Abstract
Aim
To investigate the effects of hypertension (HTN) on inner retinal thickness and macular microvasculature in patients with diabetic retinopathy (DR).
Methods
Subjects were classified into three groups: patients with type 2 diabetes mellitus (T2DM) (T2DM group), patients with DR (DR-HTN group), and patients with DR and HTN (DR + HTN group). The ganglion cell complex (GCC) thicknesses and the macular vessel density (VD) were compared. Linear regression analyses were performed to identify factors associated with the VD in the DR + HTN group.
Results
The mean GCC thicknesses were 112.2 ± 12.3, 109.2 ± 13.7, and 106.2 ± 11.2 μm in the T2DM, DR-HTN, and DR + HTN groups, respectively (P = 0.045). The mean VDs were 25.4 ± 5.0, 24.3 ± 8.9, and 21.2 ± 7.1% (P = 0.014) for the superficial capillary plexus (SCP) and 25.9 ± 4.3, 22.9 ± 8.5, and 20.2 ± 6.6% (P < 0.001) for the deep capillary plexus (DCP) in the T2DM, DR-HTN, and DR + HTN groups, respectively. In multivariate analyses, the duration of HTN was a significant factor associated with the VD of both SCP (B = -0.24, P = 0.010) and DCP (B = -0.21, P = 0.016).
Conclusions
Patients with both DR and HTN had a thinner GCC and lower VDs of SCP and DCP than those with DR alone. These outcomes could be associated with the synergistic ischemic effects in DR patients with HTN. Moreover, the duration of HTN in DR patients was significantly associated with macular VD in both SCP and DCP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR), a common microvascular complication of type 2 diabetes (T2DM), stands as a leading cause of new-onset visual impairment among the working-age population in developed countries. As the prevalence of T2DM and the lifespan of patients with T2DM increase, the prevalence of DR is also rising [1,2,3,4]. DR is caused by the activation of the polyol and hexosamine pathologic pathways, de novo synthesis of diacylglycerol-protein kinase C, and the production of free radicals and advanced glycation end-products, all of which are induced by hyperglycemia [5, 6]. Vision loss due to DR can occur through several mechanisms, such as diabetic macular edema, vitreous hemorrhages, tractional retinal detachment, neovascular glaucoma, and more [7]. Like T2DM, hypertension (HTN) is another significant systemic disease that can result in visual impairment. High blood pressure can lead to visual loss via subfoveal hemorrhages, subretinal fluids, macular exudates, macular edema, and optic nerve swelling [8, 9].
The advent of optical coherence tomography angiography (OCTA), which allows for the visualization of the fine retinal microvasculature across various layers, has fostered numerous studies that have examined retinal microvasculature damage in patients with DR and HTN. For instance, Zihan et al. [10]. found that the progression of DR was associated with an expansion of the foveal avascular zone (FAZ) area and a decrease in the vessel density (VD) of the deep capillary plexus (DCP). Durbin et al. [11]. reported a negative correlation between the VD of the superficial retinal layer and best-corrected visual acuity (BCVA), severity of DR, and age. In the context of HTN, Sargues et al. [12]. reported reduced parafoveal VD in DCP and choriocapillaris (CC) in patients with HTN, and Chua et al. [13]. found that patients with severe HTN, who exhibited changes in cardiac muscle structures, had lower VD of superficial capillary plexus (SCP) than normal subjects.
Given that both DR and HTN can damage retinal microvasculature, and considering the doubling of HTN prevalence in the presence of T2DM, it is expected that concomitant DR and HTN would lead to greater microvascular damage [14,15,16,17]. However, to date, no studies have explored this possibility in detail. Therefore, we investigated the effect of HTN on the inner retinal layer and macular microvasculature in patients with DR by comparison of inner retinal thickness using optical coherence tomography (OCT) and VDs using OCTA.
Methods
Patients
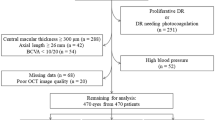
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Konyang University Hospital, Daejeon, Republic of Korea (No.2023-05-042). Due to the retrospective design of the study, the requirement for informed consent was waived. We conducted a retrospective review of the charts of 227 patients diagnosed with T2DM or DR who had undergone checkups for retinal abnormalities between January 2018 and January 2023.
Subjects were categorized into three groups: subjects with T2DM without clinical DR (T2DM group), subjects with clinical DR (DR-HTN group), and subjects with both DR and HTN (DR + HTN group). In subjects with DR, the severity of DR was graded according to the International Clinical Early Treatment Diabetic Retinopathy Study classification. Patients with mild, moderate, and severe nonproliferative DR (NPDR) were included in the study. Patients with very severe NPDR and proliferative DR, who required laser treatment or had already received laser photocoagulation, which could potentially cause retinal atrophy, were excluded. In addition, patients who had received intravitreal anti-VEGF injections to reduce DME were also excluded. All HTN patients had well-controlled blood pressure. The exclusion criteria also encompassed a history of any systemic disease other than T2DM and HTN, uncontrolled blood pressure > 140/90 mmHg even after treatment, intraocular pressure (IOP) ≥ 21 mmHg, BCVA < 20/400, central macular thickness ≥ 300 μm, any ophthalmic condition that could affect the retinal layer thickness such as glaucoma, retinal and neuro-ophthalmic diseases, and history of intraocular surgery except for cataract extraction. If both eyes met the inclusion criteria, the eye with the higher image quality was selected for analysis.
Ganglion cell complex thickness measurements using OCT
Retinal layer thicknesses were measured by an experienced examiner utilizing spectral domain OCT (SD-OCT, Spectralis; Heidelberg Engineering, Heidelberg, Germany). Built-in segmentation systems of the Spectralis device, specifically the Heidelberg Eye Explorer (version 6.9a) were employed for each layer thickness measurement from the SD-OCT. The retinal layer thickness in the parafoveal area was determined by averaging the values from the superior, nasal, inferior, and temporal segments within the 1–3 mm range. The thicknesses of segmented layers, including the retinal nerve fiber layer (NFL), ganglion cell layer (GCL), and inner plexiform layer (IPL), were measured. The ganglion cell complex (GCC) thickness, representing the thickness of the inner retinal layer, was derived by adding the thicknesses of the NFL, GCL, and IPL.
Any scans exhibiting motion artifacts, misalignment, segmentation system errors, incomplete image taking, or low resolution were excluded from the analysis.
VD measurement using OCTA
OCTA was performed using a Spectralis OCT2 device (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Images of the SCP (defined as the layer from the ILM to IPL), the DCP (defined as the layer from IPL outer border OPL), and the CC (20 μm thick layer from Bruch’s membrane) were visualized automatically by the device embedded software. A 6.0 × 4.5 mm OCTA scan, centered on the fovea, was acquired. The VD was calculated using open-source processing software (ImageJ, National Institutes of Health, Bethesda, MD, USA). In particular, 8-bit images were selected and adjusted using default threshold settings. This automatic adjustment distinguishes features of interest from the background in segmented grayscale images. Using this binarised image, the VD was subsequently calculated by dividing the area of white pixels by the total number of pixels. OCTA quality was automatically assessed by the device’s embedded software, with a scale ranging from 0 (no signal) to 40 dB (excellent quality). Images exhibiting motion artifacts, fixation loss, segmentation errors, and OCTA quality less than 30 dB were excluded.
Statistical analyses
After confirming that the data followed a normal distribution using the Kolmogorov-Smirnov test, demographic information and ocular parameters were compared among groups using ANOVA and the Bonferroni post hoc test. The chi-square test was utilized for comparisons of categorical data. To control the effect of covariates, including T2DM duration, analysis of covariance was conducted. In the DR + HTN group, univariate and multivariate linear regression analyses were performed to identify factors associated with the VD of the SCP, DCP, and CC. All statistical analyses were performed using SPSS software (version 18.0; IBM Corp., Armonk, New York, USA).
Results
Demographics
A total of 227 eyes were included; 66 eyes in the T2DM group, 78 eyes in the DR-HTN group, and 67 eyes in the DM + HTN group (Table 1). The ages of each group were 62.7 ± 12.8, 63.5 ± 9.4, and 66.1 ± 9.7 years, respectively (P = 0.120). The T2DM durations for the T2DM group, DR-HTN group, and DR + HTN group were 6.0 ± 6.0, 14.8 ± 7.9, and 15.7 ± 8.9 years, respectively (P < 0.001). The HTN duration in the DR + HTN group was 14.5 ± 9.0 years. Other factors, including HbA1c, sex, laterality, DR severity, BCVA, spherical equivalent, IOP, and axial length were not significantly different among the groups.
Inner retinal layer thicknesses in each group
The mean GCC thicknesses for the T2DM, DR-HTN, and DR + HTN groups were 112.2 ± 12.3, 109.2 ± 13.7, and 106.2 ± 11.2 μm, respectively, showing significant differences after adjusting for T2DM duration (P = 0.045) (Table 2). The DR + HTN group had a significantly thinner GCC than the T2DM group in post hoc analysis (P = 0.020). The mean GCL thicknesses were 49.3 ± 6.6, 47.6 ± 7.2, and 44.9 ± 6.2 μm (P = 0.003), and IPL thicknesses were 40.3 ± 4.1, 39.1 ± 4.9, and 39.9 ± 4.1 μm (P = 0.015) in the DM, DR, and DR + HTN groups, respectively.
VD in each group
The mean VDs of the SCP for the T2DM, DR-HTN, and DR + HTN groups were 25.4 ± 5.0, 24.3 ± 8.9, and 21.2 ± 7.1%, respectively, showing significant differences after adjusting for DM duration (P = 0.014) (Table 3). Post hoc analyses revealed significant differences when comparing the T2DM group vs. the DR + HTN group (P = 0.003), and the DR-HTN group vs. the DR + HTN group (P = 0.032). The mean VDs of the DCP in each group were 25.9 ± 4.3, 22.9 ± 8.5, and 20.2 ± 6.6%, respectively (P < 0.001 after adjustment for DM duration). In post hoc analyses, the T2DM group vs. the DR-HTN group (P = 0.031), the T2DM group vs. the DR + HTN group (P < 0.001), and the DR-HTN group vs. the DR + HTN group (P = 0.047) comparisons remained significant results. The mean VDs of the CC were 32.3 ± 4.2, 30.4 ± 8.2, and 27.6 ± 5.8% in each group, respectively (P < 0.001 after adjustment for DM duration). In post hoc analyses, the T2DM group vs. the DR + HTN group (P < 0.001) and the DR-HTN group vs. the DR + HTN group (P = 0.034) comparisons remained significant results (Fig. 1).
Bar graphs depict the thickness of the ganglion cell complex and the vessel density of the superficial capillary plexus, deep capillary plexus, and choriocapillaris in each group. T2DM group: subjects with type 2 diabetes mellitus without clinical diabetic retinopathy (DR), DR-HTN group: subjects with DR without hypertension (HTN), DR + HTN group: subjects with both DR and HTN. Error bars indicate mean ± standard deviation. *Statistically significant differences
Linear regression in the DR + HTN group
In the SCP, univariate analysis identified HTN duration (B = -0.27, P = 0.005) and BCVA (B = -14.25, P = 0.004) as significant factors associated with VD. In multivariate analysis, both HTN duration (B = -0.24, P = 0.010) and BCVA (B = -12.71, P = 0.008) continued to show significance (Table 4). In the DCP, univariate analysis revealed HTN duration (B = -0.23, P = 0.010) and BCVA (B = -9.47, P = 0.046) as significant, and multivariate analysis retained significance for HTN duration (B = -0.21, P = 0.016) (Fig. 2). No significant factors were associated with VD in the CC.
Discussion
In patients with T2DM, HTN induces upregulation of the renin-angiotensin-aldosterone system, oxidative stress, and vascular inflammation. This leads to arterial remodeling, and consequently, an increased prevalence and severity of macrovascular diseases such as cardiovascular disease and stroke [18]. Moreover, HTN exacerbates diabetic microangiopathy, including endothelial permeability and vascular smooth muscle cell dysfunction. As such, the risk for microvascular diseases like nephropathy and retinopathy significantly escalates when HTN and T2DM coexist [19]. Indeed, our previous study found that the vessel length density in the control, patients with T2DM, and those with both T2DM and HTN was 20.43 ± 1.16, 19.50 ± 1.45, and 18.19 ± 20.06 mm− 1, respectively (P < 0.001), and the GC-IPL thickness was 84.9 ± 8.8, 83.5 ± 5.8, and 78.7 ± 8.7 μm, respectively (P < 0.001), indicating greater impairment of the microvasculature and inner retinal layers in patients with T2DM with HTN than those with T2DM alone. [20] However, few studies have reported damage to the inner retinal layer and microvasculature when HTN and DR coexist. In this study, we discovered that GCC thickness tended thinner and the VDs of the SCP, DCP, and CC were significantly lower in the DR + HTN group than in the DR-HTN group. Additionally, HTN duration was significantly associated with the VDs of SCP and DCP.
Ito et al. [21]. found that inner retinal thickness tended to decrease as DR progressed. Kong et al. [22]. found that inner retinal thickness was lower in subjects with systemic HTN than those with normotensive subjects. As such, while previous studies have reported thinning of the inner retinal layers due to HTN or DR individually, there are few studies on the impact of their coexistence on inner retinal layer damage. In our study, the GCC thickness was significantly different among the groups, with the DR + HTN group presenting the thinnest GCC thickness. A previous study reported more aggressive retinal inflammatory alterations with elevated expression levels of ICAM-1, VEGF, and NF-κB in the retina of hypertensive diabetic rats compared to normotensive diabetic rats in their experimental models [23]. This condition may amplify neural apoptosis, glial cell reactivity, and various pathological biochemical pathways linked to the mechanism of diabetic retinal neurodegeneration (DRN), which would result in a more pronounced reduction of inner retinal layer thickness. Apart from intensified DRN, ischemic damage caused by HTN could also contribute to more severe inner retinal damage. Nevertheless, our post hoc analyses did not yield significant results, suggesting that further studies with larger sample sizes are needed.
The VDs of the SCP, DCP, and CC were significantly different among the groups. In particular, that of the DR + HTN group was significantly lower than that of the DR-HTN and T2DM groups. In DR, the infiltration of circulating immune cells and serum proteins into the retina and vitreous, as well as endothelial cell injuries caused by chronic hyperglycemia, can result in acellular retinal capillaries [24, 25]. Such changes are closely linked to a compromised antioxidant defense system, along with increased production of free radicals and VEGF. [26] These effects could also be influenced by altered expressions of enzymes responsible for removing free radicals, as well as epigenetic changes, including DNA methylation and post-translational modifications in the histones. [27] This, in turn, may impair macular microvasculature. In HTN, atherosclerosis, increased vascular resistance and rigidity, and vasoconstriction as an autoregulatory response may lead to ischemic retina damage, which is reflected in the impaired macular microvasculature [28]. The convergence of these two mechanisms might lead to more severe retinal microvasculature impairment. The microvascular damage due to coexisting HTN in DR patients appears to be more prominent than structural changes in the inner retinal layer, as the GCC thickness was not significantly different between the DR-HTN and DR + HTN groups in post-hoc analysis. This severe microvascular damage might be linked to the increased risk or the initial development and progression of DR due to HTN [29,30,31]. Therefore, physicians should evaluate the retinal microvasculature in patients with DR, considering that microvascular damage may be more severe when HTN is comorbid.
The VDs of SCP and DCP were significantly associated with HTN duration in the DR + HTN group. Lim et al. [32]. reported that VD and perfusion density were significantly reduced and the foveal avascular zone tended to be larger in patients with prolonged HTN. Additionally, in our previous study, we found a significant correlation between HTN duration and peripapillary RNFL thickness in patients with both T2DM and HTN [33]. This suggests that HTN duration could have a significant impact on macular microvasculature, much like it does on the thickness of peripapillary RNFL, in DR patients. On the other hand, the T2DM duration, a known factor related to retinal microvasculature in T2DM patients, was not significantly associated with macular VD in the DR + HTN group in this study [34, 35]. Indeed, Durbin et al. [11]. reported that no correlation was detected between VD or foveal avascular zone metrics and duration of diabetes in patients with DR. Thus, the duration of HTN may be a more crucial factor than T2DM duration for macular microvasculature in patients with both DR and HTN.
Previous studies have documented the relationship between visual acuity and macular microvasculature parameters in patients with T2DM [36, 37]. Meshi et al. [37]. found that VDs of the SCP and DCP were negatively correlated with logMAR visual acuity in patients with diabetes without clinical DR. This association was also observed in patients with DR. Samara et al. [38]. found a positive correlation between VD and visual acuity at both the SCP and DCP levels in patients with DR. In our study, VDs of SCP and DCP were significantly associated with BCVA in univariate analyses, suggesting a continued link between VD and BCVA even when HTN is present. However, only the VD of the SCP remained a significant association in multivariate analyses. Some studies have indeed found a significant correlation between BCVA and the VD of the SCP but not the DCP in patients with T2DM or DR [39,40,41]. This might be related to the fact that the DCP, known to be more affected in the early stages of these diseases, does not cause noticeable visual impairment at this stage [36, 42]. Therefore, SCP may have a more direct connection to visual acuity than DCP in DR patients with HTN.
The HbA1c levels among the three groups were not significantly different, and they were not significantly associated with retinal VD in the DR + HTN group in this study. Although blood glucose control is crucial in preventing or reducing retinal damage caused by DR, other factors appear to have a greater association with retinal VD when blood glucose is relatively well-controlled.
This study had certain limitations. First, its retrospective nature may have introduced some selection bias. Second, we were unable to determine the relationship between impaired retinal microvasculature and various aspects of visual function due to the lack of tests such as microperimetry, color-vision testing, or contrast sensitivity testing. Further research is needed to understand how the observed differences in this study results have affected various visual functions. Third, due to the limitations of a retrospective analysis, we could not consider various systemic factors in our analysis. Future studies that include renal function, body mass index, dyslipidemia, detailed family history, fasting blood glucose, and the use of statins will enable a more accurate analysis. Fourth, due to data scarcity, we were unable to include more diverse groups such as patients with only HTN or those with both T2DM and HTN. If these groups and normal controls had been included, a more comprehensive analysis could have been conducted. Additionally, despite adjusting for it in the analysis, the difference in the duration of T2DM among groups and the wide confidence interval range in the linear regression analysis, which may indicate relatively lower accuracy, can be the statistical limitations of this study. However, a strength of our study was that we identified more severe impairment of macular microvasculature in DR patients when HTN is present, a finding not previously reported, to the best of our knowledge.
In conclusion, HTN leads to more severe impairment of macular microvasculature in addition to structural changes in the inner retina in patients with DR. This may be linked with the accelerated deterioration of DR when HTN is concomitant. Similar to other organs, the retina also experiences exacerbated damage when T2DM is complicated by HTN. Additionally, the duration of HTN was significantly associated with VDs of SCP and DCP in DR patients with HTN, whereas the duration of T2DM was not. This suggests that HTN duration may have a greater impact on macular microvasculature than T2DM duration when DR and HTN coexist. Based on these results, further research is needed to investigate how HTN actually influences the progression of DR and various aspects of visual function.
References
Song P, Yu J, Chan KY, Theodoratou E, Rudan I (2018) Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Global Health 8(1)
Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A (2020) The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol 18(2):104–109
Kaštelan S, Orešković I, Bišćan F, Kaštelan H (2020) Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochemia Med 30(3):385–399
Teo ZL, Tham Y-C, Yu M et al (2021) Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128(11):1580–1591
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865):813–820
Sung J-Y, Lee M-W, Lim H-B, Ryu C-K, Yu H-Y, Kim J-Y (2022) The ganglion cell-inner plexiform layer thickness/vessel density of superficial vascular plexus ratio according to the progression of diabetic retinopathy. Investig Ophthalmol Vis Sci 63(6):4–4
Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ (2021) Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Invest 12(8):1322–1325
Fraser-Bell S, Symes R, Vaze A (2017) Hypertensive eye disease: a review. Clin Exp Ophthalmol 45(1):45–53
Bourke K, Patel MR, Prisant LM, Marcus DM (2004) Hypertensive choroidopathy. J Clin Hypertens 6(8):471–472
Sun Z, Tang F, Wong R et al (2019) OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study. Ophthalmology 126(12):1675–1684
Durbin MK, An L, Shemonski ND et al (2017) Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol 135(4):370–376
Remolí Sargues L, Monferrer Adsuara C, Castro Navarro V, Navarro Palop C, Montero Hernández J, Cervera Taulet E (2023) Swept-source optical coherence tomography angiography automatic analysis of microvascular changes secondary to systemic hypertension. Eur J Ophthalmol 33(3):1452–1458
Chua J, Le TT, Sim YC et al (2022) Relationship of quantitative retinal capillary network and myocardial remodeling in systemic hypertension. J Am Heart Association 11(6):e024226
Borde-Perry WC, Campbell KL, Murtaugh KH, Gidding S, Falkner B (2002) The association between hypertension and other cardiovascular risk factors in young adult African americans. J Clin Hypertens 4(1):17–22
Iglay K, Hannachi H, Joseph Howie P et al (2016) Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin 32(7):1243–1252
Ning Z, Song Z, Wang C et al (2021) How Perturbated metabolites in Diabetes Mellitus affect the pathogenesis of hypertension? Front Physiol 12:705588
Al-Azzam N, Al-Azzam S, Elsalem L, Karasneh R (2021) Hypertension prevalence and associated factors among patients with diabetes: a retrospective cross-sectional study from Jordan. Annals Med Surg 61:126–131
Petrie JR, Guzik TJ, Touyz RM (2018) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol 34(5):575–584
Lopes de Faria JB, Silva KC, Lopes de Faria JM (2011) The contribution of hypertension to diabetic nephropathy and retinopathy: the role of inflammation and oxidative stress. Hypertens Res 34(4):413–422
Lee M-W, Koo H-M, Lee W-H, Park J-H, Lee Y-H, Kim J-Y (2021) Impacts of systemic hypertension on the macular microvasculature in diabetic patients without clinical diabetic retinopathy. Investig Ophthalmol Vis Sci 62(12):21–21
Ito H, Ito Y, Kataoka K et al (2020) Association between retinal layer thickness and perfusion status in extramacular areas in diabetic retinopathy. Am J Ophthalmol 215:25–36
Kong M, Kwun Y, Sung J, Ham D-I, Song Y-M (2015) Association between systemic hypertension and macular thickness measured by optical coherence tomography. Investig Ophthalmol Vis Sci 56(4):2144–2150
Rosales MA, Silva KC, de Faria JBL, de Faria JML (2010) Exogenous SOD mimetic tempol ameliorates the early retinal changes reestablishing the redox status in diabetic hypertensive rats. Investig Ophthalmol Vis Sci 51(8):4327–4336
Cantón A, Martinez-Cáceres EM, Hernández C, Espejo C, García-Arumí J, Simó R (2004) CD4-CD8 and CD28 expression in t cells infiltrating the vitreous fluidin patients with proliferative diabetic retinopathy: a flow cytometric analysis. Arch Ophthalmol 122(5):743–749
Simó R, Stitt AW, Gardner TW (2018) Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61:1902–1912
Kern TS, Kowluru RA, Engerman RL (1994) Abnormalities of retinal metabolism in diabetes or galactosemia: ATPases and glutathione. Investig Ophthalmol Vis Sci 35(7):2962–2967
Kowluru RA, Kowluru A, Mishra M, Kumar B (2015) Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res 48:40–61
Wong TY, Mitchell P (2004) Hypertensive retinopathy. N Engl J Med 351(22):2310–2317
Klein R, Klein BE, Moss SE, Cruickshanks KJ (1998) The Wisconsin epidemiologic study of Diabetic Retinopathy: XVII: the 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 105(10):1801–1815
Silva KC, Pinto CC, Biswas SK, Lopes de Faria JB, Lopes de Faria JM (2007) Hypertension increases retinal inflammation in experimental diabetes: a possible mechanism for aggravation of diabetic retinopathy by hypertension. Curr Eye Res 32(6):533–541
Hammes H-P, Brownlee M, Edelstein D, Saleck M, Martin S, Federlin K (1994) Aminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive rat. Diabetologia 37:32–35
Lim HB, Lee MW, Park JH, Kim K, Jo YJ, Kim JY (2019) Changes in ganglion cell–inner plexiform layer thickness and retinal microvasculature in hypertension: an optical coherence tomography angiography study. Am J Ophthalmol 199:167–176
Lee M-W, Park G-S, Lim H-B et al (2021) Effect of systemic hypertension on peripapillary RNFL thickness in patients with diabetes without diabetic retinopathy. Diabetes 70(11):2663–2667
Lee M-W, Lee W-H, Ryu C-K et al (2020) Effects of prolonged type 2 diabetes on the inner retinal layer and macular microvasculature: an optical coherence tomography angiography study. J Clin Med 9(6):1849
Altinisik M, Kahraman NS, Kurt E, Mayali H, Kayikcioglu O (2022) Quantitative analysis of early retinal vascular changes in type 2 diabetic patients without clinical retinopathy by optical coherence tomography angiography. Int Ophthalmol 42(2):367–375
Kim T-Y, Song Y-Y, Na Y-J, Lee Y-H, Kim J-Y, Lee M-W (2022) The impairment of the deep vascular complex in prolonged type 2 diabetes patients without clinical diabetic retinopathy. PLoS ONE 17(6):e0269182
Meshi A, Chen KC, You QS et al (2019) Anatomical and functional testing in diabetic patients without retinopathy: results of optical coherence tomography angiography and visual acuity under varying contrast and luminance conditions. Retina (Philadelphia Pa) 39(10):2022
Samara WA, Shahlaee A, Adam MK et al (2017) Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology 124(2):235–244
Li Z, Alzogool M, Xiao J, Zhang S, Zeng P, Lan Y (2018) Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol 55:1075–1082
AttaAllah HR, Mohamed AAM, Ali MA (2019) Macular vessels density in diabetic retinopathy: quantitative assessment using optical coherence tomography angiography. Int Ophthalmol 39:1845–1859
Ghassemi F, Fadakar K, Berijani S, Babeli A, Gholizadeh A, Sabour S (2021) Quantitative assessment of vascular density in diabetic retinopathy subtypes with optical coherence tomography angiography. BMC Ophthalmol 21(1):1–13
Sambhav K, Abu-Amero KK, Chalam KV (2017) Deep capillary macular perfusion indices obtained with OCT angiography correlate with degree of nonproliferative diabetic retinopathy. Eur J Ophthalmol 27(6):716–729
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Design and conduct of the study (M.W.L.); Collection of data (H.Y.Y., J.T.K.); Analysis and interpretation of data (M.W.L., Y.Y.S.); Writing the article (H.Y.Y., M.W.L.); Critical revision of the article (M.W.L.); Final approval of the article (H.Y.Y., J.J.k, Y.Y.S., J.T.K., M.W.L.)
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance to the tenets of the Declaration of Helsinki and approved by the Institutional Review Board/Ethics Committee of Konyang University Hospital, Daejeon, Republic of Korea (No. 2023-05-027). This article does not contain any studies with animals performed by any of the authors.
Informed consent
The requirement for informed consent was waived by the Institutional Review Board/Ethics Committee of Konyang University Hospital due to the retrospective nature of the study.
Competing interests
No authors have any financial/conflicting interests to disclose. No conflicting relationship exists for any author.
Additional information
Managed by Massimo Federici.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, HY., Kim, JJ., Kim, JT. et al. Impact of systemic hypertension on inner retinal layer thickness and macular microvasculature in patients with diabetic retinopathy. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02355-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00592-024-02355-5