Abstract
There are several pieces of evidence indicating that Mycobacterium avium subspecies paratuberculosis (MAP) infection is linked to type 1 diabetes (T1D) in Sardinian patients. An association between MAP and T1D was recently observed in an Italian cohort of pediatric T1D individuals, characterized by a different genetic background. It is interesting to confirm the prevalence of anti-MAP antibodies (Abs) in another pediatric population from continental Italy, looking at several markers of MAP presence. New-onset T1D children, compared to age-matched healthy controls (HCs), were tested by indirect enzyme-linked immunosorbent assay for the presence of Abs toward the immunodominant MAP3865c/ZnT8 homologues epitopes, the recently identified C-terminal MAP3865c281–287 epitope and MAP-specific protein MptD. Abs against MAP and ZnT8 epitopes were more prevalent in the sera of new-onset T1D children compared to HCs. These findings support the view that MAP3865c/ZnT8 cross-reactivity is involved in the pathogenesis of T1D, and addition of Abs against these peptides to the panel of existing T1D biomarkers should be considered. It is important now to investigate the timing of MAP infection during prospective follow-up in at-risk children to elucidate whether Ab-titers against these MAP/ZnT8 epitopes are present before T1D onset and if so if they wane after diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1D) is one of the most frequent autoimmune diseases, characterized by a T cell-mediated destruction of insulin-secreting pancreatic β-cells. T1D results from the interaction of multiple gene variants and environmental factors, albeit the environmental factors remain poorly defined [1, 2]. Even before the clinical recognition of T1D, autoantibody responses against islet cell proteins for instance (pre–pro)insulin, glutamic acid decarboxylase-65 (GAD65), islet-associated antigen-2 (IA-2) and the zinc transporter 8 (ZnT8) become detectable providing indeed a system for disease prediction in healthy individuals with a susceptible genetic background [3]. Anderson et al. [4] recently reported that autoantibodies to either one or all three ZnT8 amino acid variants at position 325 (ZnT8RWQA) were found in 65 % of the children recently diagnosed with T1D, thus reducing by 2.8 % the frequency of autoantibody-negative patients previously tested for antibodies (Abs) to GAD65, IA-2, insulin and islet cell cytoplasm (ICA). There are several pieces of evidence suggesting that Mycobacterium avium subspecies paratuberculosis (MAP) infection is somehow linked to both T1D and multiple sclerosis in the Sardinian population [5–9]. Indeed, anti-MAP and anti-ZnT8 Abs were present in both T1D adults and newly diagnosed T1D children from Sardinia [5, 6]. What is more, an association between MAP and T1D was recently observed in an Italian cohort of pediatric T1D patients [10]. The latter being sustained by two major pieces of evidence: the presence of both MAP DNA and anti-MAP heparin-binding hemagglutinin (HBHA) Abs within the peripheral blood of this cohort of newly diagnosed T1D children. In addition, a significant correlation was found between anti-MAP HBHA Ab-positivity and the presence of HLA DQA1 *0201/DQB1*0202 [10].
In light of the recently proposed theory which envisions MAP as a new T1D environmental trigger acting through a molecular mimicry mechanism [5, 6, 11] and due to the fact that most of the evidence sustaining this hypothesis is the outcome of studies performed only on Sardinian T1D subjects, it is relevant to investigate further the prevalence of anti-MAP Abs outside Sardinia. To this end, our major objective is to assess the prevalence of anti-MAP/ZnT8 Abs in another pediatric population from continental Italy, looking at several markers of MAP presence in new-onset T1D subjects. Antibodies to insulin, GAD65, IA-2 and ZnT8, were also measured through radioimmunoassay and radioligand assays. A correlation analysis between the protein A radioimmunoprecipitation assay using 35S-labeled methionine in vitro translation products of human ZnT8 C-terminal fragments (ZnT8A) [12] and our indirect enzyme-linked immunosorbent assay (ELISA) previously described [5, 6] was also performed. Moreover, we searched for Abs directed against MAP-specific protein MptD, and the results obtained were used to perform a correlation analysis between ZnT8A and MptD Abs titers.
We asked whether the increased prevalence of MAP3865c/ZnT8 sero-positivity found in Sardinian subjects [5, 6] could be mirrored by this population of new-onset T1D children from continental Italy. Noteworthy, it is the first study to date reporting that MAP3865c/ZnT8 peptides are recognized by new-onset T1D children from continental Italy.
Material and methods
Subjects
New-onset T1D children [n = 59; 32 boys, 27 girls; mean age 9.4 ± 5 years; the median (interquartile range) of diabetes duration was 92 (32.5–129.5) days]; diagnosed in line with the American Diabetes Association criteria [13]; and healthy controls (HCs) (n = 60; 32 boys, 28 girls; mean age 9.3 ± 3.5) attending the Pediatric Diabetes Unit of Tor Vergata University Hospital of Roma were recruited. Patient’s details are provided in Table 1. Blood samples were processed as follow: 5 ml of peripheral blood was drawn in Vacutainer Serum tubes, the blood was allowed to clot undisturbed 15–30 min at room temperature, clot was removed centrifugation at 1,000–2,000×g for 10 min and the resulting supernatant (serum) was collected for use in ELISA. Frozen aliquots were stored at −80 °C and used within 6 months.
Ethical statement
Blood samples were collected after obtaining informed written consents from the guardians of all children. The study protocols were approved by the ethics committee of the Pediatric Diabetes Unit of Tor Vergata University Hospital of Roma, Italy.
Peptides
Peptides MAP3865c125–133 (MIAVALAGL) and MAP3865c133–141 (LAANFVVAL) along with their respective homologous peptides ZnT8178–186 (MIIVSSCAV), ZnT8186–194 (VAANIVLTV) and MAP3865c281–287 (HATVQID) were synthesized at >90 % purity (LifeTein, South Plainfield, NJ 07080, USA). Peptides purity was assessed by HPLC. MptD protein was expressed and purified as formerly described [14].
ELISA
Indirect ELISA to detect Abs specific for MAP3865c/ZnT8 peptides and anti-MAP-specific MptD protein was performed as previously described [5]. Receiver operating characteristic (ROC) curves were used to identify the optimal cutoff points, setting specificity at 95 % (i.e., Ab + HCs ≤5 %) for the new-onset T1D children. Results were normalized to a robustly positive control serum included in all tests, the reactivity of which was set at 10.000 arbitrary units (AU)/ml. Interassay CV for the different ELISAs ranged from 6.7 to 7.8 %. The level of statistical significance of the ELISA was assessed by Fisher’s exact test using Graphpad Prism 6.0 software.
Competitive inhibition assays
Competition inhibition assays were performed as described elsewhere [5]. Briefly, sera were pre-incubating overnight at 4 °C with saturating concentrations (10–20 μM, titrated for each individual serum) of MAP peptides, the corresponding ZnT8 peptides, irrelevant peptide (MAP3865c211–217, ILSESSP) or no peptide. Sera were then submitted to ELISA on plates coated with MAP3865c125–133 or MAP3865c133–141 at the concentration of 10 μg/ml.
Autoantibody assays
Antibodies to the ZnT8 C-terminal region (268–369, 325R, or 325 W) present in the sera were measured by protein A radioimmunoprecipitation assays as described by Lampasona et coworkers [12]. ZnT8-C-terminal constructs (RW) were expressed in vitro in a rabbit reticulocyte lysate using the TNT Quick Coupled Transcription/Translation System kit (Promega) in the presence of 40 μl of 35S-labeled methionine (PerkinElmer), purified by size-exclusion chromatography on NAP-5 columns (GE Healthcare BioSciences), and the recovered radioactivity was measured on a β-counter (PerkinElmer). A standard curve was derived from serial dilutions of a positive serum included in each assay run. Relative concentrations were expressed in arbitrary units. Threshold for Ab-positivity was set at the 99th percentile of 100 non-diabetic control subjects and corresponded to antibody levels >30 U/ml. The assay for ZnT8A (RW) showed an interassay coefficient of variation (CV) of 14 % and an intra-assay CV of 11 %.
Auto-antibodies to GAD65 and to IA-2 were routinely determined by direct radioligand assays [15] using three different commercial kits (CentAK® anti-GAD65, and CentAK® anti-IA2, Medipan, Germany) according to the manufacturer’s instruction. Results are expressed in arbitrary units derived by a standard curve established plotting the mean values of the calibrators included in each assay run. Positive values for auto-antibodies to GAD65 and to IA-2 were considered >0.9 and >0.75 U/ml, respectively.
Results
To determine whether the immunogenic MAP3865c/ZnT8 peptides in Sardinian patients are also recognized by a pediatric population from continental Italy, we tested sera from new-onset T1D pediatric individuals using our previously optimized indirect ELISA [5, 6].
Five MAP3865c/ZnT8 peptides, four belonging to the fourth transmembrane domain [5], one C-terminal peptide (MAP3865c281–287) [6] homologue to the human C-terminal region (ZnT8268–369) formerly identified [4, 16–18], together with the MAP-specific protein MptD, were examined both in 59 new-onset T1D and 60 age-matched HCs via indirect ELISA. All the peptides were highly recognized proving detectable reactivity. Results are summarized in Figs. 1 and 2.
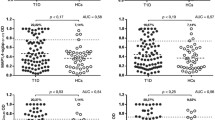
Prevalence of Abs against homologous ZnT8 and MAP3865c transmembrane epitopes in new-onset T1D and HCs children. Sera were tested for their reactivity against plate coated with MAP3865c125–133 (a) and its homologous ZnT8178–186 (b); and with MAP3865c133–141 and its homologous (c) ZnT8186–194 (d). The dotted line lines indicate the cutoff for positivity used in each assay, as calculated by ROC analysis. The percent fraction of Ab-positive sera is indicated on top of each distribution, while bars indicate the corresponding median ± interquartile range. AUC and p values are given in the top right corner. Figure shows representative experiments out of three performed
Prevalence of Abs against MAP3865c281–287 epitope and MAP-specific protein MptD in new-onset T1D and HCs children. Sera were tested for their reactivity against plate-coated MAP3865c281–287 peptides (a) and MAP-specific protein MptD in new-onset type 1 diabetes children. Data representation is the same as in Fig. 1
Prevalence of Abs against MAP3865c/ZnT8 epitopes and MAP-specific protein MptD in 59 new-onset T1D children and 60 age-matched HCs
MAP3865c125–133 Abs were detected in 45.7 % of new-onset T1D subjects and in 5 % of HCs, this difference was statistically significant (Fisher’s exact test: p < 0.0001; area under ROC curves AUC = 0.81; Fig. 1a).
ZnT8178–186 Ab reactivity was slightly higher than the one showed by its homologue MAP3865c125–133 when comparing new-onset T1D with HCs (47.4 and 5 %, respectively; p < 0.0001; AUC = 0.85; Fig. 1b).
The homologous MAP3865c133–141 and ZnT8186–194 peptides were recognized by 42.4 and 44.0 % of new-onset T1D patients, but only in 5 % of HCs (AUC 0.8 and p < 0.0001 for both; Fig. 1c–d).
MAP3865c281–287 Abs were detected in 40.6 % of new-onset T1D children and in 5 % of HCs, this difference being statistically significant (p < 0.0001; AUC = 0.81; Fig. 2a).
Antibodies against the MAP-specific protein MptD were found in 25 out of 59 new-onset T1D children (42.4 %) versus 2 out of 60 (5 %) HCs (AUC = 0.79, p < 0.0001; Fig. 2b).
Competitive inhibition assays
Abs targeting MAP3865c and ZnT8 homologous regions display similar frequencies among new-onset T1D children (45.7–47.4 and 42.4–44 %, respectively; Fig. 1), thus suggesting that Abs recognizing these epitopes could be cross-reactive. To verify whether cross-recognition between homologue epitopes occurs, competition experiments were performed. Three anti-MAP3865c125–133-positive and one anti-MAP3865c125–133-negative sera were pre-adsorbed overnight with different peptides, afterward submitted to ELISA on MAP3865c125–133-coated plates (Fig. 3a). While a control peptide caused only a very slim decline in signal, both MAP3865c125–133 and its homologous ZnT8178–186 peptide robustly inhibited the MAP3865c125–133 reactivity to a similar extent (51–88 %). As to MAP3865c133–141 reactivity, it was efficiently inhibited (37–85 %) upon serum pre-adsorption with either MAP3865c133–141 or its homologous ZnT8186–194 (Fig. 3b). In sum, these results demonstrate that anti-MAP and anti-ZnT8 Abs targeting homologous sequences are cross-reactive.
Ab reactivities against MAP3865c epitopes are inhibited by the homologous ZnT8 epitopes. a Three Ab-positive and one Ab-negative sera from new-onset T1D children were pre-incubated overnight with saturating concentrations of MAP3865c125–133 (white bars), ZnT8178–186 (hatched bars), control (gray bars) or no peptide (black bars) and their reactivity on MAP3865c125–133-coated ELISA plates subsequently tested. b The same sera were pre-incubated with MAP3865c133–141 (white bars), ZnT8186–194 (hatched bars), control (gray bars) or no peptide (black bars) and their reactivity on MAP3865c133–141-coated ELISA plates subsequently tested. Histogram bars depict mean ± SEM of triplicate wells obtained from two separate experiments performed
Autoantibody assays and correlation analyses between ZnT8A and our MAP3865c/ZnT8 indirect ELISAs
In the present population of recent-onset children with T1D, the prevalences of autoantibodies to GAD65 and IA-2 were 70 and 68.4 %, respectively.
ZnT8 antibodies (ZnT8A) in sera samples were measured by immunoprecipitation of radio-labeled (35S methionine) recombinant ZnT8 antigens. ZnT8A levels were determined searching for autoantibodies recognizing both arginine (R) and tryptophan (W) residues at position 325 of ZnT8268-369 C-terminal region. ZnT8A and GAD65 values concerning new-onset T1D children are reported in Table 1. Unfortunately, ZnT8A data are available only for 30 new-onset T1D children. Among them, 19 were assessed to be Ab-positive (63.3 %).
In order to validate the information produced analyzing these five MAP3865c/ZnT8 peptides, a correlation analysis between ZnT8A methodology and our MAP3865c/ZnT8 indirect ELISAs was performed. The analyses were carried out on the aforementioned subset of 30 new-onset T1D children, and the results are displayed in Figs. 3 and 4.
Correlation between titers of ZnT8A Abs and MAP/Znt8 reactive Abs. Correlation is shown between titers of Abs recognizing, a ZnT8A Abs and MAP3865c125–133; b ZnT8A Abs and ZnT8178–186; c ZnT8A Abs and MAP3865c133–141; d ZnT8A Abs and ZnT8186–194. Each circle corresponds to the titer of one type 1 diabetes child. The dotted line lines designate the cutoff for positivity used in each assay, as calculated by ROC analysis
Indeed, there was a discreet degree of correlation between titers of Abs recognizing MAP3865c and ZnT8 peptides with ZnT8A (Fig. 4), with MAP3865c133–141 and ZnT8186–194 (Fig. 4c, d) displaying the highest correlation (r 2 = 0.35 and p = 0.0005; r 2 = 0.36 and p = 0.0004, respectively). As regards MAP3865c125–133 and ZnT8178–186 (Fig. 4a, b), the correlation was much weaker (r 2 = 0.14 and p = 0.037; r 2 = 0.22 and p = 0.01, respectively).
The C-terminal MAP3865c281–287 peptide showed a slightly lower degree of correlation (r 2 = 0.27 and p = 0.002; Fig. 5a).
Correlation between titers of ZnT8A Abs and MAP3865c281–287 and MptD reactive Abs in new-onset T1D children. Correlation is shown between titers of Abs recognizing a ZnT8A Abs and MAP3865c281–287C-terminal epitope; b ZnT8A Abs and MptD-specific protein. Data representation is the same as in Fig. 3
At last, the correlation between titers of Abs against ZnT8A and MAP-specific protein MptD reactive Abs is shown in Fig. 5b.
As to new-onset T1D children, the high frequencies of Abs reacting against MAP-specific protein MptD, together with the discreet degree of correlation found between the ZnT8A and MAP3865c/ZnT8 assays, suggest that testing for Abs against these peptides might be a useful marker to track T1D and MAP infection in this pediatric population from continental Italy.
Discussion
It is acknowledged that MAP infection and sero-reactivity are highly prevalent among T1D Sardinian subjects [5–7, 11, 19]; in turn, MAP has been proposed as a potential environmental trigger for T1D [20]. Indeed, it was recently demonstrated that Abs against MAP3865c epitopes cross-react with ZnT8 epitopes, raising the possibility of a molecular mimicry between mycobacterial and β-cell epitopes [5, 6]. All the data accounting for this cross-recognition originate from studies conducted on Sardinian subjects, investigating both T1D adults and children [5–7, 11, 19]. Noteworthy, an association between MAP and T1D was recently observed outside Sardinia, in an Italian cohort of newly diagnosed T1D children [10], and it was verified by the presence of both MAP DNA and sero-reactivity against MAP HBHA antigen.
In the present study, we asked whether the increased prevalence of anti-MAP3865c/ZnT8 Abs found in Sardinian subjects [5, 6] could be mirrored by this pediatric population from continental Italy.
Unfortunately, thus far, no data are available on at-risk individuals. Hence, our next objective is to explore the frequency of MAP sero-reactivity in subjects prone to develop T1D, both before and after T1D diagnosis. Soon after the data produced studying new-onset T1D will be compared to the one obtained studying at-risk subject allowing us to decipher if MAP infection can be seen as a cause or a straightforward consequence of the disease. Investigating the prevalence of anti-MAP3865c/ZnT8 Abs in both new-onset T1D and at-risk subject is a mandatory step to determine whether these peptides could be used as early biomarkers of T1D.
There is evidence pointing out that systemic or local infections can trigger autoimmune reactions to β-cells, even if up until now research failed in identifying an unquestionable environmental trigger [21]. A number of reports have addressed MAP and its role in igniting autoimmunity [5–11, 19, 20, 22–24], but the role played by autoreactive effector T cells recognizing MAP3865c/ZnT8 homologous sequences still need to be explored. Thus far, it was reported that ZnT8186–194 epitope is an immunodominant CD8 (+) T cell target in a great part of T1D patients outside Sardinia [25].
The present report demonstrates that Abs against ZnT8178–186 and ZnT8186–194 in conjunction with Abs against MAP3865c125–133, MAP3865c133–141 and MAP3865c281–287 C-terminal peptides are common in new-onset T1D children from continental Italy when compared with age-matched HCs.
Noteworthy, titers against MAP3865c133–141 and MAP3865c125–133 epitopes were more or less the same displayed by the human homologous peptides (ZnT8186–194, ZnT8178–186) in new-onset T1D and healthy children, with a prevalence ranging from 42.4 to 47.4 % (Fig. 1). Indeed, Abs against MAP3865c125–133 and ZnT8178–186 had been previously found in only 34.5 % of new-onset T1D children from Sardinia, conversely MAP3865c133–141 and ZnT8186–194 were recognized by the majority of newly diagnosed Sardinian children (55.2 %) [6].
Our observations support the existence of an association between Ab-positivity for MAP and ZnT8 homologue peptides outside Sardinia in new-onset T1D children.
In conclusion, the high frequencies of Abs reacting against MAP-specific protein MptD, together with the moderate degree of correlation found between ZnT8A and MAP3865c/ZnT8 peptide ELISA assays in patients with T1D, suggest that searching for Abs against MAP3865c/ZnT8 peptides in conjunction with anti-GAD65 and anti-IA-2 measurements might be as a useful screening tool for a complete picture of new-onset T1D children. Indeed, our observations are largely consistent with our previous reports conducted on Sardinian individuals, concluding that MAP3865c/ZnT8 peptide-based ELISA [5–7] produced data somehow comparable to those obtained by ZnT8A methodology. Being able to identify the majority of recent-onset T1D children, our indirect ELISA could thus represent a cost-effective alternative to find out and track new-onset T1D subjects. We are now going to follow over time a pediatric court of children at risk to verify whether their sero-reactivity against MAP3865c/ZnT8 changes before and after T1D onset. It will help us to assess the potential of anti-MAP3865c/ZnT8 Abs as biomarker for disease prediction.
References
La Torre D (2012) Immunobiology of beta-cell destruction. Adv Exp Med Biol 771:194–218
Banin P, Rimondi F, De Togni A, Cantoni S, Chiari G, Iughetti L, Salardi S, Zucchini S, Marsciani A, Suprani T, Tarchini L, Tozzola A, Xella R, Marsella M, De Sanctis V (2010) Type 1 diabetes (T1DM) in children and adolescents of immigrated families in Emilia-Romagna (Italy). Acta Biomed 81(1):35–39
Arvan P, Pietropaolo M, Ostrov D, Rhodes CJ (2012) Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harb Perspect Med 2(8):1–20
Andersson C, Larsson K, Vaziri-Sani F, Lynch K, Carlsson A, Cedervall E, Jönsson B, Neiderud J, Månsson M, Nilsson A, Lernmark A, Elding Larsson H, Ivarsson SA (2011) The three ZNT8 autoantibody variants together improve the diagnostic sensitivity of childhood and adolescent type 1 diabetes. Autoimmunity 44(5):394–405
Masala S, Paccagnini D, Cossu D, Brezar V, Pacifico A, Ahmed N, Mallone R, Sechi LA (2011) Antibodies recognizing Mycobacterium avium paratuberculosis epitopes cross-react with the beta-cell antigen ZnT8 in Sardinian type 1 diabetic patients. PLoS ONE 6(10):e26931
Masala S, Zedda MA, Cossu D, Ripoli C, Palermo M, Sechi LA (2013) Zinc transporter 8 and MAP3865c homologous epitopes are recognized at T1D onset in Sardinian children. PLoS ONE 8(5):e63371
Masala S, Cossu D, Pacifico A, Molicotti P, Sechi LA (2012) Sardinian type 1 diabetes patients, Transthyretin and Mycobacterium avium subspecies paratuberculosis infection. Gut Pathog 274(1):24
Cossu D, Masala S, Sechi LA (2013) A Sardinian map for multiple sclerosis. Future Microbiol 8:223–232
Cossu D, Masala S, Cocco E, Paccagnini D, Frau J, Marrosu MG, Sechi LA (2012) Are Mycobacterium avium subsp. paratuberculosis and Epstein–Barr virus triggers of multiple sclerosis in Sardinia? Mult Scler 18(8):1181–1184
Bitti ML, Masala S, Capasso F, Rapini N, Piccinini S, Angelini F, Pierantozzi A, Lidano R, Pietrosanti S, Paccagnini D, Sechi LA (2012) Mycobacterium avium subsp. paratuberculosis in an Italian cohort of type 1 diabetes pediatric patients. Clin Dev Immunol 2012:785262. doi:10.1155/2012/785262
Sechi LA, Rosu V, Pacifico A, Fadda G, Ahmed N, Zanetti S (2008) Humoral immune responses of type 1 diabetes patients to Mycobacterium avium subsp. paratuberculosis lend support to the infectious trigger hypothesis. Clin Vaccine Immunol 15:320–326
Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, Songini M, Bonicchio S, Giorgino F, Bonifacio E, Bosi E, Buzzetti R (2010) Non insulin requiring autoimmune diabetes (NIRAD) study group. Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care 33(1):104–108
American Diabetes Association (2011) Diagnosis and classification of diabetes mellitus. Diabetes Care 34(1):S62–S69
Cossu A, Rosu V, Paccagnini D, Cossu D, Pacifico A, Sechi LA (2011) MAP3738c and MptD are specific tags of Mycobacterium avium subsp. paratuberculosis infection in type I diabetes mellitus. Clin Immunol 141(1):49–57
Capasso F, Rapini N, Di Matteo G, Testi M, Arcano S, Lidano R, Petrelli A, Rossi P, Piccinini S, Manca Bitti ML, Angelini F (2013) A variable degree of autoimmunity in the pedigree of a patient with type 1 diabetes homozygous for the PTPN22 1858T variant. Pediatr Diabetes 14(4):304–310
Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC (2007) The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 104:17040–17045
Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC (2008) A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57(10):2693–2697
Vaziri-Sani F, Delli AJ, Elding-Larsson H, Lindblad B, Carlsson A, Forsander G, Ivarsson SA, Ludvigsson J, Marcus C, Lernmark Å (2011) A novel triple mix radiobinding assay for the three ZnT8 (ZnT8-RWQ) autoantibody variants in children with newly diagnosed diabetes. J Immunol Methods 371:25–37
Paccagnini D, Sieswerda L, Rosu V, Masala S, Pacifico A, Gazouli M, Ikonomopoulos J, Ahmed N, Zanetti S, Sechi LA (2009) Linking chronic infection and autoimmune diseases: Mycobacterium avium subspecies paratuberculosis, SLC11A1 polymorphisms and type-1 diabetes mellitus. PLoS One 4(9):e7109
Sechi LA, Paccagnini D, Salza S, Pacifico A, Ahmed N, Zanetti S (2008) Mycobacterium avium subspecies paratuberculosis bacteremia in type 1 diabetes mellitus: an infectious trigger? Clin Infect Dis 46:148–149
Boitard C (2012) Pancreatic islet autoimmunity. Presse Med 41(2):e636–e650
Sechi LA, Scanu AM, Molicotti P, Cannas S, Mura M, Dettori G, Fadda G, Zanetti S (2005) Detection and Isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn’s disease in Sardinia. Am J Gastroenterol 100(7):1529–1536
Cossu D, Masala S, Cocco E, Paccagnini D, Tranquilli S, Frau J, Marrosu MG, Sechi LA (2013) Association of Mycobacterium avium subsp. paratuberculosis and SLC11A1 polymorphisms in Sardinian multiple sclerosis patients. J Infect Dev Ctries 7(3):203–207
Frau J, Cossu D, Coghe G, Lorefice L, Fenu G, Melis M, Paccagnini D, Sardu C, Murru M, Tranquilli S, Marrosu M, Sechi L, Cocco E (2013) Mycobacterium avium subsp. paratuberculosis and multiple sclerosis in Sardinian patients: epidemiology and clinical features. Mult Scler 19(11):1432–1437
Scotto M, Afonso G, Larger E, Raverdy C, Lemonnier FA, Carel JC, Dubois-Laforgue D, Baz B, Levy D, Gautier JF, Launay O, Bruno G, Boitard C, Sechi LA, Hutton JC, Davidson HW, Mallone R (2012) Zinc transporter (ZnT)8(186–194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia 55(7):2026–2031
Acknowledgments
This research was supported by the Sardinian Region L.R.7 2009 and 2010 Progetti di ricerca di base, PRIN MIUR Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale < Bando PRIN 2009 > Protocollo: 2009ZYECWZ and Italian Minister of Health, “Finalized Research” Protocollo: RF-2009-1545765, to LAS. LAS is the guarantor. The present publication was produced as part of the RAS research Project Number 10 selection code 06/A3-10 developed at Sassari University by Speranza Masala and supported by P.O.R. Sardinia F.S.E. 2007–2013, Axis IV human capitals, Line of Business l.3.1.
Conflict of interest
No conflicts of interest related to the manuscript to declare.
Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the institutional committee on human experimentation.
Informed Consent
Informed consent procedure does not apply as IHDR data are non identifiable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Massimo Porta.
Rights and permissions
About this article
Cite this article
Masala, S., Cossu, D., Piccinini, S. et al. Recognition of zinc transporter 8 and MAP3865c homologous epitopes by new-onset type 1 diabetes children from continental Italy. Acta Diabetol 51, 577–585 (2014). https://doi.org/10.1007/s00592-014-0558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0558-2