Abstract
Introduction
Hip osteoarthritis (OA) is a prevalent and debilitating condition, necessitating effective and safe treatment options. This systematic review aims to explore the potential of intra-articular mesenchymal stem cell (MSC) infiltrations as a therapeutic approach for hip OA.
Methods
Following PRISMA guidelines, a systematic review was conducted, encompassing PubMed, Embase, and Cochrane Library databases. Inclusion criteria involved studies focusing on intra-articular MSC injections in patients with hip OA and reporting pain relief as an outcome measure. Quality assessment utilized the Newcastle–Ottawa scale and methodological index for non-randomized studies.
Results
Ten studies were included in the review, exhibiting varied designs and sample sizes (316 patients). Outcome measures consisted of cartilage repair assessed through MRI and radiographies, pain scores (WOMAC, VAS, NRS), and functional improvements (HOS-ADL, OHS, FRI, PDQQ, LEFS). The studies reported favorable improvements in functional scores, pain relief, and cartilage repair/radiographic findings, with minimal reported adverse events.
Conclusions
Intra-articular MSC infiltrations demonstrate promise as an effective and safe therapeutic intervention for managing hip OA, offering pain relief and functional enhancements. Nevertheless, limited high-quality studies and outcome measure variations underscore the need for further research to establish definitive treatment guidelines. Future investigations should address optimal MSC utilization, long-term outcomes, and potential complications to ensure the success of MSC-based therapies for hip OA management, ultimately improving patient outcomes. The findings provide valuable insights into the potential of MSC-based treatments for hip OA, advocating further rigorous research in this field.
Trial Registration
The protocol was registered on PROSPERO database (CRD42023436973).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a chronic disease that affects the joint and surrounding tissues, causing progressive damage to the articular cartilage [1, 2]. OA is associated with health implications for the affected patients and burdens the healthcare systems worldwide [3, 4]. The lower limb, especially knee and hip, is most affected, with a worldwide overall prevalence of about 300 million [5, 6]. Currently, there are no treatments able to reverse the clinical progression of hip OA and the existing treatments primarily focus on symptom relief [5, 7]. The initial approach to early-stage hip OA involves conservative measures, including pain management, physical therapy, and lifestyle modifications [5, 8]. However, the chronic use of analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) have been associated with decreased tolerability and increased risk of gastrointestinal and cardiovascular adverse events [9]. In the advanced stages, these conservative approaches often fail and total hip arthroplasty (THA) may become necessary [5, 10]. However, although THA is effective and widely employed, patients are exposed to complications and further revision surgery [11,12,13].
In recent years, there has been growing interest in regenerative medicine [14,15,16]. Among the regenerative strategies, the use of mesenchymal stem cells (MSCs) has gained attention due to their unique properties and potential for tissue repair and regeneration [17]. MSCs are multipotent cells that derive from various tissues, including bone marrow, adipose tissue, and umbilical cord [18,19,20]. These cells have the ability to self-renew and differentiate into different cell types, including chondrocytes, which are responsible for cartilage formation and maintenance [21,22,23]. In preclinical and clinical studies, MSC-based therapies have shown promising results in the regeneration of damaged joint tissues, including the articular cartilage [24].
Besides their ability to differentiate into chondrocytes and contribute to cartilage repair, MSCs modulate inflammation, promote angiogenesis, and secrete various growth factors and cytokines which create a favorable environment for tissue healing and regeneration [21, 25, 26].
Although in recent years the use of MSCs has become popular in knee OA, there is a paucity of evidence about their application in hip OA. Nevertheless, initial reports on the use of MSCs application in hip OA have provided encouraging results [16, 27,28,29]. This systematic review investigated the efficacy and feasibility of intra-articular infiltrations of MSCs for hip OA.
Methods
This study was reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [30]. The protocol was registered on PROSPERO database (CRD42023436973).
Inclusion and exclusion criteria
The PICO framework [31] was used in developing the literature search strategy: patients (P), subjects affected by hip OA; investigated condition (I), intra-articular injection of MSCs therapies; comparator (C), none; outcome (O), pain relief; and study type (S), clinical study.
Exclusion criteria were: (a) missing information on the follow-up, (b) non-hip OA, (c) missing quantitative data, (d) preclinical studies, (e) not English language, (f) full-text unavailability, (g) a conference abstract or a review. No time constraints were used for the search. Only articles published in a peer-reviewed journal were eligible.
Outcome measures
The aim of this systematic review was to investigate the efficacy and feasibility of intra-articular infiltrations of MSCs for hip OA.
Data source and study search
A database search was performed in PubMed, Embase, and Cochrane Library using appropriate Medical Subject Headings (MeSH). The following search terms and their combinations were applied for PubMed: ((hip osteoarthritis) OR (hip OA) OR (hip degenerative joint disease)) AND ((mesenchymal stem cell) OR (adipose stem cell) OR (bone marrow stem cell) OR (fat grafting) OR (stromal vascular fraction)). References of each included article were checked to screen for additional potentially relevant studies (i.e., snowballing method). The last search date was July 1, 2023.
Selection of studies and data extraction
Two reviewers independently conducted the electronic literature search (M.A.B. and R.G.). The reference lists from the 3 databases (i.e., PubMed, Embase, and Cochrane Library) were merged, and the duplicates were removed using the reference management software EndNote (version X9.3.3, Clarivate, London, UK). After an initial screening of titles and abstracts, the full texts of pertinent papers underwent subsequent evaluation for eligibility. Discrepancies were discussed with a senior author (G.M.P.). Data extracted from selected articles were collected in Microsoft Office Excel (version 2019, Microsoft Corp, Seattle, Washington, USA) spreadsheet. Predefined variables were extracted by two authors (M.A.B. and R.G.) independently, and inconsistencies were discussed with the research team.
The following information was collected:
-
a.
Study characteristics: author, publication year, study design, sample size, and follow-up duration.
-
b.
Patient demographics: age, gender, and severity of hip OA.
-
c.
Intervention details: MSC source, route of administration, dosage, and any concomitant therapies.
-
d.
Outcome measures: cartilage repair scores, pain scores, functional assessment tools, adverse events, and radiographic findings.
Quality assessment
The risk of bias was analyzed for each study. For prospective case–control studies, the Newcastle–Ottawa Scale (NOS) [32] was used. Three domains were evaluated: (1) selection, (2) comparability, and (3) exposure. A maximum of 9 points could be allocated. For the retrospective cohort studies and case series, the methodological index for non-randomized studies (MINORS) criteria [32] was used. The MINORS tool is a validated instrument designed to assess the methodological quality of non-randomized studies. The maximum score for non-comparative studies is 16 [32].
Results
Study selection
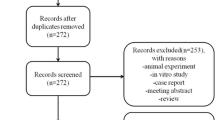
A total of 840 articles were identified through the initial literature search. After the removal of 152 duplicates, 688 articles underwent title and abstract screening. Following this process, 664 studies were excluded, while 24 studies were selected for full-text review. Ultimately, 10 studies met the inclusion criteria and were included in this systematic review (Fig. 1), (Table 1).
Characteristics of included studies
The included studies encompassed a range of study designs, including four case series, two prospective cohort studies, two retrospective studies, and one case report. The publication years of the included studies ranged from 2015 to 2023. A total of 316 patients undergoing intra-articular MSCs injection for hip OA were reported. The sample sizes varied, with the number of participants ranging from 1 to 147. The duration of follow-up in the studies varied from 3.3 to 40 months.
Intervention details
In the selected studies, MSCs were derived from various sources, including bone marrow, stromal vascular fraction, and adipose tissue. The routes of administration for MSCs included ultrasound-guided injections and fluoroscopy. The dosages and frequencies of MSC administration varied across studies. Some studies also reported the concurrent use of additional therapies. Specifically, one study involved the administration of MSCs in combination with platelet-rich plasma (PRP), while another study utilized a therapy consisting of stromal vascular fraction (SVF) injections along with PRP and hyaluronic acid.
Outcome measures
The outcome measures assessed in the included studies were diverse and included measures of cartilage repair/radiographic findings, pain relief, functional improvement, and adverse events. Cartilage repair/radiographic findings scores were evaluated using magnetic resonance imaging (MRI) and plain radiographies. Pain scores were assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [33], visual analogue scale (VAS) [34], and the numeric rating system (NRS) [35]. Specific combined hip scores were also used, such as Harris hip score (HHS) [34], modified Harris hip score (mHHS) [36], vail hip score (VHS) [37], and Japanese Orthopaedic Association hip disease evaluation questionnaire (JHEQ) [38]. Functional improvement was measured using walking distance, hip outcome score–activities of daily living (HOS-ADL) [39], Oxford hip score (OHS) [40], functional rating index (FRI) [41], Pain Disability Quality-of-Life Questionnaire (PDQQ) [42], and lower extremity functional scale (LEFS) [43].
Safety
No severe adverse events occurring during harvest procedures, injective treatment, and post-injective follow-up periods were reported. One study reported a hematoma at the donor site, which was treated conservatively. Another study reported temporary joint pain, which also resolved spontaneously.
Risk of bias and study quality assessment
The risk of bias assessment in prospective case–control studies according to the NOS is reported in Supplement 1. The only included case–control study registered a score of 7, indicating a high-quality study. Bias assessment for the remaining eight studies was performed using MINORS criteria. MINORS scores ranged from 7 to 15, with a median of 9. The major deficiencies were the lack of prospective collection of data and the calculation of study size. All studies showed a clearly stated aim, appropriate endpoints, and a small loss at follow-up. Most of the studies included consecutive patients. MINORS scores for the included studies are shown in Supplement 1.
Discussion
Interrupting the natural progression of hip OA is challenging. In this regard, the use of MSCs seems to offer an effective and safe option in the management of this condition and potentially delay the more invasive procedures. According to the main findings of the present study, intra-articular injections of MSCs for hip OA were effective and safe, providing insights into the potential benefits of a regenerative approach. The results of the included studies consistently demonstrate improvements in functional scores of the hip, indicating the positive impact of MSC injections on the overall mobility and quality of life for patients with hip OA. Despite variations in study design and patient characteristics, the potential efficacy of MSC-based therapies in managing hip OA symptoms has been demonstrated. The observed improvements in functional scores suggest that MSC therapy is viable for hip OA. These findings align with previous research highlighting the regenerative and anti-inflammatory properties of MSCs, which can contribute to the repair and regeneration of damaged joint tissues [22, 44].
Emadedin et al. conducted a case series involving five patients who received expanded bone marrow-derived MSCs (BM-MSCs) via fluoroscopy-guided injection. The study reported improvements in walking distance, WOMAC scores, and HHS at different follow-ups. Articular cartilage repair was observed on MRI imaging in three out of five patients at 6 months, thus suggesting the potential regenerative properties of BM-MSCs on damaged cartilage [28]. In the study by Mardones et al., a prospective cohort study involving ten patients (13 hips) was conducted. The patients received ex vivo expanded BM-MSCs via ultrasound-guided injection. The study reported significant improvements in VAS pain scores, WOMAC scores, HHSM scores, and VAIL scores at 16–40 months of follow-up. The study’s strengths include the use of multiple outcome measures and a relatively long follow-up period [45]. Pak et al. [46] presented a case of one woman who received SVF injections along with PRP and hyaluronic acid. The patient experienced pain relief and improved ROM after the injections, with evidence of cartilage-like tissue regeneration on MRI. Darrow et al. [47] conducted a case series involving four patients who received bone marrow concentrate (BMC) injections via ultrasound guidance. The study reported improvements in resting and active pain levels, as well as functional outcomes assessed using LEFS. Notably, the questionnaire was administered at a mean follow-up time of 3.3 months, which limits the assessment of long-term effects. Dell'Oca et al. [27] presented a case series on six patients who received fluoroscopy-guided injections of micro-fragmented adipose tissue, reporting significant improvements in HHS, WOMAC scores, and VAS pain scores at 6 months [27]. Whitney et al. [48] conducted a case series on 21 patients (16 hips) who received fluoroscopy-guided BMC injections, reporting significant improvements in NRS, WOMAC scores, mHHS, and HOS-ADL at 6 months. Burnham et al. conducted a retrospective cohort study on 30 patients who received fluoroscopy-guided BMC injections. Patients reporting ≥ 50% pain relief on the VAS and ≥ 50% improvement in PDQQ scores at 6 months were classified as “responders,” and patients not meeting these criteria were classified as “non-responders.” Eighteen of 30 patients reported ≥ 50% pain relief on the VAS and ≥ 50% improvement in PDQQ at 6-month follow-ups [49]. Heidari et al. conducted a prospective cohort study on 147 patients who received fluoroscopy-guided injections of micro-fragmented adipose tissue with or without PRP. The experimental group showed a 73% improvement in VAS scores, with 63% experiencing a significant improvement of over 20 points. In terms of the OHS, 65% showed improvement, with 73% of them being classified as super-responders. In the study group without PRP, VAS scores improved in 63% of participants, of whom 64% experiencing a significant improvement. The OHS showed improvement in 81% of participants, with 50% being super-responders. Overall, both groups demonstrated positive outcomes, but the experiment group had a higher percentage of super-responders in both measures. Temporary joint pain was reported as a complication, but no serious adverse events were mentioned. The high loss to follow-up (> 10%) is a limitation of the study [50]. Natali et al. conducted a retrospective cohort study involving 55 patients who received micro-fragmented adipose tissue injections, evidencing improvements in VAS and OHS, with the greatest improvement observed in patients with moderate OA. However, the study noted that some patients required additional treatments or total hip arthroplasty during the follow-up period. The study’s limitations include the retrospective design and potential selection bias [51]. Overall, the studies discussed above suggest that MSCs-based therapies, including BM-MSCs, SVF, BMC, and micro-fragmented adipose tissue injections, may provide pain relief and functional improvements in patients with hip OA. However, the limited sample sizes, lack of control groups, and variations in study designs and outcome measures make it challenging to draw definitive conclusions. The recent prospective case series conducted by Onoi et al. explored the application of SVF cells in treating hip osteoarthritis [52]. In the study, forty-two patients underwent a single SVF cell injection into the hip joint guided by echo imaging. Remarkable improvements were observed in the HHS, JHEQ score, and VAS at the 6-month follow-up. However, the degree of improvement varied based on the severity of osteoarthritis. Specifically, patients with Kellgren–Lawrence (KL) grade II experienced significant clinical improvement, while those with KL grade IV showed slight or minimal progress. Radiographic and magnetic resonance imaging assessments did not reveal notable changes. Nevertheless, this study demonstrates promising outcomes in terms of short-term relief and symptom alleviation for hip osteoarthritis patients.
A low rate of complications associated with MSC injections in hip OA was reported. The rate of complication was minimal: one hematoma at harvesting site [27] and one transient hip joint pain [50]. Both complications resolved without consequences. This low rate of complication suggests that MSCs-based therapy in hip OA is safe. Nonetheless, it is essential to be cautious when interpreting these results, as the long-term safety and potentially rare adverse events of MSC-based therapy require further investigations.
A previous systematic review was conducted to evaluate the effectiveness of orthobiologic injectable therapies, such as MSCs and PRP, in the treatment of hip OA [16]. The findings of this review shed light on the safety profile and potential outcomes associated with these therapies. According to the authors, the reviewed studies collectively indicated that MSCs-based injections are effective and safe.
Two studies [46, 50] reported the concurrent use of combined therapies with MSCs, and this could be a potential confounding factor. In this regard, it is important to note that several studies have shown that the addition of PRP enhances of survival MSCs [53,54,55,56,57]. This suggests that the primary mechanism of action is primarily attributed to the stem cells themselves rather than the PRP. PRP provides a supportive environment for the survival, proliferation, and differentiation of stem cells [53, 56]. It acts as a biologically active scaffold, promoting tissue repair and regeneration. Additionally, PRP stimulates endogenous stem cells and recruits beneficial cells to the site of injury [57]. While the exact mechanisms are not fully understood, the combination of PRP with MSCs shows promising results in enhancing their therapeutic potential. Further research is needed to optimize the administration and explore the precise interactions between PRP and stem cells. However, despite the promising results, several important considerations need to be addressed. Firstly, optimizing treatment protocols is essential to ensure consistent and effective outcomes. Factors such as the ideal cell source, administration methods, dosage, and timing require further investigation to maximize the potential of MSC-based therapies. Additionally, understanding the mechanisms of action by which MSCs exert their regenerative effects will provide valuable insights into their therapeutic benefits. Long-term safety and efficacy are critical considerations in the development of MSC-based therapies for hip OA. Although early studies and clinical trials have shown encouraging results, comprehensive assessments of the potential risks, such as tumor formation, immunogenicity, and long-term durability of the regenerative effects, are necessary to establish the safety profile of MSC treatments. Furthermore, it is important to address the challenges associated with translating MSC-based therapies into clinical practice. Issues such as scalability, standardization, and regulatory considerations must be carefully addressed to ensure widespread accessibility and consistency of treatment.
The present study has several limitations. Firstly, the limited number of clinical studies is available for inclusion and their overall low quality. Among the studies analyzed, only one included a control group, thus making it difficult to determine the specific effects of MSC-based therapies compared to other treatment options or a placebo. The placebo effect was analyzed in the context of intra-articular PRP injections for knee osteoarthritis. In the study by Filardo et al., the results indicate that PRP injections go beyond the placebo effect, showing a statistically and clinically significant advantage over placebo at the 12-month follow-up [58]. The absence of control groups hinders the ability to attribute the observed improvements solely to the MSC interventions. Furthermore, heterogeneity is observed in terms of the source and dosage of MSCs administered within the hip joint across the included studies. Variations in cell processing methods, such as isolation techniques, culture conditions, injection methods, and outcome measures, can impact the therapeutic potential and outcomes of MSCs. Therefore, the varying characteristics of the MSCs used among the studies may contribute to the heterogeneity in observed results. Additionally, it is important to consider the relatively short follow-up periods in most of the included studies. The limited length of follow-up does not allow to draw definitive conclusions about the long-term safety and efficacy of MSC therapy for hip OA. Longer-term studies are necessary to assess the durability of treatment effects, potential complications, and overall patient outcomes over an extended period. Furthermore, it is important to acknowledge a limitation in two studies [46, 50], as they employed additional therapies alongside the administration of MSCs. The utilization of these concomitant treatments introduces a potential confounding factor that could have influenced the outcomes. Therefore, caution should be exercised in interpreting the results, considering the possibility that the observed effects may not solely be attributed to the MSC administration itself but could be influenced by the combined effects of the supplementary therapies. Despite these limitations, the present study provides insights into the available evidence on MSC injections in hip OA. Further well-designed, controlled trials with larger sample sizes, longer follow-up durations, and standardized methodologies are needed to better evaluate the efficacy of MSC therapy in hip osteoarthritis and identify predictors of enhanced response to treatment. However, current data reported no major complications and the patients’ overall tolerance toward the procedure. In conclusion, while the present study identifies several limitations, including the scarcity of high-quality clinical evidence, heterogeneity in MSC characteristics, and relatively short follow-up periods, it serves as an important foundation for future research and clinical decision-making. Addressing these limitations through well-designed studies will enhance our understanding of the safety and long-term outcomes of MSC injections in hip OA.
Conclusions
The widespread attention and growing interest in MSCs injections for hip OA present a promising foundation for the establishment of clear and standardized treatment guidelines. The available evidence indicates that MSCs injections are safe and yield overall promising results in the management of hip OA. However, to obtain more definitive conclusions, further high-level controlled studies are required. These studies will enhance our understanding of the optimal use of MSCs intra-articular injections, including dosage, timing, and long-term outcomes. Continued research efforts in this field will contribute to the advancement of evidence-based practices and improve patient outcomes in hip OA treatment.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- BMC:
-
Bone marrow concentrate
- BM-MSCs:
-
Bone marrow-derived MSCs
- FRI:
-
Functional rating index
- HHS:
-
Harris hip score
- HOS-ADL:
-
Hip outcome score–activities of daily living
- JHEQ:
-
Japanese Orthopaedic Association Hip Disease Evaluation Questionnaire
- KL:
-
Kellgren–Lawrence
- LEFS:
-
Lower extremity functional scale
- MRI:
-
Magnetic resonance imaging
- MeSH:
-
Medical Subject Headings
- MSCs:
-
Mesenchymal stem cells
- MINORS:
-
Methodological index for non-randomized studies
- mHHS:
-
Modified Harris hip score
- NOS:
-
Newcastle–Ottawa scale
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- NRS:
-
Numeric rating system
- OHS:
-
Oxford hip score
- OA:
-
Osteoarthritis
- PDQQ:
-
Pain Disability Quality-of-Life Questionnaire
- PRP:
-
Platelet-rich plasma
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SVF:
-
Stromal vascular fraction
- THA:
-
Total hip arthroplasty
- VAS:
-
Visual analogue scale
- VHS:
-
Vail hip score
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
References
Abramoff B, Caldera FE (2020) Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am 104(2):293–311
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H et al (2015) Osteoarthritis. Lancet Lond Engl 386(9991):376–387
Hunter DJ, Bierma-Zeinstra S (2019) Osteoarthritis. Lancet Lond Engl 393(10182):1745–1759
Hunter DJ, Schofield D, Callander E (2014) The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 10(7):437–441
Katz JN, Arant KR, Loeser RF (2021) Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325(6):568–578
Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA et al (2020) Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis 79(6):819–828
Murphy NJ, Eyles JP, Hunter DJ (2016) Hip osteoarthritis: etiopathogenesis and implications for management. Adv Ther 33(11):1921–1946
Zampogna B, Papalia R, Papalia GF, Campi S, Vasta S, Vorini F et al (2020) The role of physical activity as conservative treatment for hip and knee osteoarthritis in older people: a systematic review and meta-analysis. J Clin Med 9(4):1167
Harirforoosh S, Asghar W, Jamali F (2013) Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci Publ Can Soc Pharm Sci Soc Can Sci Pharm 16(5):821–847
Learmonth ID, Young C, Rorabeck C (2007) The operation of the century: total hip replacement. Lancet Lond Engl 370(9597):1508–1519
Guo L, Yang Y, An B, Yang Y, Shi L, Han X et al (2017) Risk factors for dislocation after revision total hip arthroplasty: a systematic review and meta-analysis. Int J Surg Lond Engl 38:123–129
Kelmer G, Stone AH, Turcotte J, King PJ (2021) Reasons for revision: primary total hip arthroplasty mechanisms of failure. J Am Acad Orthop Surg 29(2):78–87
Rowan FE, Benjamin B, Pietrak JR, Haddad FS (2018) Prevention of dislocation after total hip arthroplasty. J Arthroplasty 33(5):1316–1324
Cavallo C, Boffa A, Andriolo L, Silva S, Grigolo B, Zaffagnini S et al (2021) Bone marrow concentrate injections for the treatment of osteoarthritis: evidence from preclinical findings to the clinical application. Int Orthop 45(2):525–538
Rampal S, Jaiman A, Tokgöz MA, Arumugam G, Sivananthan S, Singh RSJ et al (2022) A review of the efficacy of intraarticular hip injection for patients with hip osteoarthritis: to inject or not to inject in hip osteoarthritis? Jt Dis Relat Surg 33(1):255–262
Zaffagnini M, Boffa A, Andriolo L, Raggi F, Zaffagnini S, Filardo G (2022) Orthobiologic injections for the treatment of hip osteoarthritis: a systematic review. J Clin Med 11(22):6663
Giorgino R, Albano D, Fusco S, Peretti GM, Mangiavini L, Messina C (2023) Knee osteoarthritis: epidemiology, pathogenesis, and mesenchymal stem cells: what else is new? an update. Int J Mol Sci 24(7):6405
Muthu S, Jeyaraman M, Jain R, Gulati A, Jeyaraman N, Prajwal GS et al (2021) Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees-a panoramic review. Stem Cell Investig 8:13
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Prajwal GS, Jeyaraman N, Kanth VK, Jeyaraman M, Muthu S, Rajendran SNS et al (2022) Lineage differentiation potential of different sources of mesenchymal stem cells for osteoarthritis knee. Pharm Basel Switz 15(4):386
Barry F (2019) MSC therapy for osteoarthritis: an unfinished story. J Orthop Res Off Publ Orthop Res Soc 37(6):1229–1235
Reissis D, Tang QO, Cooper NC, Carasco CF, Gamie Z, Mantalaris A et al (2016) Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther 16(4):535–557
Shariatzadeh M, Song J, Wilson SL (2019) The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res 378(3):399–410
Goh D, Yang Y, Lee EH, Hui JHP, Yang Z (2023) Managing the heterogeneity of mesenchymal stem cells for cartilage regenerative therapy: a review. Bioeng Basel Switz 10(3):355
Mazor M, Lespessailles E, Coursier R, Daniellou R, Best TM, Toumi H (2014) Mesenchymal stem-cell potential in cartilage repair: an update. J Cell Mol Med 18(12):2340–2350
Zhao X, Zhao Y, Sun X, Xing Y, Wang X, Yang Q (2020) Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol 8:575057
Stefano B, Nicholas E, Roberto V, Elena MS, Bruno M (2019) Mesenchymal Stem Cells injection in hip osteoarthritis: preliminary results. Acta Bio Medica Atenei Parm 90(Suppl 1):75–80
Emadedin M, Ghorbani Liastani M, Fazeli R, Mohseni F, Moghadasali R, Mardpour S et al (2015) Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med 18(6):336–344
Rodriguez-Fontan F, Piuzzi NS, Kraeutler MJ, Pascual-Garrido C (2018) Early clinical outcomes of intra-articular injections of bone marrow aspirate concentrate for the treatment of early osteoarthritis of the hip and knee: a cohort study. PM R 10(12):1353–1359
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 29(372):n71
Amir-Behghadami M, Janati A (2020) Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J EMJ 37(6):387
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73(9):712–716
McConnell S, Kolopack P, Davis AM (2001) The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum 45(5):453–461
Li F, Zhu L, Geng Y, Wang G (2021) Effect of hip replacement surgery on clinical efficacy, VAS score and Harris hip score in patients with femoral head necrosis. Am J Transl Res 13(4):3851–3855
Karcioglu O, Topacoglu H, Dikme O, Dikme O (2018) A systematic review of the pain scales in adults: Which to use? Am J Emerg Med 36(4):707–714
Stasi S, Papathanasiou G, Diochnou A, Polikreti B, Chalimourdas A, Macheras GA (2021) Modified Harris hip score as patient-reported outcome measure in osteoarthritic patients: psychometric properties of the Greek version. Hip Int J Clin Exp Res Hip Pathol Ther 31(4):516–525
Saavedra M, Moraga R, Diaz P, Camacho D, Mardones R (2016) Comparative analysis of kinesiotherapy rehabilitation after hip arthroscopy, quantified by harris and vail hip scores: a retrospective study. Muscles Ligaments Tendons J 6(3):420–426
Watanabe N, Murakami S, Uchida S, Tateishi S, Ohara H, Yamamoto Y et al (2020) Validity of the Japanese Orthopaedic Association hip disease evaluation questionnaire (JHEQ) for Japanese patients with labral tear. J Hip Preserv Surg 7(3):466–473
Palmer AJR, Ayyar Gupta V, Fernquest S, Rombach I, Dutton SJ, Mansour R et al (2019) Arthroscopic hip surgery compared with physiotherapy and activity modification for the treatment of symptomatic femoroacetabular impingement: multicentre randomised controlled trial. BMJ 7(364):I185
Nilsdotter A, Bremander A (2011) Measures of hip function and symptoms: Harris hip score (HHS), hip disability and osteoarthritis outcome score (HOOS), Oxford hip score (OHS), Lequesne index of severity for osteoarthritis of the hip (LISOH), and American Academy of orthopedic surgeons (AAOS) hip and knee questionnaire. Arthritis Care Res 63(Suppl 11):S200-207
Feise RJ, Menke JM (2010) Functional rating index: literature review. Med Sci Monit 16(2):RA25-36
Burnham R, Stanford G, Gray L (2012) An assessment of a short composite questionnaire designed for use in an interventional spine pain management setting. PM&R 4(6):413–418
Mehta SP, Fulton A, Quach C, Thistle M, Toledo C, Evans NA (2016) Measurement properties of the lower extremity functional scale: a systematic review. J Orthop Sports Phys Ther 46(3):200–216
Loo SJQ, Wong NK (2021) Advantages and challenges of stem cell therapy for osteoarthritis (Review). Biomed Rep 15(2):67
Mardones R, Jofré CM, Tobar L, Minguell JJ (2017) Mesenchymal stem cell therapy in the treatment of hip osteoarthritis. J Hip Preserv Surg 4(2):159–163
Efficacy of autologous adipose tissue-derived stem cells with extracellular matrix and hyaluronic acid on human hip osteoarthritis—Record details—Embase [Internet]. [cited 2023 Jul 12]. Available from: https://www.embase.com/records?subaction=viewrecord&rid=1&page=1&id=L614605502
Darrow M, Shaw B, Darrow B, Wisz S (2018) Short-term outcomes of treatment of hip osteoarthritis With 4 bone marrow concentrate injections: a case series. Clin Med Insights Case Rep 11:1179547618791574
Whitney KE, Briggs KK, Chamness C, Bolia IK, Huard J, Philippon MJ et al (2020) Bone marrow concentrate injection treatment improves short-term outcomes in symptomatic hip osteoarthritis patients: a pilot study. Orthop J Sports Med 8(12):2325967120966162
Burnham R, Smith A, Hart D (2021) The safety and effectiveness of bone marrow concentrate injection for knee and hip osteoarthritis: a Canadian cohort. Regen Med 16(7):619–628
Heidari N, Slevin M, Zeinolabediny Y, Meloni D, Olgiati S, Wilson A et al (2022) Comparison of the effect of MFAT and MFAT + PRP on treatment of hip osteoarthritis: an observational, intention-to-treat study at one year. J Clin Med 11(4):1056
Natali S, Screpis D, Romeo M, Magnanelli S, Rovere G, Andrea A et al (2023) Is intra-articular injection of autologous micro-fragmented adipose tissue effective in hip osteoarthritis? A three year follow-up. Int Orthop 47(6):1487–1492
Onoi Y, Matsumoto T, Sobajima S, Tsubosaka M, Hayashi S, Matsushita T et al (2023) Clinical use of autologous adipose-derived stromal vascular fraction cell injections for hip osteoarthritis. Regen Ther 24:94–102
Atashi F, Jaconi MEE, Pittet-Cuénod B, Modarressi A (2015) Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng Part C Methods 21(3):253–262
Guo E, Sun L, Chen W, Liu C, Chen K, Jiang X et al (2023) Young human PRP promotes the rejuvenation of aged bone marrow mesenchymal stem cells and the therapeutic effect on ischemic heart disease. Eur J Pharmacol 5(950):175775
Hersant B, Sid-Ahmed M, Braud L, Jourdan M, Baba-Amer Y, Meningaud JP et al (2019) Platelet-rich plasma improves the wound healing potential of mesenchymal stem cells through paracrine and metabolism alterations. Stem Cells Int 2019:1234263
Seyhan N, Alhan D, Ural AU, Gunal A, Avunduk MC, Savaci N (2015) The effect of combined use of platelet-rich plasma and adipose-derived stem cells on fat graft survival. Ann Plast Surg 74(5):615–620
Yin W, Qi X, Zhang Y, Sheng J, Xu Z, Tao S et al (2016) Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med 15(14):73
Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A (2021) PRP injections for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Cartilage 13:364S-375S
Acknowledgements
Not applicable.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. Not applicable.
Author information
Authors and Affiliations
Contributions
R.G., M.A.B., and L.M. contributed to conceptualization; L.M. and F.M. were involved in methodology; R.G., M.A.B., and A.N. contributed to investigation; R.G. and M.A.B. were involved in data curation; R.G. and M.A.B. contributed to writing, original draft preparation; L.M., F.M., and G.M.P. were involved in writing, review and editing; and L.M., L.V., and G.M.P contributed to visualization and supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giorgino, R., Alessandri Bonetti, M., Migliorini, F. et al. Management of hip osteoarthritis: harnessing the potential of mesenchymal stem cells—a systematic review. Eur J Orthop Surg Traumatol (2024). https://doi.org/10.1007/s00590-024-04089-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00590-024-04089-0