Abstract
Purpose
Tranexamic acid (TXA) has been shown to be effective in reducing blood loss after total knee replacement. The purpose of this study was to prospectively assess the effectiveness of topical TXA use, to reduce blood loss after primary total knee replacement without tourniquet, and to compare these outcomes with a control group that did not receive tranexamic acid.
Methods
This is a prospective, randomized study to assess the effect of a 2-g topical tranexamic acid in 50 mL physiological saline solution in total knee replacement without tourniquet and drain. Primary outcomes were total blood loss. Secondary outcomes were hemoglobin and hematocrit level, hemoglobin and hematocrit drop, transfusion rates, length of hospital stay, deep vein thrombosis, and pulmonary embolism events.
Results
Preoperative and intraoperative data were similar between the two groups. The mean total blood loss was 620 mL in the topical tranexamic acid group and 1094 mL in the control group with significant differences (p = 0.001), which meant 43% reduction in total blood loss. The hemoglobin and hematocrit postoperative value was significantly higher in the topical tranexamic acid group than in the control group (p = 0.002). Transfusion rates were 0% in the topical tranexamic group and 4.3% in the control group. The length of stay was significantly lower in the topical tranexamic acid group (p = 0.01). There were no DVT or PE in any group.
Conclusion
A single dose of 2-g TXA in 50 mL topical administration significantly reduces blood loss and improves postoperative blood chemistries in patients undergoing unilateral primary cemented TKA without tourniquet and drain compared to a control group, without increasing the risk of thromboembolic complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several risks inherent with total knee arthroplasty (TKA). One of them is blood loss, with amounts ranging from 800 to 1800 mL. Perioperative blood loss has a significant impact on patients’ recovery after TKA. Blood-saving strategies have been suggested to reduce blood loss and minimize the use of allogenic transfusions [1, 2].
Tranexamic acid (TXA) has been one of these strategies. It is an inhibitor of fibrinolysis by binding to plasminogen at the fibrin binding site resulting in a theoretical reduction in blood loss [3]. TXA was first administrated intravenously, and level I evidence studies have been published [4]. Recent meta-analysis refers that total blood loss was less in patients with the number of intravenous TXA ≥ 2 doses of TXA. So, it needs to program during the surgery exactly the moment to administrate each dose. On the other hand, topical intra-articular administration is easy and single dose, and TXA can reach a higher concentration at the site of bleeding with a minimal systemic distribution, which may reduce adverse effects [5]. Two meta-analyses have compared the efficacy of topical intra-articular administration versus intravenous TXA in TKA [1, 5]. And one meta-analysis has compared the efficacy of topical intra-articular administration TXA versus placebo in TKA [6]. They concluded that topical intra-articular TXA had similar efficacy to intravenous TXA in reducing blood loss, postoperative hemoglobin drops, and blood transfusion, without increasing adverse effects.
Tourniquet and drain continue to be used in TKA. There is current evidence that tourniquet does not reduce total blood loss [7]. And hidden blood loss was lower in patients with tourniquet and TXA [8]. However, it seems that closed suction drainage does not affect total blood loss and blood transfusion requirement [9]. To our knowledge, there are only two studies that evaluate topical intra-articular TXA administration without the use of the tourniquet and drains in TKA [10, 11].
The purpose of this prospective, randomized study was to assess the efficacy and safety of topical intra-articular TXA in reducing blood loss after primary TKA without tourniquet and drain and to compare these outcomes with a control group that did not receive TXA. We hypothesized that there was no significant difference in postoperative blood loss between both groups.
Materials and methods
Study design
This prospective, randomized controlled study was performed between June 2017 and June 2019. The study was approved by our institutional review board, and informed consent was obtained from all recruit patients. All patients undergoing primary TKA were eligible for inclusion. Exclusion criteria were allergy to TXA, bilateral procedures, flexion deformity ≥ 30°, varus/valgus deformity ≥ 30°, history of distal venous thrombosis (DVT) or pulmonary embolism (PE), previous embolic stroke, and cardiovascular disease. Acetylsalicylic acid, antiplatelet agents, anticoagulants, or nonsteroidal anti-inflammatory agents (including cyclooxygenase inhibitors) were discontinued 7 days prior to the scheduled surgery.
Randomization
Randomization to two study groups was implemented with the use of sealed envelopes in a 1:1 ratio opened in the operation room. The topical TXA group received 2-g dose of TXA (Amchafibrin, Rottapharm) in 50 mL of normal saline solution (0.9% sodium chloride solution; Grifols) injected into the knee right before skin closure. The control group received 50 mL of normal saline solution injected into the knee joint just before skin closure. Surgeons were blinded to the treatment assignment until the operation, and the patients, nurses, physical therapists, data collector, and data analyst were blinded during the entire study.
Surgical technique and postoperative care
All surgeries were performed by the same surgeon under spinal anesthesia, without tourniquet, and through a midvastus approach. The same posterior cruciate retaining cemented TKA system was used in all patients (Triathlon, Stryker, Mahwah, New Jersey, USA). Extramedullary alignment was used for tibia, and intramedullary alignment was used for femur. All patellae were routinely resurfaced. Periarticular infiltration was performed with a 100-mL cocktail of ropivacaine (300 mg, 30 mL), adrenaline (0.3 mg, 0.3 mL), ketorolac (60 mg, 2 mL), and normal saline. Twenty mL was injected into posterior capsule external side, 30 mL into posterior capsule medial side, 30 mL into adductor canal, and 20 mL around collateral ligaments and superficial structures. No drains were used.
Antibiotic and thrombotic prophylaxis was administered according to the standard practice at our center. All patients received antibiotic prophylaxis with 2-g first-generation cephalosporin or 1-g vancomycin starting 1 h before incision. Thromboembolic prophylaxis with low-molecular-weight heparin was given from day 0 to day 30. The same multimodal postoperative analgesia was used in all patients.
Under the supervision of the physical therapist, continuous passive motion started on the same day if the patient was operated in the morning, or the next day morning if the patient was operated in the afternoon. Full weight-bearing was allowed form day 1.
Blood transfusion protocol
Blood transfusion protocol was performed when the hemoglobin was less than 70 g/L, or when the hemoglobin of a patient was higher than 70 g/L but with a bad mental status, palpitation, or pallor.
Outcomes
The primary outcomes were the total amount of blood loss calculated according to the Good [12] and Nadler [13] formula. The secondary outcomes were hemoglobin and hematocrit value, hemoglobin and hematocrit drop, transfusion rates, length of hospital stay (LOS), deep vein thrombosis (DVT), and pulmonary embolism (PE) events. The postoperative hemoglobin and hematocrit level was measured at 24 h. Patients were examined daily in the hospital for any clinical symptoms of venous thromboembolism. Any thromboembolic event or other adverse events were recorded at the time of the 6-week and 3-month follow-up visit. After discharge, patients were instructed to return to emergency if limb swelling or calf pain appeared.
Three months after surgery, clinical evaluation was performed with Knee Society Scores (KSS) [14], Western Ontario and MacMasters Universities (WOMAC) [15], and Short-Form 12 (SF12) [16]. WOMAC score was transformed to a 0–100 scale (higher value implies better outcome). Range of knee motion was assessed with a goniometer.
Statistics
Statistical analyses were conducted with SPSS software v. 20.0 (SPSS Inc., Chicago, USA). Statistical significance was considered for p values less than 0.05. Normal distribution was determined by the Kolmogorov–Smirnov test. For comparison of continuous variables, we used the Student t test or nonparametric Mann–Whitney U test for two variables and one-way analysis. For categorical variables, the Chi-square test, the Fisher exact test, or the Mantel–Haenszel was used.
A posteriori analysis of statistical power was performed with a non-inferiority test. Considering our sample size per group, a minimum difference of 120 mL in total blood loss, and alpha error (to-tailed) of 0.05, the statistical power of the study was 90%, which was considered appropriate.
Results
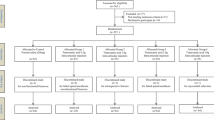
During the period of the study, 268 patients were screened. Among these, 38 patients were excluded: 18 were ineligible, and 20 declined to participate. The remaining 230 patients were included in the study. There were 115 patients in each group (Fig. 1).
Preoperative and intraoperative data of both groups are shown in Table 1. There were no statistically significant differences between the two groups. All patients included in the study were followed for 3 months.
Table 2 shows intraoperative and postoperative outcomes. There were significant differences between groups with respect to mean total blood loss, mean hemoglobin and hematocrit postoperative value, and mean hemoglobin and hematocrit drop. The topical TXA group had a reduction in total blood loss of 43%. Hemoglobin drop was 16.4% in the topical TXA group in front of a 20.6% in the control group (p = 0.001), and a 17.8% hematocrit drop in front of a 21.0% in the control group (p = 0.008).
None of the patients in the topical TXA group required intraoperative or postoperative blood transfusions. Five patients (4.3%) in the control group required postoperative blood transfusions. There were no significant differences between groups (p = 0.07).
The mean LOS was 2.5 in the topical TXA group and 3.0 in the control group, with significant differences (p = 0.01).
There was no DVT or PE in any group.
Six patients (2.6%) returned to emergency department within 30 days after discharge, 4 from the topical TXA group and 2 from the control group. There were no significant differences between groups (p = 0.67). In the topical TXA group, 1 patient suffered delayed wound healing which was controlled with dressing changes, another postoperative hematoma requiring evacuation in emergency department, and 2 calf pain with normal Doppler ultrasound. In the control group, 1 patient developed delayed wound healing which was controlled with dressing changes, and another calf paint with normal Doppler ultrasound.
At 3 months after surgery, there were no significant differences in KSS, WOMAC, SF-12, or range of motion between groups.
Discussion
The present study showed that patients undergoing TKA without a tourniquet and drain, who received 2-g TXA in 50 mL topical, had lower total blood loss after surgery.
Recently, some studies demonstrated the efficacy of topical application of 1-g up to 3-g TXA in patients undergoing TKA to reduce blood loss [1, 3, 5, 6]. As a standard practice, tourniquet and postoperative drain were used in those studies. To our knowledge, there is only one study that evaluates topical TXA in patients undergoing TKA without tourniquet and drain [11]. Wang compared three oral doses of TXA with 3-g in 100 mL (30 mg/mL) topical TXA in a randomized study of 147 patients. Mean total blood loss was 788.8 mL in the oral group and 872.4 mL in the topical group, with no statistical differences [11]. The other two studies did not use tourniquet, but they used drain [8, 17]. Huang designed a randomized study of 150 patients in three groups. The first group was treated with tourniquet and combined five IV doses of 20 mg/kg and 1-g topical TXA in 50 mL (20 mg/mL), the second group was treated without tourniquet and the same TXA dose administration, and the third group was treated with tourniquet only. Mean total blood loss was 734.5 mL, 627.7 mL, and 1584.3, respectively, with significant differences between the third group and the other two groups. They also tested inflammatory markers (C-reactive protein and interleukin-6), and they found their levels were lower in patients who received the same TXA dose administration and were operated without tourniquet, and these differences were significant. Maybe the avoidance of the tourniquet and the effect of TXA to block the fibrinolysis and reduce total blood loss are the reasons for this anti-inflammatory effect observed in those patients [8]. Tzatzairis compared three groups in a randomized study of 120 patients. The first group received no TXA, the second group received 1-g IV TXA, and the third group received 1-g topical TXA in 100 mL (10 mg/mL). Mean total blood loss was 1513.4, 1236.0, and 1205.63 mL, respectively. There were significant differences in blood loss between the three groups of patients, but no significant difference between the two routes of administration [17]. In the current study of patients undergoing TKA without tourniquet, mean total blood loss was 1094.8 mL in patients that received no TXA and 620.3 mL in patients that received 2-g (40 mg/mL) topical TXA, with significant differences. Maybe the higher concentration topical TXA was the reason for this lower value. Yue [18] proved that TXA topical concentration ≥ 20 mg/mL might be better at reducing bleeding than < 20 mg/mL topical TXA in TKA.
Recent meta-analysis reported that intra-articular TXA improved blood chemistries [6]. Postoperative hemoglobin and hematocrit were significantly higher in the intra-articular TXA group than in the control group. And drop in hemoglobin and hematocrit was significantly smaller in the intra-articular TXA group. Our results were in agreement with those published in this meta-analysis.
Blood transfusion rates dropped with the use of topical TXA when compared with a control group. In our study, transfusion requirements were 0% in the topical TXA group and 4.3% in the control group. Wang [11] reported 5% transfusion rate in the topical TXA group and 4% in the oral TXA group. Huang [8] reported 0% in patients that received combined IV and topical TXA and 8% in the control group. Tzatzairis [17] reported 12.5% in the IV TXA group, 17.5% in the topical TXA group, and 37.5% in the control group. Blood transfusion strategies are different between hospitals, and dissimilarity may be the reason for variance in transfusion rates.
It was reported that TXA could maintain a half-life of 2 to 3 h within joint fluid [19]. And when TXA is used intra-articularly, TXA enters the tissue space and accumulates up to 17 h [20]. Some authors used drain and clamped it for 1 h [17]. However, other authors suggest 2-h period with drain clamped [21]. Maybe the use of drains could reduce the effectiveness of topical TXA.
Topical TXA did not increase the risk of thromboembolic events in previous studies [5]. Systemic absorption is 70% lower with topical administration, and this should further reduce the risk [22]. There were no thromboembolic complications in the topical TXA groups in the above-mentioned studies [8, 11, 17]. No cases of DVT or PE were detected in the current study.
The difference of LOS between topical TXA and a control group was not significant according to Tzatzairis [17] (mean 6 days for the three groups). However, Huang [8] found that LOS was lower in patients that received TXA (mean 5.1) than patients who did not receive TXA (mean 5.6). In our study, LOS difference was significant between the topical TXA group and the control group. There are several factors affecting LOS. Maybe the effect of TXA in reducing total blood loss, and improving postoperative blood chemistries, plays a role to reduce hospital stay.
The strengths of this study include its prospective and randomized design with well-defined variables. The blood transfusion protocol was explicit. And outcomes were reported as a consecutive series of cases from a single surgeon, therefore eliminating inter-surgeon variability.
Nevertheless, our study has several limitations. Firstly, we recognize the lack of the control group with tourniquet use. But most of the studies about topical application of TXA were in TKA with tourniquet. Secondly, we did not use routine ultrasound examination to screen postoperative DVT. Thirdly, serum concentrations of TXA were not measured. Fourthly, our follow-up may be short. However, 3-month follow-up period was thought to be adequate for monitoring primary and secondary outcomes, side effects or important early adverse reactions.
In conclusion, a single dose of 2-g TXA in 50 mL topical administration significantly reduces blood loss and improves postoperative blood chemistries in patients undergoing unilateral primary cemented TKA without tourniquet and drain compared to a control group, without increasing the risk of thromboembolic complications.
References
Mi B, Liu G, Zhou W, Lv H, Liu Y, Zha K, Wu Q, Liu J (2017) Intra-articular versus intravenous tranexamic acid application in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 137:997–1009
Themistoklis T, Theodosia V, Konstantinos K, Georgios DI (2017) Perioperative blood management strategies for patients undergoing total knee replacement: where do we stand now? World J Orthop 8:441–454
Xiong H, Liu Y, Zeng Y, Wu Y, Shen B (2018) The efficacy and safety of combined administration of intravenous and topical tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 19:321
Zhang H, Chen J, Chen F, Que W (2012) The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 20:1742–1752
Dai W, Zhou A, Zhang H, Zhang J (2018) Most effective regimen of tranexamic acid for reducing bleeding and transfusions in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Knee Surg 3:654–663
Moskal JT, Capps SG (2018) Intra-articular tranexamic acid in primary total knee arthroplasty: meta-analysis. J Knee Surg 31:56–67
Arthur JR, Spangehl MJ (2019) Tourniquet use in total knee arthroplasty. J Knee Surg 32(8):719–729
Huang Z, Xie X, Li L, Huang Q, Ma J, Shen B, Kraus VB, Pei F (2017) Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: a prospective, randomized controlled trial. J Bone Joint Surg Am 99:2053–2061
Maniar RN, Pradhan P, Bhatnagar N, Maniar A, Bidwai R, Bindal P (2019) Role of suction drain after knee arthroplasty in the tranexamic acid era: a randomized controlled study. Clin Orthop Surg 11:73–81
Arora M, Singh S, Gupta V, Dongre A, Shetty V (2018) Comparing the efficacy of intravenous or intra-articular tranexamic acid in reducing blood loss in simultaneous bilateral knee replacement surgery without the use of tourniquet. Eur J Orthop Surg Traumatol 28:1417–1420
Wang D, Zhu H, Meng W, Wang H-Y, Luo Z-Y, Pei F-X, Li Q, Zhou Z-K (2018) Comparison of oral versus intra-articular tranexamic acid in enhanced-recovery primary total knee arthroplasty without tourniquet application: a randomized controlled trial. BMC Musculoskelet Disord 19:85
Good L, Peterson E, Lisander B (2003) Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 90:596–599
Nadler SB, Hidalgo JU, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Bellamy N, Buchanan W, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip and the knee. J Rheumatol 15:1833–1840
Ware JE Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Tzatzairis TK, Drosos GI, Kotsios SE, Ververidis AN, Vogiatzaki TD, Kazakos KI (2016) Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: a randomized controlled study. J Arthroplasty 31:2465–2470
Yue C, Pei F, Yang P, Xie J, Kang P (2015) Effect of Topical Tranexamic Acid in Reducing Bleeding and Transfusions in TKA. Orthopedics 38:315–324
Yi Z, Bin S, Jing Y, Zongke Z, Pengde K, Fuxing P (2016) Tranexamic acid administration in primary total hip arthroplasty. A randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am 98:983–991
Mannucci PM (1998) Hemostatic drugs. N Engl J Med 339:245–253
Aggarwal AK, Singh N, Sudesh P (2016) Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty 3:1442–1448
Kasparek MF, Faschingbauer M, Waldstein W, Boettner CS, Boettner F (2017) Topical tranexamic acid is equivalent to targeted preoperative autologous blood donation in total hip arthroplasty. J Arthroplasty 32:1176–1179
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Manuel Morales Santias, Jesus Mas Martinez, Javier Sanz-Reig, Enrique Martinez Gimenez, David Bustamante Suarez de Puga, and Carmen Verdu Roman declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morales Santias, M., Mas Martinez, J., Sanz-Reig, J. et al. Topical tranexamic acid in cemented primary total knee arthroplasty without tourniquet: a prospective randomized study. Eur J Orthop Surg Traumatol 30, 1003–1008 (2020). https://doi.org/10.1007/s00590-020-02656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-020-02656-9