Abstract
Although reactive oxygen species (ROS) contribute to glucose intolerance induced by the renin-angiotensin system (RAS) is well documented, the role of the newly discovered pathway of RAS, angiotensin (Ang)-(1-7)/Mas axis, in this process remains unknown. Here, we examined the effect of Ang-(1-7) on oxidative stress and glucose uptake in adipocytes. We used primary cultured epididymal adipocytes from C57 mice to study Ang-(1-7) effects on glucose uptake. We also treated fully differentiated 3T3-L1 adipocytes with exogenous Ang-(1-7) or overexpression of angiotensin-converting enzyme 2 (ACE2) to induce endogenous generation of Ang-(1-7) to clarify its effects on ROS production. Intracellular ROS was measured by flow cytometry, dihydroethidium (DHE), and nitroblue tetrazolium assay. Levels of NADPH oxidase and adiponectin mRNA were measured by real-time PCR. Ang-(1-7) improved glucose uptake both in basal and insulin-stimulated states. ROS production was slightly but significantly decreased in adipocytes treated with Ang-(1-7). Additionally, Mas receptor antagonist D-Ala7-Ang-(1-7) (A779) reversed the effect of Ang-(1-7) on glucose uptake and oxidative stress. Furthermore, treatment of adipocytes with Ang-(1-7) decreased NADPH oxidase mRNA levels. We also found that oxidative stress induced by glucose oxidase–suppressed expression of adiponectin, an insulin-sensitive protein. However, the suppression of oxidative stress by Ang-(1-7) restored adiponectin expression, while A779 agonists these changes induced by Ang-(1-7). In conclusion, Ang-(1-7) can protect against oxidative stress and improve glucose metabolism in adipocytes. These results show that Ang-(1-7) is a novel target for the improvement of glucose metabolism by preventing oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, one of the most intriguing and clinically relevant findings related to the renin-angiotensin (Ang) system (RAS) inhibitors is their ability to delay the onset of type 2 diabetes mellitus (T2DM) in high-risk populations [1–4]. Meanwhile, several new components of the RAS have been discovered. Among them, new findings related to ACE2 have forced a re-evaluation of the original cascade. ACE2, a negative regulator of the RAS, can degrade Ang-II to Ang-(1-7), which takes the opposite effect of Ang-II through the Mas receptor.
In the adipose tissue, Ang-II induces insulin resistance via oxidative stress [5]. Ang-II stimulates the overexpression of cytosolic proteins involved in the activation of NADPH oxidase [6, 7], which favors the production of reactive oxygen species (ROS). Furthermore, Ang-II treatment causes a diminution of IRS-1-dependent insulin signaling and insulin-stimulated GLUT-4 translocation that is associated with the activation of NADPH oxidase and ROS production [8].
Recently, it was shown that Ang-(1-7) overcomes the inhibition of insulin-induced phosphorylation of Akt by Ang-II [9, 10]. The action of Ang-(1-7) on insulin pathways indicated a role in metabolic processes. In addition, Santos et al. [10] reported that Mas-knockout mice presented a metabolic syndrome state. These findings support a putative function of Ang-(1-7) in the improvement of insulin resistance and glucose homeostasis. However, the potential effect of Ang-(1-7) on oxidative stress in adipocytes and whether this effect is involved in glucose metabolism has not been evaluated.
Our previous studies using ACE2-knockout mice reported that ACE2 is an important regulator in glucose homeostasis as well as T2DM and establish ACE2 as a new target for the treatment of T2DM [11, 12]. These findings are keeping with our results and support a putative new physiological function of Ang-(1-7) in oxidative stress and glucose metabolism.
In the present report, we investigated the effects of Ang-(1-7) on protecting against oxidative stress and improving glucose metabolism by enhancing glucose uptake and insulin-sensitizing protein adiponectin expression in adipocytes. We also found that Ang-(1-7) decreased NADPH oxidase mRNA levels, indicating that Ang-(1-7) may decrease ROS production through NADPH oxidase pathway. By use of the selective receptor antagonist A779, we analyzed the involvements of the Mas receptor in these effects.
Materials and methods
Primary culture of adipocytes and 2-deoxy glucose uptake
Adipocytes were isolated under sterile conditions from epididymal fat pads of C57 mice according to the Rodbell method [13] and maintained in primary culture in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone) containing 25 mmol/l glucose, 10% fetal bovine serum (Hyclone), 20 unit/ml penicillin, 20 mg/ml streptomycin (Invitrogen), and 1% bovine serum albumin (BSA) (Beyotime). Isolated adipocytes were diluted (1:120, weight/volume) and placed in sterile airtight tubes with cells floating on the medium. The cells were incubated in the presence or absence of insulin (1 μmol/l). Each group was treated with 10−6 mol/l [14] A779 (Bachem, Switzerland), 10−9 mol/l [14] Ang-(1-7) (Bachem, Switzerland), or pioglitazone (positive control). All tubes were incubated at 37°C. At the end of the incubation, samples were collected to measure 2-deoxy glucose (2DG) uptake. The optimal time of collection and Ang-(1-7) concentration were determined in preliminary experiments.
The 2DG uptake assay was performed by a non-radioactivity enzyme assay protocol as previously described [15–17]. Briefly, the differentiated 3T3-L1 were incubated with 150 μl/well of DMEM with 10% FBS in the presence of insulin and/or a test compound for the indicated time. After incubation, the cells were washed twice and incubated with Krebs–Ringer–phosphate–Hepes (KRPH) buffer (pH 7.4, 20 mmol/l Hepes, 5 mmol/l KH2PO4, 1 mmol/l MgSO4, 1 mmol/l CaCl2, 136 mmol/l NaCl, 4.7 mmol/l KCl) containing 1 mmol/l 2DG and 0.1% BSA for the indicated time at 37°C in 5% CO2. The cells were then washed three times with KRPH buffer containing 0.1% BSA and then 25 μl of 0.1 N NaOH was added. To degrade NADPH, NAD(P)+, and other enzymes, the culture plate was incubated for 40 min at 85°C in a temperature-controlled bath. The components were neutralized by the addition of 25 μl of 0.1 N HCl, then 25 μl of 150 mmol/l TEA buffer (pH 8.1) was added. Uptake of 2DG into the cells was measured by the enzymatic fluorescence assay: fluorescence at 590 nm following excitation at 530 nm was measured by a MTP-32 microplate reader (Corona Electric, Hitachinaka, Japan) to detect the resorufin derived from reduced resazurin. A standard curve was generated by placing 2DG standard solutions in wells of the culture plate without cells (Fig. S1). Effect of cytochalasin B (CyB) (Sigma-Aldrich, St. Louis, MO), an inhibitor of the hexose carrier on insulin-stimulated 2DG-uptake, was used as a negative control (Fig. S2) and effect of insulin on 2DG-uptake was used as positive control (Fig. S3).
Cell culture
Mouse pre-adipose cell line 3T3-L1 (China Center for Type Culture Collection) were grown to confluence in growth medium (DMEM containing 25 mmol/l glucose and 10% fetal bovine serum), as described previously [18]. After 48 h, confluence cells were induced to differentiate into adipocytes by replacing the medium with differentiation medium (DMEM supplemented with 10% fetal bovine serum, 5 mg/ml recombinant human insulin, 0.5 mmol/l 3-isobutylmethylxanthine, and 0.25 mmol/l dexamethasone) for 72 h. Cells were used 9–10 days following differentiation induction when exhibiting 80–90% adipocyte phenotype. The cells were incubated under basal conditions or insulin-stimulating conditions in the presence of either 10−6 mol/l A779, 10−9 mol/l Ang-(1-7) or both [Ang-(1-7) and A779]. Oxidative stress was induced by adding glucose oxidase (GO) (type II from Aspergillus niger, Sigma) in serum-free DMEM to make final concentrations from 25 to 100 mU/ml.
Plasmid constructs and transfection
Rat ACE2 gene was cloned into the EcoRI restriction site of the pcDNA3.1/myc-His(-)B (PCDB) (purchased from SinoGenoMax, China). The recombinants were sequenced for identification the right insertion of the ACE2 gene. 3T3-L1 cells were transfected with plasmid DNA (5 μg) by Lipofectamine TM 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The pcDNA3.1-transfected 3T3-L1 adipocytes were used as empty vector cells. The cells transfected with ACE2 or empty vector for 48 h can be used. The infected efficiency was detected by fluorescence microscopy (×200) (Olympus 1X51, Japan) by transfection of pcDNA3.1-GFP in mature 3T3-L1 cells (Fig. S4).
Measurement of intracellular ROS
Intracellular ROS was measured by flow cytometry and using an oxidation-sensitive probe, dihydroethidium (DHE) (Sigma-Aldrich, St Louis, Mo., USA), and an nitroblue tetrazolium (NBT) assay [19]. In brief, 5 μmol/l DHE [20], dissolved in dimethyl sulfoxide (Sigma-Aldrich, St Louis, Mo., USA), was added to the sections and incubated at 37°C for 60 min. After washing three times with PBS, ethidium staining was visualized using a fluorescent microscope (Leica DMLB; Leica, Wetzlar, Germany) equipped with a Leica DC 200 digital camera. Fluorescence intensity was detected by FACScan flow cytometer (Biosciences, San Jose, CA, USA) equipped with FACSort Cell Quest software. For each sample, 10,000 events were collected. For NBT assay, the 3T3-L1 mature cells were incubated for 90 min in phosphate-buffered saline (PBS) containing 0.2% NBT. Then, the resultant formazan formed was dissolved in 50% acetic acid, and the absorbance was read at 560 nm using Microplate Reader Model 550 (BIO-RAD, Tokyo, Japan).
Reverse transcription and real-time polymerase chain reaction
Total RNAs were extracted using trizol reagent (Invitrogen, Carlsbad, CA) [21]. Two micrograms of RNAs was subjected to ReverTraAce qPCR RT Kit (TOYOBO, Osaka, Japan) according to the manufacturer’s protocol. Real-time PCR amplification was performed in triplicate using the following primers:
-
p22 phox : 5′-TGGCCTGATCCTCATCACAG-3′;5′-AGGCACGGACAGCAGTAAGT-3′
-
gp91 phox /Nox2: 5′-CCAGTGAAGATGTGTTCAGCT-3′;5′-GCACAGCCAGTAGAAGTAGAT-3′
-
Nox4: 5′-AGTCAAACAGATGGGATA-3′; 5′-TGTCCCATATGAGTTGTT-3′
-
p47 phox : 5′-TCACCGAGATCTACGAGTTC-3′; TCCCATGAGGCTGTTGAAGT-3′
-
p67 phox : 5′-CAGTTCAAGCTGTTTGCCTG-3′; TTCTTGGCCAGCTGAGCCAC-3′
-
Tumor necrosis factor - alph a (TNF - α) : 5′-CGT CGT AGC AAA CCA CCA AG-3′; 5′-TTG AAG AGA ACC TGG GAG TAG ACA-3′
-
Interleukin - 6(IL - 6): 5′-TGG GAA ATC GTG GAA ATG AG-3′; 5′-CTC TGA AGG ACT CTG GCT TTG-3′
-
Adiponectin : 5′-TGG AGA GAA GGG AGA GAA AGG-3′; 5′-TGG TCG TAG GTG AAG AGA ACG-3′
-
ACE2: 5′-CATTGGAGCAAGTGTTGGATCTT3′; 5′-GAGCTAATGCATGCCATTCTCA-3′
-
18S: 5′-TCAAGAACGAAAGTC-GGAGG-3′; 5′-GGACATCTAAGGGCATCACA-3′.
Each PCR mixture contained 5 ml of cDNA, 400 nmol/l concentration of each primer, and 25 ml of SYBR1 Green Real-Time PCR Master Mix (TOYOBO, Osaka, Japan) in a 50 ml reaction mixture. Real-time PCR was performed using ABI GeneAmp 5700 Real-Time PCR system (Applied Biosystems, Foster City, CA). The quantity of each mRNA (Ct of each subunits) was normalized by subtracting the quantity of 18S mRNA (Ct of 18S) (internal control) to obtain a normalized value of each one. The relative quantity of mRNA was obtained using the value of 2−ΔΔCt, according to the manufacturer’s protocol.
Statistical analysis
Statistical analyses were performed in GraphPad Prism, and data are expressed as means ± S.E.M. A one-way ANOVA followed by a Tukey’s or Dunnett’s test was used to compare all groups or selected groups to control. P values less than 0.05 were considered significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Effects of Ang-(1-7) on glucose uptake in primary cultural adipocyte
To examine whether Ang-(1-7) affects insulin sensitivity in adipocytes, we first investigated the effect of Ang-(1-7) on glucose uptake. Epididymal adipocytes were isolated from C57 mice and incubated under basal or insulin-stimulating conditions in high-glucose medium in the presence of 10−9 mol/l [14] Ang-(1-7) or together with 10−6 mol/l [14] A779, a Mas-receptor inhibitor. Pioglitazone was used as positive control. The analysis of glucose uptake showed a twofold increase in both basal and insulin-stimulated glucose uptake levels (P < 0.001) (Fig. 1). These data indicate the ability of Ang-(1-7) to improve glucose-transporting via Mas receptor in adipocytes.
Protective effect of Ang-(1-7) on basal and insulin-stimulated 2DG uptake in primary culture epididymal adipocytes of C57 mice. a 2DG uptake in basal state. b 2DG uptake in insulin-stimulated state. The adipocytes exposed to Ang-(1-7) and pioglitazone were significantly higher (1–3 fold) in basal and insulin-stimulated 2DG uptake compared with control cells, which were blocked by A779. ***P < 0.001. Data are presented as means ± S.E.M. (n = 3)
Ang-(1-7) decreases ROS production in 3T3-L1 adipocytes via Mas receptor
To elucidate the mechanism of the improvement of insulin sensitivity by Ang-(1-7) in adipocytes, we used mature 3T3-L1 adipocytes, which were incubated with 25 mmol/l glucose and Ang-(1-7) (exogenous treatment) or overexpress ACE2 (endogenous generation), to investigate the influence of Ang-(1-7) on ROS production. Intracellular ROS was measured by DHE staining, NBT assay, and flow cytometry (Fig. 2). Ang-(1-7) treatment slightly, but significantly, decreased intracellular concentration of ROS by staining with DHE (Fig. 2a) and NBT assay (Fig. 2b). The DHE fluorescence intensity (Fig. 2a) and NBT reduction level (Fig. 2b) exhibited a significant decrease in Ang-(1-7)-treated adipocytes. Meanwhile Ang-(1-7) agonist, A779 increased ROS production (Fig. 2a, b). Furthermore, analysis of fluorescence intensity by flow cytometry also revealed a significant decrease in superoxide formation in Ang-(1-7) infused in the concentration of 10−9 mol/l (Fig. 2c) and ACE2-overexpressed 3T3-L1 adipocytes (Fig. 2d), which were counteracted by A779. Accordingly, ACE2 expression was markedly increased as measured by real-time PCR at 48 h post-transfection. (*, P < 0.05) (Fig. S5). The infection efficiency was detected by a null vector, PcDNA3.1-GFP at 6 h, 24 h, and 48 h (Fig. S4) after transfection in 3T3-L1 cells by fluorescence microscopy. These results indicate that Ang-(1-7) protected against oxidative stress in adipocytes, the effect of which is blocked by A779.
Ang-(1-7) decreased ROS production in 3T3-L1 adipocytes via Mas receptor. The 3T3-L1 adipocytes were treated with 10−9 mol/l Ang-(1-7) or together with 10−6 mol/l A779 for 24 h. a DHE staining in adipocytes. The DHE intensity was showed blow. b NBT staining in adipocytes. The NBT reduction was showed blow. c Flow cytometry using DHE in 3T3-L1 adipocytes. The intensity of staining was measured blow. d Flow cytometry using DHE in ACE2-overexpressor adipocytes. The intensity of staining was measured blow. The increase in the area blow M1 was measured (except for the negative group). Both Ang-(1-7) treatment and ACE2 overexpress can slightly, but significantly, decreased ROS production compared with the control group, which was blocked by A779. *P < 0.05; **P < 0.01; ***P < 0.001. Data are presented as means ± S.E.M. (n = 3)
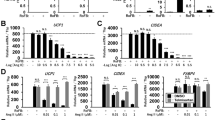
Ang-(1-7) decreases NADPH oxidase subunits mRNA expression in adipocytes
One of the major sources of ROS generation and oxidative stress in many cells and tissues is an activation of phagocyte-type NADPH oxidase [16]. The NADPH oxidase complex consists of six subunits, including two plasma membrane–associated proteins, gp91phox (homolog of NOX1-5) and p22phox, and four cytosolic factors, p47phox, p67phox, p40phox, and rac [16]. To investigate the mechanism of Ang-(1-7) protecting against oxidative stress, mRNA expressions of p22phox, gp91phox, p67phox, p47phox, and NOX4 were tested by real-time PCR. Expressions were markedly inhibited (2–4 fold) by Ang-(1-7) in 3T3-L1 compared with controls (Fig. 3a), which was counteracted by A779 (Fig. 3b). p22phox mRNA level was also decreased in ACE2-overexpression adipocytes (Fig. S6). These observations suggest that Ang-(1-7) may protect against oxidative stress in adipocytes by inhibiting NADPH oxidase expression via the Mas receptor.
Ang-(1-7) decreased mRNA expression of NADPH oxidase subunits. p22phox, gp91phox, p67phox, p47phox and NOX4levels were evaluated by real-time PCR in 3T3-L1 adipocytes. a 3T3-L1 adipocytes were treated with 10−9 mol/l Ang-(1-7). b 3T3-L1 adipocytes were treated with 10−6 mol/l A779 or together with Ang-(1-7) for 24 h. p22phox, gp91phox, p67phox, p47phox, and NOX4 mRNA expressions decreased in adipocytes treated with 10−9 mol/l Ang-(1-7), which was blocked by A779. *P < 0.05; **P < 0.01. Data are presented as means ± S.E.M. (n = 3)
Ang-(1-7) induces adiponectin expression by protecting against oxidative stress in 3T3-L1
To investigate the molecular mechanism of changes in glucose uptake and oxidative stress in adipocytes, we analyzed the levels of insulin-sensitizing components and cytokines in adipocytes. Although the IL-6 and TNF-α mRNA levels were not altered (Fig. 4a, b), the anti-insulin-resistance protein adiponectin mRNA expression was markedly increased in Ang-(1-7)-treated adipocytes and decreased in A779-treated adipocytes (Fig. 4c).
Ang-(1-7) enhanced adiponectin mRNA levels by suppressing oxidative stress. Total RNA was isolated from the cells and reverse transcribed. a, b Prior treatment with Ang-(1-7) or together with A779. Quantification of IL-6 and TNF- alpha was performed by real-time PCR. c Fully differentiated 3T3-L1 adipocytes were preincubated with Ang-(1-7) and/or GO, A779. Adiponectin level was measured by real-time PCR. d Adipocytes were serum-starved for 2 h and then exposed to H2O2 generated by adding different concentrations of GO to medium for 12–16 h. Quantification of adiponectin was performed by real-time PCR. **P < 0.01; ***P < 0.001. Data are presented as means ± S.E.M. (n = 3)
To examine the role of oxidative stress in this process, fully differentiated 3T3-L1 adipocytes were exposed to growth medium (DMEM containing 25 mmol/l glucose and 10% fetal bovine serum) with 25–100 mU/ml GO for 12 h. It was shown that adiponectin levels exhibited a dose-dependent decrease (Fig. 4d), indicating that oxidative stress decreased adiponectin expression. Intriguingly, Ang-(1-7) restored the ROS-induced decrease in adiponectin mRNA expression (Fig. 4c). This suggests that Ang-(1-7) plays a crucial role on improving insulin resistance through decreasing ROS-induced oxidative stress pathway.
Discussion
Oxidative stress is known to play a critical role in the initiation and development of T2DM. A constellation of clinical and preclinical findings has suggested the involvement of oxidative stress in the pathogenesis of T2DM [22, 23]. Both O2 − and H2O2, with strong oxidative characteristics, are classified as ROS and are responsible for blood glucose disturbances and target organ injuries [24, 25]. Oxidative stress reduces the sensitivity of peripheral tissues to circulating insulin and causes reduced metabolism of blood glucose.
In this study, we documented for the first time that both exogenous Ang-(1-7) treatment and endogenous Ang-(1-7) generated by overexpression of ACE2 decreased ROS production in fully differentiated 3T3-L1 adipocytes.
NADPH oxidase is a highly regulated membrane-bound enzyme complex that catalyzes the one-electron reduction of oxygen to superoxide anion via the oxidation of cytosolic NADPH [8]. In this study, we observed that Ang-(1-7) decreased mRNA levels of NADPH oxidase subsets (p22phox, gp91phox, p47phox, p67phox and NOX4) in adipocytes, which was prevented by A779. These results suggest Ang-(1-7) play a pivotal role of protecting against oxidative stress in adipocytes and it could be an important therapeutic target for the treatment of insulin resistance associated with oxidative stress in T2DM.
Insulin resistance is the reduced ability of cells or tissues to respond to physiological levels of insulin and is characteristic of T2DM [26]. Adipose tissue is the primary tissue responsible for the post-prandial uptake of glucose from the blood [16]. In a recent study, Mas-knockout mice exhibit an insulin-resistant state as shown by the decrease in insulin sensitivity, glucose tolerance, and a decrease in glucose uptake in adipose tissue [10]. This suggests that the Ang-(1-7)/Mas axis plays a role as an insulin sensitizer.
Ang-(1-7) is the endogenous legend for the receptor Mas, a seven-transmembrane protein with domains containing sequences characteristic of G protein-coupled receptors [27]. The Mas protooncogene, first detected in vivo by tumorigenic properties originating from rearrangement of its 5′ flanking region [28, 29], encodes a protein with seven hydrophobic transmembrane domains, considered to be an “orphan” G protein-coupled receptor [30]. In this study, we report first that Ang-(1-7) directly increases glucose uptake both in basal and insulin-stimulated states in adipocytes. The potency of such insulin-sensitizing effects is comparable to that seen in pioglitazone-treated cells and is blocked by Ang-(1-7) antagonist A779. This report provides evidence that Ang-(1-7) exerts its insulin-sensitizing effects directly on adipocytes.
Despite unchanged TNF-α and IL-6 mRNA levels, Ang-(1-7) treatment showed an increased adiponectin mRNA expression. Adiponectin is a plasma adipokine that plays a pivotal role in modulating lipid metabolism, insulin sensitivity, and anti-inflammatory activity [31]. Decreased adiponectin levels in blood serum are a common feature of obesity and insulin-resistant state in both humans and rodents [32]. These results suggest that Ang-(1-7) may improve insulin resistance through increasing adiponectin expression. Additionally, fully differentiated 3T3-L1 adipocytes exhibited a decrease in adiponectin mRNA levels when exposed to low concentrations of H2O2 and adiponectin gene expression is negatively modulated by oxidative stress [33].
In this study, we showed that Ang-(1-7) protection against oxidative stress was associated with an elevation of adiponectin levels. Caused increases in H2O2 concentration to micromolar levels in medium by GO generated low concentrations of H2O2 significantly decreased adiponectin mRNA levels in a dose-dependent manner. Thus, it is possible that increased adiponectin expression to protecting against oxidative stress is one mechanism by which Ang-(1-7) improves insulin resistance.
It has been demonstrated consistently that adipose tissue is an important endocrine organ and plays an essential role in lipid and glucose metabolism [34]. Many RAS components are found in adipose tissue [35]. Many studies have shown that Ang-(1-7) can counteract Ang-II actions and act as an ACE inhibitor [36–38]. Ang-II-induced oxidative stress enhanced insulin resistance [39]. Ang-(1-7), as the opposite role of Ang-II in the RAS, could be involved in reducing insulin resistance via its anti-oxidative stress effects [40]. Furthermore, the Ang-(1-7)/Mas axis activates the phosphoinositide-3 kinase (PI3 K)/Akt pathway in adipose tissue, liver, skeletal muscle [41] endothelial cells [42], and in the heart [9], which might be direct metabolic effects of Ang-(1-7). Ang-(1-7) induces the phosphorylation of Akt at both threonine (308) and serine (473) sites in rat adipose tissues. Selective antagonism of the Mas receptor with A779 blocks the Ang-(1-7)-induced Akt phosphorylation in adipose tissues [41]. In fructose-fed rats, a model of metabolic syndrome, chronic Ang-(1-7) treatment, results in the normalization of insulin signaling involving the IR/IRS-1/PI3 K/Akt pathway in adipose tissues [43]. These results show that Ang-(1-7) increases glucose uptake via a mechanism that could involve the modulation of insulin signaling.
In addition, some studies suggest that oxidative stress was able to impair PI3 K and Akt insulin signaling steps in cultured insulin-sensitive cell lines such as 3T3-L1 adipocytes and L6 myocytes [44, 45]. The results suggest a causal relationship between the Ang-(1-7)-mediated negative regulation of NADPH oxidase (with a resulting decrease in ROS) and an increase in glucose uptake. To the best of our knowledge, this is the first study to implicate Ang-(1-7) as a role of anti-oxidative stress.
Reports about the circulation levels of Ang-(1-7) in the serum of are rare. Yamada et al. [46] reported that levels of Ang-(1-7) in the sera of Sprague–Dawley (SD) rats and spontaneously hypertensive rats (SHR) are 1.37 ± 0.18 and 3.77 ± 0.88 (×10−10 mol/l), respectively. In the present study, the concentration of Ang-(1-7) as 10−9 mol/l was based on the previous study [14], which were about 2–7 times the levels of angiotensin in the sera of SD and SHR rats.
A weakness of the current study is that we did not considered the role of Ang-(1-7) on mitochondrial ROS generation. However, mitochondrial ROS is known to be important for the disruption of cellular metabolism, and the NADPH oxidase-associated ROS may alter parameters of signal transduction, insulin secretion, and cell proliferation or cell death. The increase in ROS generation in adipocytes that leads to insulin resistance mainly depends on superoxide generation by NADPH oxidase but not by the mitochondrial respiratory chain, another major source of superoxide in the cell [47]. Another question that arises from our study is whether the changes observed in adipocytes are also observed in other extracardiac cells. Ongoing experiments in our laboratory preliminary suggest that Ang-(1-7) also decreases ROS and NADPH oxidase expression in hepatic and skeletal muscle cells in vitro.
In conclusion, in current study, we provide novel data suggesting that Ang-(1-7) improved glucose uptake and protected against oxidative stress via Mas receptor in adipocytes. The primary mechanisms involved in this effect appear to include an increase in adiponectin and a decrease in NADPH oxidase. Together with the recently reported phenotype of Mas-knockout mice [10], our current findings reinforce the notion that Ang-(1-7) possesses a role in protecting against oxidative stress, at least partly, through NADPH oxidative pathway and improving metabolic processes.
References
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 342:145–153
Kjeldsen SE, Dahlof B, Devereux RB, Julius S, Aurup P, Edelman J, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn S, Wedel H (2002) Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a losartan intervention for endpoint reduction (LIFE) substudy. JAMA 288:1491–1498
McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley, BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, and Califf RM (2010) Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 362:1477–1490
Cao Z, Cooper ME (2011) Efficacy of renin-angiotensin system (RAS) blockers on cardiovascular and renal outcomes in patients with type 2 diabetes. Acta Diabetol. doi:10.1007/s00592-011-0328-3
Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S (1987) Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 36:54–59
Strazzullo P, Galletti F (2004) Impact of the renin-angiotensin system on lipid and carbohydrate metabolism. Curr Opin Nephrol Hypertens 13:325–332
Muscogiuri G, Chavez AO, Gastaldelli A, Perego L, Tripathy D, Saad MJ, Velloso L, Folli F (2008) The crosstalk between insulin and renin-angiotensin-aldosterone signaling systems and its effect on glucose metabolism and diabetes prevention. Curr Vasc Pharmacol 6:301–312
Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS (2006) Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281:35137–35146
Giani JF, Gironacci MM, Munoz MC, Pena C, Turyn D, Dominici FP (2007) Angiotensin-(1 7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol 293:H1154–H1163
Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA (2008) Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57:340–347
Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM (2008) Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 34:56–61
Yang JK, Lin SS, Ji XJ, Guo LM (2010) Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47:193–199
Rodbell M (1964) Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239:375–380
Santos SH, Braga JF, Mario EG, Porto LC, Rodrigues-Machado Mda G, Murari A, Botion LM, Alenina N, Bader M, Santos RA (2010) Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler Thromb Vasc Biol 30:953–961
Yamamoto N, Kawasaki K, Sato T, Hirose Y, Muroyama K (2008) A nonradioisotope, enzymatic microplate assay for in vivo evaluation of 2-deoxyglucose uptake in muscle tissue. Anal Biochem 375:397–399
Yamamoto N, Sato T, Kawasaki K, Murosaki S, Yamamoto Y (2006) A nonradioisotope, enzymatic assay for 2-deoxyglucose uptake in L6 skeletal muscle cells cultured in a 96-well microplate. Anal Biochem 351:139–145
Ueyama A, Sato T, Yoshida H, Magata K, Koga N (2000) Nonradioisotope assay of glucose uptake activity in rat skeletal muscle using enzymatic measurement of 2-deoxyglucose 6-phosphate in vitro and in vivo. Biol Signals Recept 9:267–274
Iida K, Takahashi Y, Kaji H, Yoshioka S, Murata M, Iguchi G, Okimura Y, Chihara K (2003) Diverse regulation of full-length and truncated growth hormone receptor expression in 3T3-L1 adipocytes. Mol Cell Endocrinol 210:21–29
Fukuoka H, Iida K, Nishizawa H, Imanaka M, Takeno R, Iguchi G, Takahashi M, Okimura Y, Kaji H, Chihara K, Takahashi Y (2010) IGF-I stimulates reactive oxygen species (ROS) production and inhibits insulin-dependent glucose uptake via ROS in 3T3-L1 adipocytes. Growth Horm IGF Res 20:212–219
Munzel T, Afanas’ev IB, Kleschyov AL, Harrison DG (2002) Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol 22:1761–1768
Menghini R, Marchetti V, Cardellini M, Hribal ML, Mauriello A, Lauro D, Sbraccia P, Lauro R, Federici M (2005) Phosphorylation of GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissue-related inflammation: a novel pathway linking obesity to atherosclerosis. Circulation 111:1946–1953
Maritim AC, Sanders RA, Watkins JB 3rd (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G (2010) Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud 7:15–25
Li N, Frigerio F, Maechler P (2008) The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans 36:930–934
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Cerbone AM, Macarone-Palmieri N, Saldalamacchia G, Coppola A, Di Minno G, Rivellese AA (2009) Diabetes, vascular complications and antiplatelet therapy: open problems. Acta Diabetol 46:253–261
Young D, O’Neill K, Jessell T, Wigler M (1988) Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci USA 85:5339–5342
Young D, Waitches G, Birchmeier C, Fasano O, Wigler M (1986) Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45:711–719
Rabin M, Birnbaum D, Young D, Birchmeier C, Wigler M, Ruddle FH (1987) Human ros1 and mas1 oncogenes located in regions of chromosome 6 associated with tumor-specific rearrangements. Oncogene Res 1:169–178
Zohn IE, Symons M, Chrzanowska-Wodnicka M, Westwick JK, Der CJ (1998) Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol Cell Biol 18:1225–1235
Sarfstein R, Gorzalczany Y, Mizrahi A, Berdichevsky Y, Molshanski-Mor S, Weinbaum C, Hirshberg M, Dagher MC, Pick E (2004) Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox-Rac1 chimeras. J Biol Chem 279:16007–16016
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935
Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ (2003) Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52:1355–1363
Hattori Y, Akimoto K, Gross SS, Hattori S, Kasai K (2005) Angiotensin-II-induced oxidative stress elicits hypoadiponectinaemia in rats. Diabetologia 48:1066–1074
Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C, Ganten D, Bader M (1998) Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem 273:11867–11873
Giacchetti G, Faloia E, Mariniello B, Sardu C, Gatti C, Camilloni MA, Guerrieri M, Mantero F (2002) Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens 15:381–388
Ferreira AJ, Santos RA (2005) Cardiovascular actions of angiotensin-(1-7). Braz J Med Biol Res 38:499–507
Zhou JB, Yang JK (2010) Angiotensin-converting enzyme gene polymorphism is associated with proliferative diabetic retinopathy: a meta-analysis. Acta Diabetol 47:187–193
Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR (1996) Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA 93:12490–12495
Yuan L, Li X, Li J, Li HL, and Cheng SS (2010) Effects of renin-angiotensin system blockade on the islet morphology and function in rats with long-term high-fat diet. Acta Diabetol. doi:10.1007/s00592-010-0210-8
Munoz MC, Giani JF, Dominici FP (2010) Angiotensin-(1-7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept 161:1–7
Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM (2007) Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49:185–192
Giani JF, Mayer MA, Munoz MC, Silberman EA, Hocht C, Taira CA, Gironacci MM, Turyn D, Dominici FP (2009) Chronic infusion of angiotensin-(1-7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am J Physiol Endocrinol Metab 296:E262–E271
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Nakanishi S, Yamane K, Kamei N, Nojima H, Okubo M, Kohno N (2005) A protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostane. Metabolism 54:194–199
Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM (1998) Converting enzyme determines plasma clearance of angiotensin-(1-7). Hypertension 32:496–502
Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R (2007) Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol 583:9–24
Acknowledgments
This study was supported by grant No. 81070644, No. 30871187 and No. 30671001 from National Natural Science Foundation of China.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Liu and X.-H. Lv contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, C., Lv, XH., Li, HX. et al. Angiotensin-(1-7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol 49, 291–299 (2012). https://doi.org/10.1007/s00592-011-0348-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-011-0348-z