Abstract
We hypothesize that type 2 diabetic patients with different phenotypes may show different response to incretin-based therapies. Therefore, we tested whether the presence of metabolic syndrome (MS) influences glycemic response to these drugs. We prospectively followed 211 patients, treated with the GLP-1 analog exenatide (n = 102) or a DPP-4 inhibitor (n = 109) for at least 4 months. Treatment was decided on clinical grounds. We collected baseline data (age, sex, BMI, waist, systolic and diastolic blood pressure, lipid profile, data on diabetic complications and concomitant treatment) and HbA1c at subsequent visits. Patients were divided into groups according to the presence/absence of MS. Compared to patients on exenatide, patients on DPP-4 inhibitors were older and had lower BMI, waist, diastolic blood pressure, fasting plasma glucose, and HbA1c. At means of baseline values, HbA1c reduction was similar in patients treated with exenatide or DPP-4 inhibitors. Patients on exenatide showed significantly higher HbA1c reduction if they had MS (−1.55 ± 0.22%; n = 88) than if they had not (−0.34 ± 0.18%; P = 0.002). Conversely, patients on DPP-4 inhibitors showed significantly lower HbA1c reduction if they had MS (−0.60 ± 0.12%; n = 73) than if they had not (−1.50 ± 0.24%; P < 0.001). Type of MS definition (ATP-III, IDF or WHO) poorly influenced these trends. The interaction between type of therapy (exenatide vs. DPP-4 inhibitors) and MS remained significant after adjusting for age, baseline HbA1c, BMI, and concomitant medications. In conclusion, the presence of MS appears to modify the response to incretin-based therapies. Given the non-randomized nature of this study, these data need to be replicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous incretins (glucagon-like peptide-1 [GLP-1] and glucose-dependent insulinotropic peptide [GIP]) regulate insulin secretion and exert a variety of other effects through receptor binding in several organs and tissues [1]. GLP-1 is rapidly degraded by dipeptidyl peptidase (DPP)-4 [2]. Incretin-based therapies, including synthetic GLP-1 analogs and DPP-4 inhibitors (DPP-4i), have widened the therapeutic armamentarium and are increasingly used in the management of type 2 diabetes [3, 4] for their ability to improve beta-cell function. Synthetic analogs rise GLP-1 concentrations above physiologic values and exert ancillary effects, such as slowing of gastric emptying and stimulation of satiety, that favor weight loss. DPP-4i block endogenous degradation of GLP-1, restoring physiological incretin signaling, but are weight neutral [5, 6]. Emerging data suggest that widespread expression of DPP-4 may modulate pleiotropic effects of these drugs [7].
The two cornerstones of type 2 diabetes pathophysiology are insulin resistance and beta-cell dysfunction. Indeed, type 2 diabetes phenotype ranges from prevalent beta-cell failure to prevalent insulin resistance, with most patients showing some degree of both defects. Incretin-based therapies counter beta-cell dysfunction, while it is not clear whether or not they also modify insulin resistance [8]. In preclinical studies, mice lacking DPP-4 have shown protection against diet-induced insulin resistance [9], and GLP-1 analogs appear to prevent insulin resistance in rats [10, 11]; however, no clear effects have been found with GLP-1 analogs or DPP-4i on insulin resistance in humans [12, 13].
Despite acting on the same biological system, GLP-1 analogs and DPP-4i have different mechanisms of action and ancillary effects. Therefore, diabetic patients may show different responses to GLP-1 analogs versus DPP-4i based on clinical characteristics and phenotype. At present, there are no studies showing clinical determinants of glycemic efficacy in patients on incretin-based therapies. It has been only shown that patients treated with the GLP-1 analog exenatide achieve HbA1c improvements that are directly proportional to initial HbA1c values [14, 15].
This study was designed to find clinical determinants of glycemic efficacy (HbA1c reduction) in patients on incretin-based therapies, analyzed altogether or per drug type (GLP-1 analog vs. DPP-4i). We specifically focus on the metabolic syndrome (MS), which is a surrogate indicator of insulin resistance in type 2 diabetic patients [16]. Moreover, several features of MS appear to modulate endogenous incretins [17] and may affect response to drugs that act on the incretin system.
Materials and methods
Patients
The study was approved by local institutions and carried out according to the Declaration of Helsinki. Inclusion criteria were type 2 diabetes and initiation of therapy with either a GLP-1 analog or a DPP-4 inhibitor for clinical indication. Decision to start incretin-based therapy in these patients was taken purely on clinical basis. At the time, exenatide was the only GLP-1 analog available in Italy, while DPP-4i included vildagliptin and sitagliptin. Exclusion criteria were missing key baseline data and early drug discontinuation due to side effects. At baseline, we collected the following data: age, sex, weight, height, waist circumference, systolic and diastolic blood pressure, cigarette smoking, lipid profile, fasting plasma glucose, HbA1c, and duration of diabetes. The metabolic syndrome was diagnosed according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP)-III criteria, or the International Diabetes Federation (IDF), or the World Health Organization (WHO) criteria [18]. Patients were also characterized regarding chronic complications. Retinopathy was defined based on digital fundus photography; nephropathy was defined as either a urinary albumin excretion rate >30 mg/g of creatinine or an estimated GFR <60 mL/min/m2. Peripheral neuropathy was defined based on symptoms (DNI questionnaire) and eventually confirmed by an electromyogram. Macroangiopathy was defined as either coronary artery disease (stable or unstable angina, or a history of myocardial infarction, or angiographic evidence of hemodynamically relevant coronary stenosis) or peripheral arterial disease (history of intermittent claudication or rest pain, or an ankle brachial index of less than 0.9, or angiographic evidence of hemodynamically relevant stenosis) or cerebrovascular disease (a history of stroke or transient ischemic attack, or instrumental evidence of carotid atherosclerosis). Data on concomitant medications were collected. We recorded HbA1c values during follow-up.
Statistical analysis
Data are expressed as mean ± standard deviation or as percentage. Comparisons of continuous variables between two or more groups were analyzed using 2-tail unpaired Student’s t test and ANOVA, respectively. The χ2 test was used for categorical variables. Univariate linear associations between change in HbA1c and baseline data were analyzed with Pearson’s r or Spearman’s rho coefficient of correlation for normal and categorical data, respectively. A multivariate regression analysis was run to identify variables independently associated with change in HbA1c, among variable showing significant association upon univariate analyses. A generalized linear regression model was used to detect an interaction between type of treatment and metabolic syndrome, controlling for potential covariates, such as clinical parameters different between the two drug groups. Statistical significance was accepted at P < 0.05 and SPSS ver. 15 was used.
Results
Characteristics of the study population
Between February 2008 and June 2010, 242 type 2 diabetic patients were treated with either exenatide or a DPP-4i for at least 4 months. Of these, 211 had complete records for baseline data and follow-up HbA1c levels, 102 on exenatide, and 109 on a DPP-4i (32 patients on vildagliptin and 77 on sitagliptin). There were no significant differences in age and sex between patients excluded for missing data and patients finally included in the analysis. Compared to patients on exenatide, patients on DPP-4i were significantly older had lower baseline BMI, waist circumference, diastolic blood pressure, fasting plasma glucose, and HbA1c (Table 1). The prevalence of MS at baseline was 67% (ATP-III), 71% (IDF), and 61% (WHO) among patients on DPP-4i and 84% (ATP-III), 86% (IDF), and 82% (WHO) among patients on exenatide.
Determinants of glycemic efficacy
Patients were followed for an average 6.7 ± 0.3 months (7.3 ± 0.4 with exenatide and 6.2 ± 0.4 with DPP-4i). In the entire study population, exenatide was associated with a higher drop in HbA1c compared to DPP-4i (−1.38 ± 0.20% vs. −0.90 ± 0.12%; P = 0.03); weight loss was also greater with exenatide than with DPP-4i (−5.0 ± 0.7 vs. −2.2 ± 0.7 kg; P = 0.006). However, these differences were lost after adjusting for baseline HbA1c and weight, respectively, both of which were higher in the exenatide group. Mean change in HbA1c was comparable between sitagliptin and vildagliptin (−0.92 ± 0.13% vs. −0.83 ± 0.26%, respectively; P = 0.730).
To look for determinants of glycemic efficacy, we performed univariate linear regression analyses between HbA1c reduction and clinical parameters. When patients on exenatide and DPP-4i were analyzed altogether, we found a highly significant direct correlation between baseline HbA1c and drop in HbA1c (r = 0.70; P < 0.001), while other clinical parameters were not correlated. Among diabetic complications, patients with macroangiopathy showed a lower drop in HbA1c than patients without, independently of baseline HbA1c (0.85 ± 0.12% vs. −1.50 ± 0.25%; P = 0.012), while microangiopathies were not associated with glycemic efficacy. When patients on DPP-4i were examined separately, drop in HbA1c was still directly correlated to baseline HBA1c (r = 0.47; P < 0.001) and also negatively correlated with BMI (r = −0.22; P = 0.02) and the presence of high blood pressure (rho = −0.34; P < 0.001). On multivariate analysis, these three parameters remained significantly and independently associated with HbA1c reduction (Table 2). When patients on exenatide were examined separately, change in HbA1c was directly correlated to baseline HbA1c (r = 0.77; P < 0.001) and to the presence of high blood pressure (rho = 0.21; P = 0.03). Change in HbA1c was not correlated to diabetes duration or change in body weight in the whole population or in drug groups separately.
Glycemic efficacy and metabolic syndrome
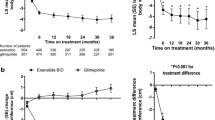
Patients were first divided according to the presence/absence of ATP-III MS and its components. Patients on exenatide showed significantly higher HbA1c reduction in the presence than in the absence of MS (−1.55 ± 0.22% vs. 0.34 ± 0.18%; P = 0.002; Fig. 1a). There was a significant direct correlation between drop in HbA1c and number of MS components (rho = 0.25; P = 0.011). Among MS components, high blood pressure (n = 85, 83%) was associated with a significant higher HbA1c reduction than its absence. Presence of obesity (n = 97, 95%) showed a similar trend, which was not statistically significant, owing to the low number of patients on exenatide who were not obese according to the ATP-III definition of MS (Fig. 1d).
HbA1c reduction in relation to metabolic syndrome and its components. Patients on DPP-4i or exenatide were divided into groups according to the presence or absence of ATP-III a, IDF b, and WHO c metabolic syndrome (MS) (ANOVA P < 0.05 for all; *significant vs. no MS; †significant vs. exenatide). Patients on exenatide d or DPP-4i e were then divided according to the presence or absence of individual ATP-III MS components. *P < 0.05 yes versus no MS (borderline P values are also shown). TG triglycerides, hBP high blood pressure. Numbers of patients are shown inside columns
Patients on DPP-4 inhibitors showed significantly lower HbA1c reduction in the presence than in the absence of MS (−0.60 ± 0.12% vs. −1.5 ± 0.24%; P < 0.001; Fig. 1a). There was a significant inverse correlation between drop in HbA1c and number of MS components (rho = −0.29; P = 0.002). Among MS components, high blood pressure and low HDL were associated with significantly lower HbA1c reduction, and a similar non-significant trend was found for obesity (Fig. 1e).
When patients were divided according to IDF MS instead of ATP-III MS, HbA1c changes in DPP-4i and exenatide-treated patients were quite similar (Fig. 1b); with the WHO definition of MS, HbA1c reductions showed a similar trend, but the difference did not reach statistical significance in exenatide-treated patients (Fig. 1c).
In the linear generalized multivariate model, we found that the interaction between type of incretin therapy (exenatide or DPP-4i) and ATP-III MS was independent of age, baseline HbA1c, BMI, and concomitant anti-diabetic medications (Table 3).
As high blood pressure was a significant determinant of glycemic efficacy in both groups, we analyzed the relationship between anti-hypertensive medications and HbA1c reduction: In the whole cohort and in drug groups separately, we found no differences in HbA1c change in patients taking or not taking any of the anti-hypertensive medications listed in Table 1.
Discussion
This was a prospective, single-center, observational study of patients treated in a real-life setting. The most important finding is that MS modified the response to incretin-based therapies. Strikingly, patients showed opposite responses to exenatide and DPP-4i according to the presence or absence of MS. At means of age, BMI, baseline HbA1c, and concomitant anti-diabetic medications, patients without MS were more likely to respond to a DPP-4i than to exenatide and vice versa. If confirmed, this observation would have important implications in the treatment of type 2 diabetic patients with this class of drugs. However, owing to its non-randomized nature, data from this cohort need to be replicated by independent investigators, possibly within a clinical trial.
At present, the reasons why patients on exenatide responded better if they had than if they did not have MS are unclear and warrant further investigation. In a double-blind, randomized cross-over study, DeFronzo et al. [5] found that exenatide was superior to sitagliptin in reducing post-prandial glucose in type 2 diabetic obese patients, many of which likely had MS. In type 2 diabetic patients, diagnosis of MS has not a clear clinical value [19, 20], but the number of MS components is a surrogate indicator of the degree of insulin resistance [16]. Thus, supraphysiological GLP-1 signaling achieved with exenatide might counter insulin resistance, as suggested by preclinical studies [11, 21, 22]. In addition, MS patients may have developed “GLP-1 resistance”, mediated by the low expression of GLP-1 receptors on beta cells and resulting in low incretin-stimulated insulin secretion [23, 24]. The possibility to force this resistance with high GLP-1 levels may explain the better response to exenatide than to DPP-4i in MS patients.
Interestingly, patients without MS showed a better response to DPP-4i than to exenatide. Weight loss might influence glycemic efficacy, but the fact that patients without MS were leaner cannot explain the worse response to exenatide, and we found no correlation between change in HbA1c and change in body weight at follow-up. It should be acknowledged that the number of patients without MS in the exenatide group was low, because GLP-1 analogs are not usually prescribed to lean patients.
Another possible explanation comes from the observation that DPP-4 activity is increased in patients with non-alcoholic fatty liver disease [25] and obesity [26, 27], both of which are features of MS and insulin resistance. In addition, methylation of the DPP4 gene, which blocks production of the DPP-4 enzyme, is directly related to HDL cholesterol [28], another MS component. Finally, an interaction, although complex, between DPP-4 activity and hypertension has been shown [29]. Thus, an increased DPP-4 activity in the presence of MS and its components would explain why DPP-4i were less effective in patients with MS than in those without.
With different MS syndrome definitions, the trends of HbA1c reduction in the 4 groups were preserved, thus strengthening the clinical value of the study. However, the WHO definition reduced statistical significance in the exenatide-treated group, indirectly suggesting that results are more robust in DPP-4i-treated patients.
It has been shown that DPP-4i modulates the hemodynamic response to ACE inhibitors in patients with the MS [30, 31]. We examined whether, vice versa, ACE inhibitors modulated the effects of DPP-4i on HbA1c. We found that there was no relationship between use of ACE inhibitors and HbA1c response. This was true for patients on DPP-4i or exenatide and for all classes of anti-hypertensive medications.
We confirm that the baseline HbA1c value is a strong determinant of decrease in HbA1c after treatment, as it happens regularly in studies with diabetic patients [32]. Moreover, mean HbA1c reduction in this cohort was higher with both exenatide and DPP-4i compared to what reported in the literature [33]. This is probably related to the difference between the routine practice and randomized control study settings and to the fact that other interventions may have occurred in parallel. Diabetes duration was not a determinant of glycemic efficacy in this cohort of patients treated with exenatide or a DPP-4i. While there had been initial suggestions that disease duration could affect the choice of incretin-based therapies, several investigators have subsequently showed that disease duration does not affect response to DPP-4i [34] or GLP-1 [35].
This study has several limitations owing to the small sample size and because it has been conducted in a real-life setting, without excluding patients with confounding factors. Treatment was decided on clinical ground and was not randomly assigned. Moreover, patients who withdrew the drug due to early side effects were excluded for the unavailability of at least one follow-up HbA1c value. As a result of these selection biases, differences between patients on DPP4i and patients on exenatide make these groups not directly comparable. However, remarkable differences in glycemic efficacy were found within each group according to MS status. The results in the exenatide group appear less solid than in the DPP-4i group, as the criteria used (ATP-III and IDF vs. WHO) modified the glycemic efficacy difference between MS and non-MS patients, likely because of the low number of non-MS patients treated with exenatide. Residual confounding may account for observed results, and efforts were done to uncover eventual biases and potential confounders. To this end, a generalized linear regression model was run and showed that the interaction between type of drug and MS in determining change of HbA1c persists after adjusting for age, BMI, baseline HbA1c, and concomitant medications, which were different between the two groups. Age was not a determinant of glycemic efficacy. The association between macroangiopathy and glycemic efficacy in both drug groups may even suggest that occult comorbidities, not accounted for, may affects results.
In conclusion, we show that exenatide and DPP-4i have opposing glycemic efficacy according to MS status independently of age, baseline HbA1c, BMI, and concomitant medications. These results may have direct clinical implications in the individualization of therapy, but due to the limitations inherent to the study design, they should be considered hypothesis generating and need to be replicated.
References
Nauck MA (1998) Glucagon-like peptide 1 (GLP-1): a potent gut hormone with a possible therapeutic perspective. Acta Diabetol 35:117–129
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157
Giugliano D, Standl E, Vilsbøll T et al (2009) Is the current therapeutic armamentarium in diabetes enough to control the epidemic and its consequences? What are the current shortcomings? Acta Diabetol 46:173–181
Rotella CM, Pala L (2008) Time to insulin in type-2 diabetes: high hurdles or Santiago way? Acta Diabetol 45:67–74
DeFronzo RA, Okerson T, Viswanathan P et al (2008) Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 24:2943–2952
Vilsboll T, Holst JJ (2004) Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia 47:357–366
Pala L, Pezzatini A, Dicembrini I et al. (2011, in pres) Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells. Acta Diabetol
Hansen BF, Jensen P, Nepper-Christensen E, Skjolstrup B (1998) Effects of glucagon-like peptide-1 (7–36)amide on insulin stimulated rat skeletal muscle glucose transport. Acta Diabetol 35:101–103
Conarello SL, Li Z, Ronan J et al (2003) Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 100:6825–6830
Li L, Yang G, Li Q et al (2008) Exenatide prevents fat-induced insulin resistance and raises adiponectin expression and plasma levels. Diabetes Obes Metab 10:921–930
Park S, Hong SM, Ahn IS (2010) Exendin-4 and exercise improve hepatic glucose homeostasis by promoting insulin signaling in diabetic rats. Metabolism 59:123–133
DeFronzo RA, Triplitt C, Qu Y et al (2010) Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care 33:951–957
Perreault L, Man CD, Hunerdosse DM, Cobelli C, Bergman BC (2010) Incretin action maintains insulin secretion, but not hepatic insulin action, in people with impaired fasting glucose. Diabetes Res Clin Pract 90:87–94
Buysschaert M, Preumont V, Oriot PR et al (2010) One-year metabolic outcomes in patients with type 2 diabetes treated with exenatide in routine practice. Diabetes Metab 36:381–388
Preumont V, Hermans MP, Brichard S, Buysschaert M (2010) Six-month exenatide improves HOMA hyperbolic product in type 2 diabetic patients mostly by enhancing beta-cell function rather than insulin sensitivity. Diabetes Metab 36:293–298
Bonora E, Targher G, Formentini G et al (2004) The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med 21:52–58
Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ (2011) Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54:10–18
Assmann G, Guerra R, Fox G et al (2007) Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European populations. Am J Cardiol 99:541–548
Kahn R, Buse J, Ferrannini E, Stern M (2005) The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 48:1684–1699
Erbas T (2003) Metabolic syndrome. Acta Diabetol 40(Suppl 2):S401–S404
Liu Q, Adams L, Broyde A et al (2010) The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt-sensitive rats. Cardiovasc Diabetol 9:32
Shang Q, Saumoy M, Holst JJ, Salen G, Xu G (2010) Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol 298:G419–G424
Tseng CC, Boylan MO, Jarboe LA, Usdin TB, Wolfe MM (1996) Chronic desensitization of the glucose-dependent insulinotropic polypeptide receptor in diabetic rats. Am J Physiol 270:E661–E666
Lynn FC, Pamir N, Ng EH et al (2001) Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes 50:1004–1011
Firneisz G, Varga T, Lengyel G et al (2010) Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: a novel liver disease biomarker. PLoS One 5:e12226
Carr RD, Larsen MO, Jelic K et al (2010) Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 95:872–878
Reinehr T, Roth CL, Enriori PJ, Masur K (2010) Changes of dipeptidyl peptidase IV (DPP-IV) in obese children with weight loss: relationships to peptide YY, pancreatic peptide, and insulin sensitivity. J Pediatr Endocrinol Metab 23:101–108
Turcot V, Bouchard L, Faucher G et al. (2010) DPP4 Gene DNA Methylation in the omentum is associated with its gene expression and plasma lipid profile in severe obesity. Obesity (Silver Spring) 19:388–395
Jackson EK, Dubinion JH, Mi Z (2008) Effects of dipeptidyl peptidase IV inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol 35:29–34
Marney A, Kunchakarra S, Byrne L, Brown NJ (2010) Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension 56:728–733
Jackson EK (2010) Dipeptidyl peptidase IV inhibition alters the hemodynamic response to angiotensin-converting enzyme inhibition in humans with the metabolic syndrome. Hypertension 56:581–583
Gale EA, Beattie SD, Hu J, Koivisto V, Tan MH (2007) Recruitment to a clinical trial improves glycemic control in patients with diabetes. Diabetes Care 30:2989–2992
Amori RE, Lau J, Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. Jama 298:194–206
Matthews DR, Dejager S, Ahren B et al (2010) Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab 12:780–789
Toft-Nielsen MB, Madsbad S, Holst JJ (2001) Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 86:3853–3860
Acknowledgments
The authors declare no conflict of interest related to the topic covered by this manuscript. GPF, SVdK, and AA received lecture fees from manufacturers of GLP-1 analogs and/or DPP-4 inhibitors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fadini, G.P., de Kreutzenberg, S.V., Gjini, R. et al. The metabolic syndrome influences the response to incretin-based therapies. Acta Diabetol 48, 219–225 (2011). https://doi.org/10.1007/s00592-011-0296-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-011-0296-7