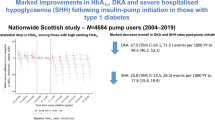
Abstract
Aims
To examine contemporary rates of severe hypoglycemia (SH) and identify the effect of predictors of SH in a pediatric type 1 diabetes population.
Methods
The national diabetes register provided data on children residing in Denmark from 2008 to 2013 in this register-based population study. Robust Poisson regression models were applied.
Results
The study population [n = 2,715 (50.9 % boys), mean (SD) age at onset; 8.1 (4.0) years, diabetes duration; 5.6 (4.9) years] comprised 7,390 person-years of data and 561 events of SH. The overall incidence of SH was 7.6 per 100 person-years. The incidence rate peaked with 16.0 per 100 person-years in 2008 reaching a nadir of 4.9 in 2011. Overall, insulin pump reduced the rate of SH with 27 % compared to any pen treatment (P = 0.003). When stratifying pen treatment, premixed insulin increased the rate of SH by 1.9-fold (P = 0.0015) and NPH increased the rate by 1.6-fold (P = 0.003) versus pump treatment, whereas long-acting insulin analogues were comparable with pump treatment (P = 0.1485). We found no association of SH with glycemic control (P > 0.05).
Conclusions
A nationwide halving in rates of severe hypoglycemia was observed during the study period independent of the prevailing average HbA1c level. Changes in diabetes care and successful educational programs may have influenced the lower incidence rate of severe hypoglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycemic regulation in young type 1 diabetes patients is often like a “rollercoaster-ride” with blood glucose “racing up and down” between the extremes of hyper- and hypoglycemia. The interplay between the fear of acute and long-term complications are potential drivers for regulating blood glucose to sometimes the extremes. The patient’s fear of chronic complications is often surpassed by the fear of the unpleasant symptoms accompanying severe hypoglycemia (SH) causing unconsciousness or seizure, and ultimately without intervention, death [1, 2]. Consequently, a deliberate raise in blood glucose levels is often undertaken to avoid hypoglycemia.
The DCCT study established that tight glycemic regulation prevents or delays long-term complications to diabetes [3–5], but was paralleled by an increase in SH [6]. Recent studies including a meta-analysis did not report this association [7–10]. We have previously shown that the incidence of SH in the 10-year period until 2009 was unchanged with a simultaneous improvement in glycemic control [11]. Clearly, diabetes treatment has changed markedly within the past few years.
The aim of this study was to give an update on contemporary rates of SH during a 5-year time period and determine the effect of potential predictors on the risk of SH after recent changes in insulin therapy.
Materials and methods
Study population
Participants were children and adolescents with type 1 diabetes aged <17 years at diagnosis from the national Danish population-based diabetes register (DanDiabKids) attending one of the 18 diabetes centers for children in Denmark during the period January 1, 2008 to December 31, 2012. During this period, there were 3,006 patients in the register. Patients with diabetes duration <6 months (n = 237 with expected residual-beta cell function), missing data for SH (n = 36) and missing data for treatment regimen (n = 18) during follow-up were excluded. A total of 2,715 patients were eligible for inclusion. The 291 (9.7 %) patients who did not meet the inclusion criteria were comparable with the study population in terms of age at diagnosis, sex, BMI, HbA1c and ethnicity (but had shorter diabetes duration).
Severe hypoglycemia
Severe hypoglycemia was strictly defined according to ISPAD guidelines as a hypoglycemic event leading to altered/loss of consciousness and/or seizures [12]. The time of day and reports of hypoglycemic events that did not include these symptoms were not considered an outcome for this report.
Data collection
Children and adolescents with newly diagnosed type 1 diabetes according to the WHO’s criteria [13] are recorded on a standard registration form submitted (online) to DanDiabKids. Patients are prospectively followed until they are transferred to an adult clinic. The register has data completeness currently at 99 % [14].
Data were collected retrospectively during quarterly assessments in the clinics and summarized in annual reports submitted to the register. The physicians/nurses responsible for data collection at the respective centers attend annual audits to validate outliers, and here, new procedures (definitions) are agreed upon. Data included demographics, records of diabetes treatment regimens and frequency of acute complications (SH). We stratified patients into insulin pen and pump users. Insulin pen users were further categorized according to insulin type use: premixed human insulin (a combination of rapid-acting and intermediate-acting insulin in a fixed proportion), intermediate-acting human insulin NPH (neutral protamine Hagedorn) and long-acting insulin analogues (insulin glargine and detemir). The use of rapid-acting insulin analogues (insulin aspart, lispro and apidra) in combination with one of the above categories was the basis for pen treatment (recommendations were ≥5 injections daily). Insulin pump treatment was based on the use of rapid-acting insulin analogues prescribed to basal and bolus insulin (recommended basal/bolus insulin ratio was 40/60). Daily insulin dose (U kg-1 day-1), insulin types, injection frequency (pump downloads or patient reports if pen treated), frequency of daily blood glucose measurements (glucose meter downloads), percent of rapid-acting insulin (vs. total dose) and annual recordings of centralized measured HbA1c were recorded. The frequency of severe hypoglycemia was recorded during quarterly assessments as a discrete numeric variable (the number of events) and validated by confirming that hypoglycemia was accompanied with loss of consciousness or seizure. Missing registry data were validated by assessing medical records and translated into missing values if they could not be extracted. Ethnicity was based on ethnic origin of the child (native Danish or non-Danish).
Glycemia
HbA1c was measured annually at a central national Diabetes Control and Complications Trial (DCCT) standardized laboratory at Herlev Hospital, Copenhagen, using a high-pressure liquid chromatographic method (Tosoh Bioscience®, South San Francisco, CA, USA). The (DCCT) HbA1c non-diabetic reference range was 4.3–5.8 % corresponding to 23–40 mmol/mol (IFCC). The HbA1c values were validated twice monthly by the European Reference Laboratory and aligned with DCCT values.
The study was performed according to the criteria of the Helsinki II Declaration and was approved by the local ethics committee.
Statistical analyses
The analysis set consisted of multiple records for each patient, and all relevant covariates contributed with events and patient-years to the stratum. The number of contributing patient-years was calculated based on the time at inclusion to the next clinic visit and the time that elapsed between these visits (inter-visit periods). The number of SH was modeled in terms of rates by robust Poisson regression accounting for intra-person correlation. The risk time was calculated based on the length of inter-visit periods (expressed as 100 person-years). To assess the association between glycemic control and severe hypoglycemia, we analyzed the HbA1c level prior to the occurrence of SH. P values for comparisons corresponded to robust Wald test for testing the rate ratio equal to one.
Firstly, we calculated the overall rates of SH for the respective variables (adjusted for calendar year and the variables listed in the respective tables, hereafter multiple adjustments). Proportionality of the incidence rates could be assumed (P = 0.96), and the treatment effect could be summarized as rate ratios [95 % CI]. Secondly, we evaluated the rates of SH for the treatment regimens (pen/pump) separately. This was undertaken because the two treatments differ clinically by the recommended frequency of blood glucose measurements, bolus injections and basal/bolus ratio. The variables were consequently stratified according to groups that were clinically applicable. Out of the 18 pediatric centers reporting to the register, 10 centers care for >100 patients. Center size was stratified accordingly (levels: ≤100 or >100 patients).
Lastly, we assessed the effect of risk factors (multiple comparisons) by means of backward elimination with a cutoff value of 5 % for inter-visit periods. The effect of risk factors was modeled and expressed as rate ratios [95 % CI]. The two sub-categories (and not the actual patients) “blood glucose measurements per week: 0–14,” and “number of daily bolus injections: 0–2” (for pump users) were omitted from the analysis due to no events of SH in these categories. Furthermore in these analyses, no risk time was allocated to the year 2008, and therefore, we could not estimate the baseline rate in 2008. Analyses were conducted using SAS version 9.2.3 (SAS Institute, Inc. Cary, NC, USA) and the statistical programming platform R version 3.0.0 (www.r-project.org).
Results
Demographics of the study cohort
We enrolled 2,715 children [mean (SD) age at diagnosis 8.1 (4.0); range 1–15.9 years] in the study, of whom 1,382 (50.9 %) were boys. Mean age at latest follow-up was 13.7 (3.6), and duration of diabetes was 5.6 (4.9) years. Non-Danish ethnicity was present in 7.6 % of patients.
Incidence of severe hypoglycemia
In total, 7,390 person-years of data and 561 events of SH were recorded in the study period convening an overall incidence of 7.6 per 100 person-years. Of all patients, 12.3 % experienced one or more events of SH; 7.6 % (n = 207) had one event, 2.7 % (n = 73) had two events and 1.2 % (n = 33) had three events and 0.8 % (n = 21) had more than three episodes of SH. The rate of SH (events per 100 person-years) decreased markedly over the 5-year study period, peaking with 16.0 in 2008 (9.1 and 6.1 in 2009 and 2010, respectively) and reached a nadir of 4.9 in 2011. Hereafter, we observed an insignificant increase to 6.6 per 100 person-years in 2012. The overall baseline rate of SH decreased significantly (multiple adjustments, P = 0.001 for trend). The incidence of SH stratified by treatment is illustrated in Fig. 1.
Time trends in insulin therapy and the risk of SH
Change in insulin therapy over time is illustrated in Fig. 2. The influence of risk factors on the rate of SH is shown in Table 1 (multiple adjustments). There was no difference in the rate of SH according to sex, ethnicity, diabetes duration, HbA1c or center size. The incidence of SH stratified by treatment is illustrated in Table 2 (multiple adjustments). The youngest children (under 5 years) had a nonsignificant trend toward a lower frequency of SH. There was a beneficial effect of pump treatment quantified as a 27 % reduction in the rate of SH [rate ratio 0.73 (0.60–0.90)] compared to any type of pen treatment (P = 0.003).
We compared pen- and pump-treated patients; pen-treated patients were subdivided by the use of insulin types. The analysis revealed a significant effect of the respective insulin types compared to pump therapy (P < 0.0001, multiple adjustments). Patients using premixed human insulin had an 89 % increased rate of SH [rate ratio 1.89 (1.27–2.79), P = 0.0015], and for NPH users, a 63 % increased rate [rate ratio 1.63 (1.18–2.26), P = 0.0031] was observed compared to pump users. Patients on long-acting insulin analogues [rate ratio 1.22 (0.93–1.60)] were comparable with pump users (P = 0.1485).
Insulin pen therapy and SH rates
The baseline rate of SH for pen therapy changed with calendar time, but did not change at a constant rate (P = 0.02), which means that decrease in the rate of SH for pen users is not solemnly explained by the variables included in the model. We found no difference in the rate of SH between sexes, ethnic groups, duration of diabetes, the number of weekly blood glucose measurements, HbA1c or center size, when performing multiple comparison of predictors for pen-treated patients (Table 3a). Patients aged 5–15 years had higher rates of SH compared to adolescents aged 15–18 years (P = 0.02). There was colinearity between insulin pen therapy and the number of bolus injections; consequently, this individual analysis was not valid for interpretation. Backward elimination resulted in a simplified (final) model with the number of bolus injection as the major risk factor (P < 0.02). Results from this analysis revealed an increased rate of SH when using one bolus or less daily [rate ratio 1.78, (1.12–2.83), P = 0.01] and two to three boluses [rate ratio 2.32 (2.08-2.60), P < 0.0001] compared to ≥6 daily insulin boluses. Lastly, an intervention to six daily bolus injections for all patients on pen therapy would have resulted in an additional reduction of 54.3 % of SH during the study period.
Pump therapy and SH rates
The baseline rate of SH for pump therapy also changed with calendar time, but the rate changed at a constant rate when adjusted for multiple confounders (P = 0.60). The effect of the various variables for pump patients is listed in Table 3b. The model was now reduced through backward elimination and revealed significant effects of ethnicity [native Danish rate ratio 0.35, (0.14–0.86) vs. non-Danish, P = 0.02] and percentage of bolus vs. basal insulin [rate ratio 0.10 (0.021–0.48), P = 0.004]. Furthermore, by changing the percentage of bolus insulin to 75 % of total daily insulin dose for all patients on insulin pump therapy, we detected a 36.8 % reduction in the frequency of SH in the pump group.
Glycemic control
The HbA1c levels did not change significantly over time, and there was no difference in HbA1c levels [mean (SD)] between pen 8.5 (3.5) % [69 (15) mmol/mol] and pump users 8.1 (3.4) % [65 (14) mmol/mol, P > 0.05].
Discussion
We report more than a halving in the national rates of severe hypoglycemia to a stable low level over only a 5-year period and no association to glycemic control. Insulin pump usage was associated with a lower risk of SH; Danish ethnic background and high percentage of bolus (vs. basal) insulin decreased the risk of SH for pump users. Patients on pen therapy with few daily bolus injections were most susceptible to SH, albeit, the use of long-acting insulin glargine and detemir had beneficial effects on SH. Overall, the youngest children irrespective of treatment had a tendency to lower rates of SH.
Changes in clinical practice after 2007 with implementation of ADA/ISPAD guidelines [15, 16] in Denmark with glycemic target settings [HbA1c ≤7.5 % (58 mmol/mol)] and intensive education programs have prompted a more streamlined diabetes care within the country. National multi-disciplinary courses have been conducted since 2005 [17], and systematic education (carbohydrate counting and use of bolus calculators) was implemented in 2009. These changes combined have most likely affected this fall in SH rates. Insulin pump use is an important contributor to this excellent finding in the overall incidence of SH, but there was also a simultaneous decrease for patients on pen therapy. Furthermore, the rate of SH was not constant for pen users, so the decreasing use of premixed insulin and more frequent use of long-acting analogues cannot solemnly explain the decrease in SH for pen user. Consequently, there must be additional variables that we have not measured that contribute to this pleasant finding. The meticulous recording of SH has been thoroughly validated and quantified by number of events, and false reporting to our register is consequently minimized. There is no selection bias as all children with type 1 diabetes in Denmark are reported to the register. The reported finding is therefore considered a valid insight into the contemporary rates of SH in this pediatric type 1 diabetes population from a clinical setting.
The overall incidence of SH reported here is comparable to a recent Australian study by Cooper et al. [18] who reported an incidence rate of 6.2 per 100 person-years in 2011; however, our data are national rates rather than center specific. There was a quantitative increase in the rates of SH in the last year of our study; however, this finding was not statistically significant, as overall baseline rates of SH for both pen and pump users reached a steady state after 2009. The overall reports of patients experiencing SH differed between this and the Australian study (22.6 vs. 12.3 %, respectively), and a higher proportion of the Australian patients had recurrent events of SH (10.7 vs. 4.7 %). The populations are difficult to compare, as there are distinct differences in study design (age at inclusion, frequency of pump use, etc.). Notwithstanding, both studies report reductions in SH rates over the recent years, and the youngest age group (<5 years) are not as susceptible to SH as previously reported. Also, a weaker association of good glycemic control with severe rates is reported [18], in alignment with others [9].
We previously reported unchanged rates of SH preceding 2008 (start of pump therapy), but this period coincided with a marked nationwide reduction in HbA1c [11]. The dramatic reduction in SH after 2008 is plausible multifactorial. The overall effect is most likely influenced by the combined effect of pump therapy, better insulin analogues when on pen therapy, improved quality of diabetes care and new features accompanying pump use. Novel sensor-augmented insulin pump (SAP) with a threshold-suspend feature which protects against SH [19, 20] and the use of bolus calculators are more frequently used after 2008, but have not reached steady state yet. Unfortunately, though, we do not have complete data in the register to evaluate the quality of diabetes care and use of SAP/bolus calculators at this time, so their specific effects remain speculative. Nevertheless, we believe the aforementioned disciplines have improved on the hands of patients and professionals and contribute to the marked reduction in SH till 2013. An inspiring multicenter Swedish study emphasizes the importance of a quality improvement collaborative and access to a quality register, which resulted in improved glycemic control [21]. This approach requires defined target settings available for evaluation and emphasizes the need to continuously expand the register.
Younger children had a trend toward lower rates of SH compared to older children and adolescents: a favorable finding in light of the possible hypoglycemic-induced changes to the young developing brain [22, 23]. Continuous glucose monitoring (CGM) reduced the frequency of SH [24, 25], and we have administered SAP with suspension feature and CGM to the youngest and most vulnerable children which may have led to the observed shift in a reduction in SH for the youngest children. Ethnical differences may be explained by several socioeconomic and cultural factors (e.g., language, numeracy, literacy and health-seeking behavior among others) that unfortunately are not available for evaluation in this study.
Long-acting insulin analogues (glargine and detemir) for patients on pen treatment protected against SH and seemed to predominate over premixed and NPH when evaluating SH as the outcome in this cohort. This is not a novel finding. The use of premixed insulin declined in the study period, whereas long-acting insulin analogues were used more frequently, but these therapies alone cannot explain the decrease in SH rates for pen users. Frequent (rapid-acting) insulin injections in the correct doses reduce glucose variability and may prevent extreme low blood glucose levels to occur. Interestingly, our analysis revealed an expected and additional 54 % reduction in the frequency of SH, if all pen patients intensified to six or more daily bolus injections. Notably, low basal insulin levels conferring a decrement in between-meal hypoglycemia protects the patient on pump therapy from SH. In light of this assertion, it is of interest that by increasing bolus (vs. basal) insulin for pump users, we observed a marked reduction in the rate of SH. These findings portray a statistical intervention, and cautious interpretation of data is needed.
Strengths and limitations
Our findings highlight the importance of continually evaluating the quality of diabetes care. In the register, the variable for SH is carefully validated by means of default settings in the software to secure correct data recording. Although SH is self-reported by the patient (and/or parents), the variable is validated in accordance with the health care provider responsible for the patient, so that recall bias is limited. We have not expanded the quality of data registration after novel therapies have been implemented (i.e., SAP, carbohydrate counts and bolus-guide). A continuous improvement in data registration is required to unravel various factors affecting the outcome in question. Thus, a large population size and long follow-up period in concert with centralized HbA1c measurements enable us to tease out important predictors associated with SH. As this study is an observational study, we can however only give reports of associations and not causality.
A national halving in the frequency of severe hypoglycemia is an uplifting and novel finding for our childhood population. We believe that implementation of novel therapies and national guidelines have influenced this finding. A tailored prevention based on the identified predictors would enable us to promote behavioral modifications in susceptible patient groups and further reduce this feared complication.
Abbreviations
- CSII:
-
Continuous subcutaneous insulin infusion
- DANDIABKIDS:
-
Danish National Diabetes Register in Childhood and Adolescence
- DCCT:
-
Diabetes control and complications trial
- HbA1c:
-
Glycated hemoglobin HbA1c
- IFCC:
-
International Federation of Clinical Chemistry
- NPH:
-
Neutral protamine Hagedorn
- RCT:
-
Randomized control trial
- SH:
-
Severe hypoglycemia
References
McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA (2012) Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 35(9):1897–1901
Cryer PE (2010) Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am [Internet] 39(3):641–54. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2923455&tool=pmcentrez&rendertype=abstract
Nathan DM, Cleary PA, Backlund J-YC, Genuth SM, Lachin JM, Orchard TJ et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353(25):2643–2653
White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV et al (2001) Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 139(6):804–812
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med (1993) 329(14):977–86
Bulsara MK, Holman CDJ, Davis EA, Jones TW (2004) The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care 27(10):2293–2298
O’Connell SM, Cooper MN, Bulsara MK, Davis EA, Jones TW (2011) Reducing rates of severe hypoglycemia in a population-based cohort of children and adolescents with type 1 diabetes over the decade 2000–2009. Diabetes Care 34(11):2379–2380
Pickup JC, Sutton AJ (2008) Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med J Br Diabet Assoc 25(7):765–774
Karges B, Rosenbauer J, Kapellen T, Wagner VM, Schober E, Karges W et al (2014) Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med 11(10):e1001742
Wagner VM, Rosenbauer J, Grabert M, Holl RW (2008) German initiative on quality control in pediatric diabetology. Severe hypoglycemia, metabolic control, and diabetes management in young children with type 1 diabetes using insulin analogs–a follow-up report of a large multicenter database. Eur J Pediatr 167(2):241–242
Johansen A, Kanijo B, Fredheim S, Olsen B, Hertz B, Lauridsen M, Andersen M, Mortensen H, Svensson J, the Danish Society for Diabetes in Childhood (2014) Prevalence and predictors of severe hypoglycemia in Danish children and adolescents with diabetes. Pediatr Diabetes. doi:10.1111/pedi.12171
Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ (2008) Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 9(2):165–174
Surveillance WHOD of ND. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. http://apps.who.int/iris/handle/10665/66040
Svensson J, Marinelli K, Eising S (2007) Danish childhood diabetes register, national discharge register. [Comparison of registration of data from the Danish childhood diabetes register and the national discharge register]. Ugeskr Laeger 169(2):122–125
Bangstad H-J, Danne T, Deeb LC, Jarosz-Chobot P, Urakami T, Hanas R et al (2007) ISPAD clinical practice consensus guidelines 2006–2007. Insulin treatment Pediatr Diabetes 8(2):88–102
Swift PGF (2007) International society for pediatric and adolescent diabetes. ISPAD clinical practice consensus guidelines 2006–2007. Diabetes education. Pediatr Diabetes 8(2):103–109
Nørgaard Kirsten, Olsen Birthe (2011) Changing Denmark from a non-user country to a place using insulin pumps: a story on the initiatives taken. Infusystems Int 10(4):25–29
Cooper MN, O’Connell SM, Davis EA, Jones TW (2013) A population-based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia [Internet] http://www.ncbi.nlm.nih.gov/pubmed/23832082
Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW (2013) Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 310(12):1240–1247
Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN et al (2011) Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care 34(11):2403–2405
Peterson A, Hanberger L, Akesson K, Bojestig M, Andersson Gäre B, Samuelsson U (2014) Improved results in paediatric diabetes care using a quality registry in an improvement collaborative: a case study in Sweden. PLoS One 9(5):e97875
Hershey T, Lillie R, Sadler M, White NH (2004) A prospective study of severe hypoglycemia and long-term spatial memory in children with type 1 diabetes. Pediatr Diabetes 5(2):63–71
Holemans X, Dupuis M, Misson N, Vanderijst JF (2001) Reversible amnesia in a Type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med J Br Diabet Assoc 18(9):761–763
Pickup JC, Freeman SC, Sutton AJ (2011) Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 343:d3805
Choudhary P, Ramasamy S, Green L, Gallen G, Pender S, Brackenridge A et al (2013) Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care 36(12):4160–4162
Acknowledgments
We are grateful to patients and their families, and our colleagues throughout the country who provided us with patient data. We acknowledge our bioanalyst Jette Høgsmose for expert handling of blood samples and data management. This work was supported by “Beckett Foundation,” “Dr. Louise’s Children Hospitals Research Grant,” and the Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Conflict of interest
JS has received lecture funding from Novo Nordisk, Sanofi Aventis and is part of the Bayer Advisory Board. SMS and JS are stock/shareholders in Novo Nordisk. All other authors declare no conflict of interest.
Ethical standard
The study was performed according to the criteria of the Helsinki II Declaration and was approved by the local ethics committee.
Human and animal rights disclosure
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent disclosure
Informed consent for participation in register studies is not required according to Danish law.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Fredheim, S., Johansen, A., Thorsen, S.U. et al. Nationwide reduction in the frequency of severe hypoglycemia by half. Acta Diabetol 52, 591–599 (2015). https://doi.org/10.1007/s00592-014-0697-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0697-5