Abstract
Aims
Advanced glycation end products (AGEs) have been implicated in pulmonary and renal fibrosis. Herein, we investigated whether AGEs are associated with liver fibrosis and examined the underlying mechanism by focusing on hepatic stellate cell (HSC) activation and autophagy induction.
Methods
Liver fibrosis was assessed by transient elastography (FibroScan). Serum AGE levels were determined by ELISA. Rat primary HSCs and HSC-T6 were treated with BSA–AGEs, cell proliferation was examined by WST-1 assay, and cell activation was evaluated by qPCR for transcripts of α-SMA and collagen type Iα1 and by Western blotting. Autophagy was measured by detection of LC3-II lipidation, p62 degradation, and puncta GFP-LC3 formation. Receptor of AGE (RAGE)-blocking antibodies and soluble RAGE were employed to inhibit AGE–RAGE signaling.
Results
First, elevated AGE levels were observed in CHC patients than patients with chronic hepatitis B, especially in those with insulin resistance. Second, compared to controls, AGE-treated rat primary HSCs displayed an enhanced cell proliferation (1.39-fold), increased transcripts of α-SMA (2.40-fold) and proCOL1A1 (1.76-fold), and a higher level of α-SMA protein (1.85-fold). Moreover, AGE-induced HSC activation improved autophagy flux, as evidenced by significantly more LC3-II lipidation, p62 degradation, as well as GFP-LC3 puncta formations. In addition, our results showed that AGE-induced HSC autophagy and HSC activation could be reduced by RAGEs.
Conclusion
AGEs were found to induce autophagy and activation of HSCs, which subsequently contributes to the fibrosis in CHC patients. Blocking AGE–RAGE signaling may be a promising way to alleviate fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 170 million people worldwide are chronically infected with hepatitis C virus (HCV). Chronic HCV infection is the leading cause for the development of liver fibrosis. While fibrosis remains largely asymptomatic for years, it slowly progresses to cirrhosis, which is an end-stage liver disease and the major cause of HCV-related morbidity and mortality. Treatment with pegylated interferon and ribavirin can prevent advanced liver fibrosis. A recent discovery suggests that insulin resistance (IR) can weaken the efficacy of anti-HCV therapy, resulting in hepatic fibrosis [1]. Although efforts have been devoted to characterize the biological mechanisms, the primary cause remains poorly understood.
Advanced glycation end products (AGEs), a heterogeneous group of irreversible reactive derivatives formed by non-enzymatic glycation and oxidation of proteins and lipids, elicit oxidative stress generation, subsequently evoke inflammation [2], and have been implicated in pulmonary [3] and renal fibrosis [4]. AGE levels are elevated in non-alcoholic steatohepatitis patients (NASH) [5] and chronic alcohol misuse patients [6, 7]. There are no data on AGE levels in hepatitis C patients; however, AGE levels independently correlate with IR [8]. Our clinical data previously have shown that IR is closely associated with HCV infection and advanced liver cirrhosis [9]. These observations led us to speculate that AGEs could also play a role in the pathogenesis of liver fibrosis, especially in those with IR.
Hepatic stellate cells (HSC) play a central role in liver fibrosis [10]. Activation of HSCs is a pivotal event in the development of cirrhosis. In HSC line LI90, glycer-AGEs increased cell proliferation and upregulated transcripts encoding α-SMA and TGF-β [11]. Beside this, little is known about the impact of AGEs on HSCs. Recently, several studies and our data support that autophagy contributes to HSC activation [12, 13]. Moreover, AGEs binding to murine HSCs can stimulate the generation of reactive oxygen species, which subsequently induces autophagy [14]. Finally, an increased autophagosome number is observed in liver specimens from hepatitis C-infected patients by using electron microscopy [15]. However, knowledge about the capability of AGEs activating HSC and its underlying molecular mechanism is limited.
To address the role played by AGEs in live fibrosis, we examined whether AGEs were associated with fibrosis, especially in those with IR, and investigated the effect of AGEs on autophagy in HSCs.
Materials and methods
Subjects
A total of 79 patients with CHC were recruited for this study. All patients attended the First Teaching Hospital of Xi’an JiaoTong University from May 2013 to April 2014 and were recently diagnosed with chronic HCV infection. Clinical parameters including liver stiffness, fasting insulin level, liver biochemistry, coagulation function, HCV RNA load, and upper abdominal CT scan were evaluated. Esophageal varices were also assessed for most patient participants using electronic gastroscopy or barium radiography. Contemporarily, a group of 79 age- and liver function-matched patients with hepatitis B virus infection were enrolled as control. All patients provided written informed consent to participate in this research, which was approved by the Ethics Committee of First Affiliated Hospital of Xi’an Jiaotong University.
Measurements
Liver stiffness was measured by transient elastography (FibroScan, Echosens, Paris). Liver fibrosis was assessed using APT-to-platelet ratio index (APRI) [AST (U/L)/upper limit of normal platelet (109/L)]. An APRI equivalent to or above 1.5 was considered significant fibrosis (corresponding to a biopsy score ≥F2). IR was evaluated in homeostatic model assessment (HOMA) [fasting insulin (mIU/L) × fasting plasma glucose (mmol/L)/22.5] [9].
Serum AGE assay
Carboxy-methyl lysine (CML) is one of the most prevalent AGEs and widely used as a representative biomarker of glycosylation in numerous animal and human studies [12, 16]. Serum CML concentration was measured by AGE–CML enzyme-linked immunosorbent assay (ELISA; MicroCoat GmbH, Bernried, Germany), and the results were reported in μg/mL.
Reagents
GFP-LC3 construct was kindly provided by Dr. Zixu Mao (Emory University, Atlanta, USA). Anti-RAGE (#6479), anti-LC3 (#8899), and anti-SQSTM1/p62 (#5114) antibodies were obtained from cell signaling (Danvers, MA). Anti-α-SMA (ab5694) antibody from Abcam (Cambridge, MA), and anti-GAPDH (sc-74424), anti-actin (c-11), and RAGE-blocking antibodies (n-16 and c-20) were acquired from Santa Cruz biotechnology (Dallas, TX). BSA–AGEs were purchased from BioVision Inc (Milpitas, CA).
Isolation, culturing, and treatment of rat primary HSCs
HSCs were isolated from killed rat via sequential digestion of the liver with pronase and collagenase, followed by gradient centrifugation, as previously described [17, 18]. Adequate cell viability (95 %) was determined using trypan blue exclusion staining accompanied with 90–99 % purity. Rat HSCs were then cultured in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO/BRL; Grand Island, NY) supplemented with 10 % fetal bovine serum and 2 mM l-glutamine and maintained at 37 °C and 5 % CO2-humidified atmosphere.
Culture of HSC-T6
HSC-T6 cells were subcultured as described elsewhere [19]. Briefly, cells were maintained in Waymouth MB 752/1 medium (Gibco, NY) supplemented with 10 % fetal bovine serum (FBS) and 50 U/ml penicillin/streptomycin at 37 °C under 5 % CO2. Cells were split when 70–80 % confluence was reached. For matrigel culture, HSC-T6 cells were trypsinized and 0.5 mL cell suspension (106 cells/mL) was seeded on plastic dishes coated with matrigel (50 μL/cm2 of growth surface). Gel was prepared according to the manufacturer’s instructions (BD Biosciences, Bedford, MA).
Cell proliferation assay
To test the proliferative effect of AGEs on HSC, BSA–AGEs, and non-glycated BSA were added to the culture medium. Approximately 104 HSC cells were introduced to individual well in a 96-well plate and subcultured for 48 h prior to spectrophotometric analysis. Ten microliters of WST-1 reagent (Roche Applied Science) was then added into each well and incubated for 1 h. Absorbance at 450 nm was measured using a Synergy HT Multi-Mode Microplate Reader (Bio-Tek).
Quantitative real-time PCR
Total RNA was isolated using TRIzol® (Gibco/BRL). Single-stranded complementary DNA was synthesized from 2 μg of RNA using RETROscript® First Strand Synthesis kit (Ambion, AM1710) and followed by the qPCR reactions with primer sets as follows: α-SMA, 5′- ACTGGGACGACATGGAAAAG-3′ (F) and 5′-TAGATGGGGACATTGTGGGT-3′ (R); proCOL1A1, 5′- GCTCCTCTTAGGGGCCACT-3′ (F) and 5′-CCACGTCTCACCATTGGGG-3′ (R); and actin, 5′-AAGGACTCCTATAGTGGGTGACGA-3′ (F) and 5′-ATCTTCTCCATGTCGTCCCAGTTG-3′ (R). qPCR was performed using SYBR Green assay contained 12.5 μL SYBR Green PCR Master Mix (Bio-Rad) [20, 21].
SDS-PAGE and Western blot analysis
Western blots were performed in SDS-PAGE sample buffer containing protein extracts of HSC-T6 cells and primary rat HSC cultures. A total of 50 µg of total proteins were loaded onto a 4–12 % polyacrylamide gel and then electrically blotted on nitrocellulose membrane (Bio-Rad). Filters were hybridized with antibodies against RAGE (at a 1:1000 dilution) or LC3-II (1:1000) or SQSTM1/p62 (1:1000) or GAPDH (1:10,000) and then detected using HRP-conjugated specific secondary antibodies and ECL. ImageJ was used to quantify bands.
Measurement of autophagic flux
HSC-T6 cells were plated at 1.5 × 105 cells/well in 12-well plates and transfected with 50 ng of GFP-LC3 with Lipofectamine 2000 (Invitrogen, California). For the detection of the autophagic flux following AGE exposure, GFP-LC3 puncta were visualized using a Leica DM6000B microscope at 24 h after AGE treatment. Quantitative micrograph analysis was carried out using ImageJ software (National Institute of Health).
Statistical analysis
Data were presented as mean ± standard deviation (range) unless otherwise specified. Statistical analysis was performed by SPSS software 13.0 (SPSS Inc., Chicago, IL). The following variables were included in the univariate analysis: demographics (age, sex), alcohol intake, viral titer, and other test results (e.g., ALT, AST, ALP, GGT, CHOL, TBIL, DBIL, TP, ALB, BUN, Cr, Glu, UA, Na, K, Cl, RBC, WBC, PLT, PT, APTT, FIB, LSM, AGEs, and APRI). All continuous variables were analyzed with Student’s t test or one-way ANOVA followed by post hoc Tukey’s analysis or Kruskal–Wallis test depending on the data distribution, and category variables were compared by x 2. A two-tailed P value of <0.05 was considered statistically significant.
Results
AGEs were elevated in CHC patients with IR
Previous studies have shown that pulmonary fibrosis and renal fibrosis are associated with a higher AGE level [3, 4]. However, AGE level in HCV-related fibrosis is rarely reported. Our earlier study demonstrated that CHC is associated with IR [22], assessed by HOMA–IR. To evaluate the AGE level in patients with CHC, a cohort of 79 HCV patients was enrolled, and concurrently, age- and sex- matched patients with chronic hepatitis B were included as control subjects. Differences in disease duration, BMI, alcohol intake, and cholesterol and glucose concentrations that have potential impacts on both IR and AGE levels were insignificant between the two groups (Table 1). In comparison with patients with hepatitis B, a remarkable elevation in AGE level was observed in CHC patients (0.77 ± 0.82 μg/mL vs. 1.21 ± 0.71 μg/mL, P = 0.013), which is consistent with our previous findings [9]. Next, patients with CHC were divided into IR or non-IR groups based on HOMA–IR, among which 33 chronic HCV patients (41.72 %) were determined as IR, while 46 patients (58.23 %) were non-IR. Notably, CHC patients without IR did not exhibit an increased AGE level when compared to CHB patients (0.77 ± 0.82 μg/mL vs. 0.82 ± 0.23 μg/mL, P = 0.24), whereas the highest levels (0.82 ± 0.23 μg/mL vs. 1.52 ± 0.65 μg/mL, P = 0.002) were found in the CHC patients with IR (Table 2). Thus, elevated AGE level was associated with IR, but not HCV infection.
AGE-associated fibrosis in patients with IR
Next, association between IR and fibrosis, determined by AST-to-platelet ratio index (APRI) [23] and transient elastography [24], was also investigated. Opposed to non-IR CHC patients, CHC patient with IR displayed a higher APRI (0.81 ± 0.04 vs. 2.04 ± 0.15, P < 0.0001) and increased liver stiffness (9.66 ± 4.73 kpa vs. 14.00 ± 6.07, P = 0.0012) (Fig. 1). Our results indicated that AGE level was correlated with IR and IR associated with fibrosis, and it was thus postulated that whether AGEs were also involved in IR-related fibrosis. As shown in Fig. 2b, a positive correlation between liver stiffness and serum AGEs was identified (r = 0.5731, P < 0.001). Furthermore, our data show that the correlation between liver stiffness and serum AGEs was insignificant in non-IR group (r = 0.0774, P = 0.596) (Fig. 2c), whereas it was more significant in IR group (r = 0.6640, P < 0.001) (Fig. 2d). Thus, the role of AGEs in the pathogenesis of fibrosis needs further investigation.
AGEs stimulate primary rat HSC proliferation
HSCs have been considered as the key cellular source of extracellular matrix synthesis in the liver. Morphology changes of HSCs from quiescent to myofibroblast-like cells indicate the enhanced synthesis of extracellular matrix components. To determine whether AGEs affect the proliferation properties of HSCs, primary rat HSC cells were incubated with different concentrations of BSA–AGEs ranging from 12.5 to 200 µg/mL for 48 h, and cell proliferation was measured using WST-1 assay. In contrast to non-glycated BSA control, significant increase in cell viability was seen in AGE-treated cells, and the maximum cell proliferation was observed at 100 μg/ml BSA–AGEs (Fig. 3).
AGEs induce HSC activation
Consistent with cell proliferation data, qPCR results also suggested a dose-dependent increase in α-SMA and proCOL1A1 transcripts (Fig. 4a, b). Next, we tested whether the protein levels were changed following AGE treatment. HSC-T6 cells were incubated with non-glycated BSA control or 100 μg/mL BSA–AGE for 24 h and analyzed by Western blotting. As expected, AGE treatment significantly stimulated the production of α-SMA protein and demonstrated 1.85- and 3.3-fold increases compared to blank and non-glycated BSA control, respectively (Fig. 4c, d). As a result, treatment with AGEs increased α-SMA and proCOL1A11 at both transcript level and protein level, not only in HSC-T6 cell line but also in primary rat HSCs, showing that HSCs undergo activation process by AGEs.
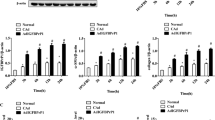
AGEs induce HSC activation. Rat primary cells on day 4 were incubated with non-glycated BSA or BSA–AGEs at the concentration indicated for 4 h, followed by qPCR measurement of mRNA encoding α-SMA (a) and proCOL1A1 (b), transcript level normalized to housekeeping gene GAPDH. Data are shown as mean ± SD from three independent experiments. **P < 0.01 compared to the value of non-glycated BSA control. HSC-T6 cells were treated with BAS or BAS–AGEs at the final concentration of 100 μg/mL for 24 h. Western blotting analysis of α-SMA and LC3-II with specific antibodies as indicated. GAPDH used as protein loading control. Images presented are representative of three independent experiments (c). Protein α-SMA and LC3-II ratios (normalized to GAPDH) were used to quantify fold change relative to control and are shown in (d). *P < 0.05; **P < 0.01 compared to untreated control
AGEs induce autophagy in HSCs
Next, the underlying mechanism for HSC activation by AGEs was investigated. Recent studies suggested that autophagy provides the energy that is essential for HSC activation [12, 13]. Autophagy is a catabolic process through which cytoplasmic components are degraded by their own lysosome. We explored whether AGEs induce autophagy in HSCs by detecting the lipidated form of LC3-II, a widely monitored marker offering biochemical insights on autophagy [25]. Western blot results showed a more intense band of LC3-II, indicating the occurrence of autophagy during HSC activation induced by AGEs (Figs. 4c, d, 5a, b, 6a, c–f).
AGE-induced autophagy in HSC. HSC-T6 cells were treated with non-glycated BSA or BSA–AGEs in the presence or absence of bafilomycin A1 for 24 h. Lysates were analyzed for the protein levels of LC3-II and SQSTM1/p62 as indicated. Images presented are representative of three independent experiments (a). Protein was quantified by densitometry and normalized by GAPDH expression levels. Data were represented as mean ± SD from three independent experiments. **P < 0.01 (b). HSC-T6 cells were transfected with GFP-LC3 for 12 h and then treated with BSA–AGEs or non-glycated BAS control for 24 h. Images are representative of three independent experiments (c). The average number of GFP-LC3 dots per cell was quantified in >10 cells per sample (d). The data shown represent the mean ± SD from three independent experiments. **P < 0.01
Blocking AGE–RAGE axis attenuates autophagy and HSC activation. a HSC-T6 cells in quiescent state highly expressed RAGE 50 KD, and dim RAGE 46 KD was also detected. Upon BAS–AGE stimulation, HSC-T6 cells expressed both 50 KD and 46 KD RAGE in a time-dependent manner. b RAGE was detected in human liver tissue from an CHC patients with IR (left), rat liver tissue, and HSC-T6 cells. HSC-T6 cells were treated with RAGE-blocking antibody at the final concentration of 10 μg/mL (Ab1) or 30 μg/mL (Ab2) for 2 h, followed by AGE exposure for additional 24 h, non-glycated BSA as a control. Lysates were analyzed for the protein levels of LC3-II and α-SMA as indicated. Images are representative of three independent experiments (c). Protein was quantified by densitometry and normalized to GAPDH. The data shown represent the mean ± SD from three independent experiments. **P < 0.01. HSC-T6 cells were treated with sRAGE (20 μg/mL) for 2 h, followed by AGE exposure for additional 24 h, non-glycated BSA as a control. Lysates were analyzed for the protein levels of LC3-II and α-SMA as indicated. Images are representative of three independent experiments (e). Protein was quantified by densitometry and normalized to GAPDH. The data shown represent the mean ± SD from three independent experiments (f). **P < 0.01 (b)
LC3-II itself behaves as a substrate of autophagy and can also be degraded by lysosomes. If cells are treated with the lysosomal proton pump inhibitor bafilomycin A1 (BA), the degradation of LC3-II is blocked, resulting in the accumulation of LC3-II [26]. It is a well-established method for monitoring autophagosome synthesis [25]. In the case that LC3-II further accumulates in the presence of BA, it suggests the enhancement of the autophagic flux; if the LC3-II level maintains unchanged, the autophagosome accumulation is likely attributed to inhibition of autophagic degradation. As shown in Fig. 5a, b, the overall LC3 lipidation was increased (lane 2 vs. lane 1) following AGE treatment, and more importantly, incubation with AGEs and BA together had introduced a further increase in LC3-II lipidation. These results demonstrated that the accumulation of autophagosomes was indeed attributed to the increase in autophagic flux rather than the inhibition of autophagic degradation. Alternatively, autophagic flux can be characterized by measuring the degradation of p62 (SQSTM1/sequestosome), a long-lived scaffolding protein. Differences in p62 expression level between AGE-treated and non-treated cells have been noticed, suggesting an enhanced p62 degradation by AGEs (Fig. 5b).
The puncta formation by GFP-LC3 is a reliable autophagy marker for autophagosomes [25]. Increased formation of LC3 puncta reflects that more autophagosomes have been developed. Figure 5c, d shows the comparison of LC3 puncta formation in GFP-LC3-transfected HSC-T6 cells. The outcome indicated that more autophagosomes were formed during AGE-induced HSC activation.
Taken together, our data showed that AGEs increase LC3 lipidation, autophagic flux, long-lived protein degradation, and autophagosome formation.
Blocking RAGE attenuates HSC activation and autophagy induction
RAGE, receptor of AGEs, is physiologically highly expressed in lung and pathologically expressed in the setting of diabetes, aging, inflammation, neurodegeneration, and tumors; however, its role in HCV-related liver fibrosis remains controversial [27]. In our current attempt, the RAGE expression data were used to delineate its role in HSC activation and proliferation. As shown in Fig. 6a, only 50 KD RAGE was expressed in quiescent HSC-T6 cells, and 46 KD RAGE was merely detected. Upon BAS–AGE stimulation, both 50 KD and 46 KD RAGE bands were expressed, and over the time, the 46 KD RAGE displayed increased levels with the highest band intensity at 48 h of AGE stimulation. In addition, RAGE expression in liver sample from CHC patients with IR and from rat was confirmed by Western blot analysis (Fig. 6b).
To further confirm whether autophagy induction and HSC activation by AGEs are RAGE dependent, RAGE-blocking antibody was employed. As shown in Fig. 6c, d, AGEs alone induce LC3 lipidation and α-SMA expression, which is consistent with our pervious finding, suggesting HSC activation and autophagy induction. However, RAGE-blocking antibody attenuated such effects, especially in HSCs treated with higher concentration. Soluble form of RAGE (sRAGE) binds RAGE ligands, thereby preventing downstream signaling and damage [28]. Next, sRAGE was used to block AGE–RAGE signaling. Consistent with blocking antibody, sRAGE also reduced AGE-induced LC3-II lipidation and α-SMA expression, suggesting that blocking RAGE signaling inhibits AGE-induced autophagy and HSC activation.
Discussion
HSC activation plays a central role in liver fibrosis. Here, our clinical data showed that serum AGE levels were elevated in patients with CHC and correlated with liver stiffness in patients with IR, but not patients without IR, indicating the involvement of AGEs in IR-related liver fibrosis. Next, we found that AGEs stimulate primary rat HSC proliferation and induce α-SMA and proCOL1A1 mRNA transcription and protein expression, suggesting that HSCs were activated by AGEs. Recently, autophagy has been implicated in HSC activation [29]. Our data uncovered AGE-induced autophagy in HSCs. Moreover, blockade RAGE signaling attenuated both autophagy and HSC activation. Our results integrate AGEs, HSC activation, and autophagy into a common framework that underlies the molecular mechanism of IR-related fibrosis.
Chronic HCV infection is more likely to favor IR by the viral protein. IR plays a pivotal role in accelerating the severity of hepatic fibrosis in CHC patients. In non-obese, non-diabetic patients, serum AGE level is correlated with HOMA–IR. This phenomenon drives us to investigate the role AGEs played in fibrosis in HCV-infected patients. Two important clues were found from our clinical data. First, serum AGE level elevated only in patients with HCV infection, but not in patients with HBV infection, and the concentration was much higher in those with IR. Second, AGE level correlated with liver stiffness. The correlation was more apparent in those patients with IR, but not those without IR, which indicated that AGE level may be a useful noninvasive biomarker for assessing fibrosis. Both kidney and liver are involved in the removal of AGEs [30]. In our study, the kidney function of all subjects was normal, and the liver function was compared between HBV group and HCV group. Thus, the accumulation of AGEs was conceivable as a consequence of increased formation de novo. AGE formation can be enhanced by oxidative stress or non-oxidative reactions of sugars and their adducts to proteins and lipids. Compared with chronic hepatitis B, chronic hepatitis C is particularly more effective at generating oxidative stress [31]. This notion could partially explain our observation that AGEs were specifically increased in CHC patients. Finally, our data indicated that elevated AGE level was associated with IR, but not HCV infection. Similar findings were reported by Arsov et al. [32] in 2010, with the conclusion that independent markers of AGE accumulation were age, diabetes mellitus, and cardiovascular disease, but not hepatitis C.
Furthermore, the molecular mechanism of HSC activation by AGEs was investigated. We demonstrated here that AGEs induce autophagy, as indicated by increased LC3 lipidation, autophagosome formation, autophagy flux, and long-lived protein degradation. These results point to autophagy as a possible target in the prevention of HSC activation and liver fibrosis. AGEs exert their effect by binding to different ligands including RAGEs. High constitutive RAGE expression is restricted to the lung, while other tissues display low expression. Although whether HSCs express RAGE remained controversial, we confirmed that RAGE is expressed not only in liver sample from CHC patients and rat, but also in HSC-T6 cells.
More importantly, RAGE antibody reversed AGE-induced autophagy induction and HSC activation, and this observation was reinforced by blocking RAGE signaling using sRAGE. The results in current study not only indicated that autophagy and HSC activation may be coupled, but also suggested that AGE–RAGE axis plays a role in HSC activation and subsequent liver fibrosis. Reduction in the AGE–RAGE signaling, by avoiding overcooked foods, sensitizing insulin function, controlling of high glucose, and oxidant supplement digestion [33], may be a promising way to lower the risk of fibrosis, especially in those CHC patients with IR and increased AGEs.
Abbreviations
- AGEs:
-
Advanced glycation end products
- ALB:
-
Albumin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- APRI:
-
AST-to-platelet ratio index
- APTT:
-
Activated partial thromboplastin time
- AST:
-
Aspartate transaminase
- α-SMA:
-
α-Smooth muscle actin
- BA1:
-
Bafilomycin A1
- BUN:
-
Blood urea nitrogen
- CHOL:
-
Serum cholesterol
- CML:
-
Carboxy-methyl lysine
- CREA:
-
Creatinine
- DBIL:
-
Direct bilirubin
- Fbg:
-
Fibrinogen
- GGT:
-
Gamma glutamyl transpeptidase
- Glu:
-
Glucose
- Hb:
-
Hemoglobin
- HCV:
-
Hepatitis C virus
- HOMA:
-
Homeostatic model assessment
- HSCs:
-
Hepatic stellate cells
- IR:
-
Insulin resistance
- LC:
-
Liver cirrhosis
- LSM:
-
Liver stiffness measurement
- PLT:
-
Platelet
- PT:
-
Prothrombin time
- proCOL1A1:
-
Procollagen α 1 chain type I
- RAGE:
-
Advanced glycation end products
- RBC:
-
Red blood cell count
- TBIL:
-
Total bilirubin
- TP:
-
Total protein
- γ-GGT:
-
gamma Glutamyl transpeptidase
- UA:
-
Uric acid
- WBC:
-
White blood cell count
References
Harrison SA, Hamzeh FM, Han J, Pandya PK, Sheikh MY et al (2012) Chronic hepatitis C genotype 1 patients with insulin resistance treated with pioglitazone and peginterferon alpha-2a plus ribavirin. Hepatology 56:464–473
Piarulli F, Sartore G, Lapolla A (2013) Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta Diabetol 50:101–110
Kyung SY, Byun KH, Yoon JY, Kim YJ, Lee SP et al (2014) Advanced glycation end-products and receptor for advanced glycation end-products expression in patients with idiopathic pulmonary fibrosis and NSIP. Int J Clin Exp Pathol 7:221–228
Zhou GH, Li C, Cai L (2004) Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol 165:2033–2043
Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M et al (2007) Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 22:1112–1119
Marta K, Tomas Z, Petr P, Pavel S, Martin B et al (2004) Advanced glycation end-products in patients with chronic alcohol misuse. Alcohol Alcohol 39:316–320
Goodwin MA, Herath CB, Jia Z, Coughlan M, Forbes JM et al (2009) The role of rage (receptor for advanced glycation endproducts) in hepatic fibrogenesis. J Gastroenterol Hepatol 24:A304
Choi EY, Kwon HM, Ahn CW, Lee GT, Joung BY et al (2005) Serum levels of advanced glycation end products are associated with in-stent restenosis in diabetic patients. Yonsei Med J 46:78–85
He YL, ZY, Wang K, Chen TY, Liu M, Ye F, Zhang SL, Lin SM, Zhao YR (2013) HCV infection and advanced liver cirrhosis were associated insulin resistance. The International Liver Congress™, 2013. Amsterdam, The Netherlands
Yin CY, Evason KJ, Asahina K, Stainier DYR (2013) Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Investig 123:1902–1910
Iwamoto K, Kanno K, Hyogo H, Yamagishi SI, Takeuch M et al (2008) Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol 43:298–304
Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI et al (2012) Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142:938–946
Thoen LFR, Guimaraes ELM, Dolle L, Mannaerts I, Najimi M et al (2011) A role for autophagy during hepatic stellate cell activation. J Hepatol 55:1353–1360
Guimaraes ELM, Empsen C, Geerts A, van Grunsven LA (2010) Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepatol 52:389–397
Thoen LFR, Guimaraes EL, van Grunsven LA (2012) Autophagy: a new player in hepatic stellate cell activation. Autophagy 8:126–128
Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ et al (2008) A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol 172:583–591
Wang X, Tang X, Gong X, Albanis E, Friedman SL et al (2004) Regulation of hepatic stellate cell activation and growth by transcription factor myocyte enhancer factor 2. Gastroenterology 127:1174–1188
He Y, Jin L, Wang J, Yan Z, Chen T, et al (2014) Mechanisms of fibrosis in acute liver failure. Liver Int 35:1–9
Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC et al (2000) An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res 41:882–893
Iwamoto K, Kanno K, Hyogo H, Yamagishi S, Takeuchi M et al (2008) Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol 43:298–304
Petrasek J, Bala S, Csak T, Lippai D, Kodys K et al (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Investig 122:3476–3489
He YL, Yan Z, Wang K, Chen TY, Liu M et al (2010) HCV infection and advanced liver cirrhosis were associated insulin resistance. J Hepatol 52:S88
Lackner C, Struber G, Liegl B, Leibl S, Ofner P et al (2005) Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 41:1376–1382
Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J et al (2008) Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134:960–974
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E (2005) Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1:84–91
Basta G, Navarra T, De Simone P, Del Turco S, Gastaldelli A et al (2011) What is the role of the receptor for advanced glycation end products-ligand axis in liver injury? Liver Transpl 17:633–640
Xu YL, Toure F, Qu W, Lin LL, Song F et al (2010) Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem 285:23231–23238
Thoen LF, Guimaraes EL, Dolle L, Mannaerts I, Najimi M et al (2011) A role for autophagy during hepatic stellate cell activation. J Hepatol 55:1353–1360
Sebekova K, Kupcova V, Schinzel R, Heidland A (2002) Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis—amelioration by liver transplantation. J Hepatol 36:66–71
Clement S, Pascarella S, Negro F (2009) Hepatitis C virus infection: molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses-Basel 1:126–143
Arsov S, Graaff R, Morariu AM, van Oeveren W, Smit AJ et al (2010) Does hepatitis C increase the accumulation of advanced glycation end products in haemodialysis patients? Nephrol Dial Transplant 25:885–891
Raffaelli F, Vignini A, Giulietti A, Alidori A, Borroni F et al (2014) In vitro effects of resveratrol on oxidative stress in diabetic platelets. Acta Diabetol 51:61–69
Acknowledgments
This study was supported by grants from the China National Natural Science Foundation (No. 30700712), and the Major National Science and Technology Projects for Infectious Diseases (11th and 12th Five Year, China) (No. 2008ZX10002-007, No. 2012ZX10002007) and the Fundamental Research Funds for the Central Universities. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Human and Animal Rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Managed by Massimo Federici.
YingLi He and JinQiu Zhu have equally contributed to this work.
Rights and permissions
About this article
Cite this article
He, Y., Zhu, J., Huang, Y. et al. Advanced glycation end product (AGE)-induced hepatic stellate cell activation via autophagy contributes to hepatitis C-related fibrosis. Acta Diabetol 52, 959–969 (2015). https://doi.org/10.1007/s00592-015-0763-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0763-7