cutoff value of 5 cm is currently used in several staging systems for HCC, there were no significant differences between patients with tumors >5 and <5 cm in the incidence of lung metastasis in our study (data not shown). Interestingly, there was no significant difference in the hepatic recurrence rate associated with the tumor size. This might be partially because hepatic recurrence develops not only as intrahepatic metastasis, but also due to the multicentric carcinogenesis of HCC [15, 16].
Although the exact reason for the high frequency of lung metastasis in patients with large HCC tumors was not elucidated in this study, it is possible that lung micrometastases may have already been present before resection, or that the surgical procedures performed during hepatic resection could have disseminated tumor cells systemically via the hepatic veins and the inferior vena cava [17–19]. Careful and skillful surgical manipulations are necessary to avoid touching tumors during surgery. Liver mobilization is often required during conservative hepatectomy. On the other hand, liver mobilization is preceded by hepatic resection and ligation of both the hepatic inflow and outflow in hepatectomy by the anterior approach. Therefore, the anterior approach for hepatectomy might be helpful to reduce lung metastasis in patients with huge HCC tumors [20–22].
Sorafenib is currently the only agent that has been scientifically demonstrated to be effective as systemic therapy for HCC [8, 23]. In other words, the therapeutic options for HCC patients with lung metastasis are either sorafenib or palliative treatments [1, 24–26]. The indications for surgery are very limited, because lung metastases usually show multiple loci. The current findings suggested that patients with huge tumors should be recognized as a high-risk group for lung metastasis after hepatic resection. Although there is no evidence that sorafenib is as effective as an adjuvant therapy after HCC surgery at present, sorafenib could be used for postoperative adjuvant chemotherapy [26, 27]. Further studies should be performed to explore the possibility of using sorafenib as adjuvant therapy in high-risk patients.
In conclusion, this study described the relationship between the tumor size and presence of lung metastasis after HCC surgery as determined by a retrospective single institution analysis. We found that tumors larger than 7 cm in diameter were a risk factor for lung metastasis, even when they were solitary and did not invade major vessels, thus resulting in a poor prognosis in the patients with large HCCs. These findings suggest the necessity of using the anterior approach for hepatectomy for large HCC tumors, and postoperative adjuvant chemotherapy with agents such as sorafenib.
References
Bruix J, Sherman M, Practice Guidelines Committee AeAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36.
Takada Y, Uemoto S. Liver transplantation for hepatocellular carcinoma: the Kyoto experience. J Hepatobiliary Pancreat Sci. 2010;17(5):527–32.
Shirai K, Tamai H, Shingaki N, Mori Y, Moribata K, Enomoto S, et al. Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011;41(8):738–45.
Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):S44–57.
Shah SA, Wei AC, Cleary SP, Yang I, McGilvray ID, Gallinger S, et al. Prognosis and results after resection of very large (>or =10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11(5):589–95.
Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14(10):2817–23.
Harimoto N, Shirabe K, Kajiyama K, Gion T, Takenaka M, Nagaie T, et al. Spontaneous regression of multiple pulmonary recurrences of hepatocellular carcinoma after hepatectomy: report of a case. Surg Today. 2012;42(5):475–8.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Yamamoto Y, Ikai I, Kume M, Sakai Y, Yamauchi A, Shinohara H, et al. New simple technique for hepatic parenchymal resection using a Cavitron ultrasonic surgical aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23(10):1032–7.
Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81(4):195–202.
Hao K, Luk JM, Lee NP, Mao M, Zhang C, Ferguson MD, et al. Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer. 2009;9:389.
Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141(2):196–202.
Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94(3):320–6.
Tanaka K, Shimada H, Matsuo K, Takeda K, Nagano Y, Togo S. Clinical features of hepatocellular carcinoma developing extrahepatic recurrences after curative resection. World J Surg. 2008;32(8):1738–47.
Ikeda K, Arase Y, Kobayashi M, Saitoh S, Someya T, Hosaka T, et al. Significance of multicentric cancer recurrence after potentially curative ablation of hepatocellular carcinoma: a longterm cohort study of 892 patients with viral cirrhosis. J Gastroenterol. 2003;38(9):865–76.
Taura K, Ikai I, Hatano E, Yasuchika K, Nakajima A, Tada M, et al. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142(5):685–94.
Zhong C, Wei W, Su XK, Li HD, Xu FB, Guo RP. Serum and tissue vascular endothelial growth factor predicts prognosis in hepatocellular carcinoma patients after partial liver resection. Hepatogastroenterology. 2012;59(113):93–7.
Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15(5):1375–82.
Chun JM, Kwon HJ, Sohn J, Kim SG, Park JY, Bae HI, et al. Prognostic factors after early recurrence in patients who underwent curative resection for hepatocellular carcinoma. J Surg Oncol. 2011;103(2):148–51.
Liddo G, Buc E, Nagarajan G, Hidaka M, Dokmak S, Belghiti J. The liver hanging manoeuvre. HPB (Oxf). 2009;11(4):296–305.
Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244(2):194–203.
Wu TJ, Wang F, Lin YS, Chan KM, Yu MC, Lee WC. Right hepatectomy by the anterior method with liver hanging versus conventional approach for large hepatocellular carcinomas. Br J Surg. 2010;97(7):1070–8.
Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–37.
Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Takami K, Ohigashi H, et al. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. Am J Surg. 2006;192(1):46–51.
Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, et al. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40(7):667–85.
Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–64.
Ishii H, Yamamoto J, Ikari T. Adjuvant treatments for resectable hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15(5):459–62.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishii, T., Hatano, E., Yasuchika, K. et al. High risk of lung metastasis after resection of hepatocellular carcinoma more than 7 cm in diameter. Surg Today 44, 1900–1905 (2014). https://doi.org/10.1007/s00595-013-0792-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-013-0792-1