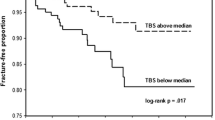
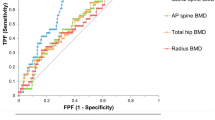
Abstract
Purpose
Kidney transplant recipients are prone to metabolic bone diseases and consequent fractures. This study aimed to evaluate the incidence of incipient vertebral fractures, osteopenia, osteoporosis, and the clinical factors associated with incipient vertebral fractures in a group of kidney transplant patients.
Methods
Two hundred sixty-four patients (F/M 124/140, 45.3 ± 13 years) who had undergone kidney transplantation in tertiary care centers were included. Vertebral fractures were assessed semiquantitatively using conventional thoracolumbar lateral radiography in 202 of the patients.
Results
Vertebral fractures were observed in 56.4% (n = 114) of the study group. The frequency of osteoporosis was 20.0% (53 of 264 patients), and osteopenia was 35.6% (94 of 264 patients). Bone mineral density (BMD) levels were in the normal range in 40.3% (n = 46) of the subjects with vertebral fractures. It was in the osteoporotic range in 20.1% (n = 23) and the osteopenic range in 40.3% (n = 46). Vertebral fractures were associated with age, duration of hemodialysis, BMI, and femoral neck Z score (R2 37.8%, p = 0.027).
Conclusion
As incipient vertebral fractures can be observed in patients with normal BMD levels in kidney transplant recipients, conventional X-ray screening for vertebral fractures may be beneficial for a proper therapy decision of metabolic bone disease in kidney transplant recipients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplant recipients are prone to metabolic bone diseases and have a high risk of fractures. A high prevalence of osteoporosis (ranged 13.9% to 53%) [1,2,3,4,5] and osteopenia (ranged 7% to 52.5%) [3, 5,6,7,8,9], leading to fractures, has been reported after kidney transplantation (KT). Although hip fractures often present with clinical signs, most vertebral fractures occur atypically [10]. Patients may benefit from radiographic screening for vertebral fractures. Vertebral fractures are often overlooked, but even undiagnosed vertebral fractures negatively affect physical function [11], quality of life [12], and mortality [13].
It has been well demonstrated that rapid bone loss occurs primarily in the first six months after KT. Following this initial period, the decrease proceeds at a slower rate or stabilizes. Approximately 2.9% to 6.8% bone loss at the spine over the first six months after kidney transplantation was detected [9, 14,15,16]. After the eighteenth month, the annual bone loss rate decreases to 1.7% [17]. Bone loss after kidney transplantation is mainly associated with glucocorticoids and other immunosuppressive drugs and previous renal osteodystrophy. Other risk factors for bone loss include hyperparathyroidism, decreased vitamin D, diabetes mellitus, post-transplant metabolic acidosis, kidney/pancreas transplantation, and the predictors of osteoporosis in the general population such as age, smoking, female gender, and low body mass index (BMI). As a consequence of these factors, renal transplant recipients have shown three times increased bone fracture risk compared to age-matched controls [18, 19].
The incidence of fractures in the first five years after kidney transplantation has been reported between 8 and 45% in previous studies [4, 5, 18, 20, 21]. Bone fractures mainly occur within two years after transplantation [5]. Peripheral fractures (involving hands, ankles, feet, and femur) are detected more commonly than spinal fractures [22].
In clinical studies, there is a wide range of distribution of vertebral fracture (VF) ratios, ranged between 1.8 and 38.5% [4, 18, 23,24,25], and there are conflicting results about predictors of fracture.
This descriptive clinical study aimed to determine vertebral fracture prevalence using lateral spinal radiographs and the factors associated with vertebral fractures and to evaluate bone mineral density measurements in kidney transplant recipients on a multicenter basis.
Material and methods
Clinical and laboratory evaluation
We included 264 patients who underwent renal transplantation and followed in endocrinology and nephrology clinics of in tertiary care centers. All study participants underwent their first transplantation over 12 months, and in this period, graft function was stable. Immune-suppressive treatment was not changed in the last year.
Patients who underwent kidney–pancreas transplantation, patients already on bisphosphonate or denosumab treatment, or unstable renal function were excluded from the study. Patients with celiac disease, chronic liver failure, inflammatory bowel disease, autoimmune disorders, multiple myeloma, and hyperthyroidism were also excluded. All subjects were screened for comorbidities and other diseases predisposing secondary osteoporosis before renal transplantation.
The study protocol was approved by the Ethics Committee (09.2017.518) and conducted following the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
The induction treatment consisted of 1 g of methylprednisolone along with anti-thymocyte globulin or basiliximab, and maintenance therapy included calcineurin inhibitors (tacrolimus or cyclosporine), mammalian target of rapamycin (mTOR) inhibitors (sirolimus or everolimus), anti-proliferative agents (mycophenolate mofetil/mycophenolic acid or azathioprine), and steroids. After induction, the steroid dose is gradually tapered to 5 mg/day for maintenance.
Clinical characteristics of patients detailed medical history (duration of hemodialysis, peritoneal dialysis, fracture history, medical history), demographic parameters (age and sex), body mass index, and laboratory data [blood urea nitrogen (BUN), creatinine, calcium, phosphorus, albumin, 25-OH vitamin D, alkaline phosphatase (ALP)], routine lateral thoracal and lumbar X-ray images and bone mineral density measurements at the last visit were obtained retrospectively from medical records.
Calcium levels were measured using a photometric color test, phosphorous levels were analyzed using a photometric ultraviolet (UV) test, and ALP and creatinine were analyzed using a kinetic color test (Jaffé method) with an AU5800 Clinical Chemistry Analyzer (Beckman Coulter, USA). Serum PTH was determined in all cases using an immunoassay method (Roche, modular system, E170, Germany). 25-OH vitamin D was analyzed using an immunoassay method on a Unicel DXI 800 automated analyzer (Beckman Coulter, USA).
Bone mineral density measurements
Dual-energy X-ray absorptiometry (DXA) was used to determine bone mineral density (BMD). DXA measured the lumbar spine (L1–L4) in the anteroposterior (AP) projection and the three sites of the right and left hip (femoral neck, Ward's triangle, and trochanter) using Lunar, DPX-L. The coefficient of variation (CV) for three sets of measurements was 1.0%.
Osteopenia was defined according to the classical WHO criteria, as a value for BMD more than 1.0 but less than 2.5 SD below the young adult reference mean (T score less than − 1 and greater than − 2.5 SD).
Osteoporosis was defined as a value for BMD 2.5 or more standard deviation below the young adult reference mean (T score less than or equal to − 2.5 SD).
Vertebral fracture evaluation
Lateral chest and lumbar radiographs were used for the diagnosis of vertebral fractures. A semiquantitative method defined by Genant et al. was used [26]. Vertebral fractures were graded as normal (grade 0), grade 1 (mild, approximately 20–25% reduction in anterior, middle, and/or posterior height and a reduction of area 10–20%), grade 2 (moderate, approximately 25–40% reduction in any height and a reduction of area 20–40%), and grade 3 (severe, approximately 40% reduction in any height and area).
All radiographs were reread in consensus readings and verified by an expert radiologist (OB). On average, we evaluated 10.8 ± 1.4 vertebrae per patient.
Statistical analyses
Continuous variables were summarized using descriptive statistics presented as mean and standard deviation (SD). Categorical variables were summarized using counts and percentages. Categorical data were analyzed using the chi-square (χ2) test or Fisher’s exact test appropriately. Student's t-test and analysis of variance were used for parametric variables.
A multiple regression analysis was performed to define the relationship between vertebral fractures and the following variables: age, gender, duration of chronic renal failure (CRF), duration of hemodialysis, time from renal transplantation, BMI, creatinine, PTH, creatinine, lumbar spine BMD, lumbar spine Z score, femoral neck BMD, and femoral neck Z score.
Two different multiple regression analyses were also performed to determine the relationship between lumbar BMD and femoral neck BMD and parameters. Age, gender, duration of CRF, duration of hemodialysis, time from renal transplantation, BMI, creatinine, PTH, creatinine, and 25-OH vitamin D were used as independent variables.
Multiple regression analysis parameters with a possible influence on PTH were added as covariates: age, gender, duration of CRF, BMI, calcium, phosphorous, and 25-OH vitamin D.
The results were evaluated at a 95% confidence interval, and p < 0.05 was considered statistically significant. All statistical analyses were performed using software (GraphPad InStat 3.0; GraphPad Software, Inc., San Diego, CA, USA).
Results
A total of 264 transplant recipients who underwent bone metabolism evaluation during the study period was under steroid treatment as immune suppression.
Twelve percent of our cohort consisted of cadaver kidney recipients (n = 32). The most common etiologies for primary kidney diseases were as follows: diabetes mellitus in 16.3% (n = 43), glomerulonephritis in 23.5% (n = 62), hypertensive nephropathy in 19.3% (n = 51), polycystic kidney disease in 11.4% (n = 30), and unknown etiology in 29.5% (n = 78) of the patients.
Patient characteristics and biochemical parameters
Male preponderance [F/M 124 (47%)/140 (53%)] with mean age 45 ± 13 years was observed. Clinical and laboratory parameters are summarized in Table 1. The main renal replacement treatment was hemodialysis in most cases, whereas 14% (n = 37) of patients were treated with peritoneal dialysis before renal transplantation. The median duration of follow-up post-transplantation was 6.7 ± 4.8 years (range 1–33 years).
Vertebral fracture
History of any fracture was reported in 42 cases, mostly located in ankles, feet, radius, and femur. Radiological vertebral fracture evaluation was available for 202 patients. Patients' clinical, laboratory parameters, and DEXA results according to the presence of vertebral fractures are summarized in Table 2. Patients with vertebral fractures were older than patients without vertebral fractures (46.8 ± 11.4 years vs. 41.7 ± 11.9 years, p = 0.012).
One hundred and fourteen (56.4%) patients had grade 1 and more vertebral fragility fractures. While mild fractures were detected in 26.2% (n = 53/202) of the patients who were evaluated with X-ray, severe vertebral fractures (grade two and more) were observed in 30.7% (n = 62/202) of the patients. Thirteen patients (6.4%) had grade 3 vertebral fractures. The mean number of lumbar and thoracic vertebral fractures was 3.1 ± 1.7, while the mean number of grade 2 fractures was 2.2 ± 1.5 (min–max 1–10).
The majority of vertebral fractures were observed more frequently in patients with normal BMD. Forty-six patients (40.3%) with vertebral fractures had normal lumbar and femoral neck BMD, whereas 23 (20.1%) and 45 (39.5%) patients with vertebral fractures were osteoporotic and osteopenic.
Bone mineral density
The mean Z score and BMD levels of the lumbar spine were − 0.810 ± 1.348 and 1.036 ± 0.196 g/cm2; and those were − 0.693 ± 1.196, and 0.855 ± 0.158 g/cm2 in the femoral neck, respectively. While osteoporosis was defined in 20% (n = 53), osteopenia was present in 35.6% (94) of the whole study group. There was no statistically significant difference between clinical, laboratory data of patient groups according to the presence of osteopenia, osteoporosis, or normal BMD (p > 0.05 for all), except age (Table 1). Diabetic patients had lower BMD levels both in the femoral neck (p < 0.001) and lumbar spine (p = 0.035).
Correlation and multivariate regression analyses
Correlation analyses are shown in Table 3. A negative correlation between femoral neck BMD and age (r − 0.21, p < 0.001) was observed. Femoral neck BMD levels were positively correlated with BMI (r 0.29, p < 0.001) and lumbar BMD (r 0.54, p < 0.001) (Table 3). Lumbar BMD was positively correlated with BMI (r 0.34, p < 0.001) and the duration of CRF (r 0.22, p < 0.001).
In multiple regression analyses, femoral neck BMD was associated with age and BMI (R2 35.4%, p < 0.001), lumbar BMD was associated with BMI (R2 18.6%, p = 0.040). None of the variables (gender, duration of CRF, time from renal transplantation, PTH, creatinine, femoral neck BMD, lumbar BMD, lumbar Z score) were statistically associated with vertebral fractures in multiple regression analysis. However, those were associated with age, duration of hemodialysis, BMI, femoral neck T score, and femoral neck Z score (R2 37.8%, p = 0.027). None of the clinical and laboratory parameters were associated with PTH levels (R2 12.5%, p = 0.130).
Discussion
In this retrospective study, vertebral fragility fractures were detected in 56.4% (n = 114) of the patients. Forty-six (40.3%) patients with VF had normal DEXA. Osteoporosis and osteopenia were observed in 20% and 35.6% of the whole study group. Diabetic patients had lower BMD levels and Z scores both in the femoral neck and lumbar spine.
Age and BMI, which have already known as predictive factors of osteoporosis in the general population, were found as the factors associated with femoral neck BMD scores in renal transplant recipients in our study. Techawathanawanna et al. also stated that the decreasing BMI and increasing age were correlated with osteoporosis of the hip region [3].
In the Rotterdam Study, which included a healthy population, vertebral fractures were higher in women than men [27]. In Akaberi et al., Durieux et al., and Ball et al.’s studies with renal transplant recipients, female gender was found as a risk factor for fracture [4, 5, 28]. In our study, there was no significant association between the presence of fracture and gender (p = 0.793). In Grotz et al., Braga et al., and O’Shaughnessy et al.’s studies, the presence of fractures was also independent of gender [25, 29, 30]. We also observed no difference between genders in lumbar and femoral BMD levels (p = 0.160, p = 0.715). High BMD levels may have been detected due to the high rate of male subjects in our patient group. However, most of the renal transplant recipients in the literature consisted of the predominantly male population [4, 5, 8, 31], similar to our study.
The standardized incidence ratio of vertebral fractures was 23.1% in Vautour et al.'s study [24]. Vertebral fractures were detected in 1.8% of patients in the O'Shaughnessy et al. study [25]. On the other hand, in Nam et al., Durieux et al., Vautour et al., Schreiber et al., Patel et al., and Pichette et al. study, the prevalence of VF was 38.5%, 28.8%, 15.1%, 14.3%, 9.1%, and 5.7%, respectively. [4, 17, 18, 23, 24, 32] While Nam et al. [23] and Patel et al. [18] evaluated VF, respectively, with X-ray morphometric criteria and semiautomated method, the rest of the studies used a semiquantitative method. This wide distribution rate among studies on vertebral fracture frequency can be attributed to different definition criteria in the fracture decision.
Increased fracture risk was associated with diabetes mellitus in previous reports [25, 33]. In Vautour et al.’s study [24], increasing age and diabetes as the cause of end-stage renal disease were found as independent predictors of overall fracture risk. While the standardized incidence ratio of vertebral fracture was 33.5% in the diabetic patient, it was 21.2% in the non-diabetic group [24]. While the presence of diabetes was not associated with vertebral fractures in our study, diabetic patients had lower BMD levels both in the femoral neck and lumbar spine.
In Giannini et al.’s study [34], high PTH levels were significantly associated with VF. In our study, the frequency of secondary hyperparathyroidism was 20.4% (n = 54). However, when we compared the groups with and without secondary hyperparathyroidism in terms of the presence of VF (p = 0.420) and the number of fractures (p = 0.065), there was no statistical significance between the two groups. Although some studies did not find a significant relationship with PTH similar to our study [4, 30, 35, 36], many studies supported that persistent PTH elevation is associated with VF [34, 37,38,39]. Our study excluded subjects with persistent PTH elevation after renal transplantation. We can attribute this to not including patients with primary or tertiary hyperparathyroidism.
Low BMD in the femoral neck and lumbar spine was associated with an increased risk of vertebral fractures [23]. In our study, femoral neck Z scores were found to be associated with vertebral fractures.
In a study with 238 kidney transplant recipients, patients with osteoporosis were found to have a 3.5-fold increased risk of fracture than patients with normal BMD [5]. Contrary to Nam et al.'s study [23], BMD levels showed no significant differences between patients with VF and without VF in our study. In the Marcel et al. study, vertebral fractures were primarily observed in osteoporotic patients, but 43% of patients with VF had normal lumbar BMD [6]. In our study, the VF ratio in patients with normal BMD was 40.3%. Although our osteopenia and osteoporosis rates are lower than most previous reports, lower prevalences have been reported [5, 17]. This difference between studies may be attributed to false signals from extra‐osseous calcifications or falsely high values obtained from unrecognized mechanical bone deformation or preexisting renal osteodystrophy.
Lumbar BMD losses have been reported as 3–10% in the first six months following kidney transplantation [9]. Although there are no well-established therapeutic approaches to prevent early post-transplantation bone loss, the most recommended manners to provide bone anabolic effects in renal transplant recipients are: minimizing the dose of corticosteroids and using vitamin D supplements, active vitamin D (calcitriol or alfacalcidol), or bisphosphonates [40,41,42]. In addition, determining the high-risk group for fractures with routine vertebral radiological imaging can be helpful to decide to give treatment to renal transplant recipients in the early period.
The major limitation of this study is that the evaluation of vertebral fractures was made using a semiquantitative method. Second, we evaluated retrospectively, limiting our ability to determine the exact timing of the occurrence of VF, whether it had occurred before transplantation. To determine the occurrence time of VF, prospective studies with long-term follow-up with a routine evaluation of spine X-rays at regular intervals before and after transplantation are needed. Although patients with predisposing factors for secondary osteoporosis were excluded, we included subjects with a history of secondary hyperparathyroidism before renal transplantation, which also may be responsible for a high frequency of VF. We evaluated BMD with DXA in this study. However, as it is known, these measurements can be confounded by various factors, such as positioning errors, artifacts, vertebral fractures, vertebral osteoarthritis, scoliosis, arterial calcifications, BMI, or inadequate internal hip rotation. Considering that aortic calcifications are common in dialysis patients, we may detect relatively high BMD levels. To rule out false measurements with DXA, studies using other measurement methods, such as evaluation of trabecular bone score and quantitative computed tomography, could be planned.
This study found a high vertebral fracture prevalence regardless of osteoporosis and CRF duration. Vertebral fractures were observed more frequently in patients with normal BMD. Besides the BMD evaluation, vertebral fracture evaluation may help a proper treatment for metabolic bone disease in kidney transplant recipients.
References
Park WY, Han S, Choi BS, Park CW, Yang CW, Kim YS, Kim JI, Moon IS, Chung BH (2017) Progression of osteoporosis after kidney transplantation in patients with end-stage renal disease. Transplant Proc 49:1033–1037. https://doi.org/10.1016/j.transproceed.2017.03.038
Unal A, Kocyigit I, Sipahioglu MH, Tokgoz B, Kavuncuoglu F, Oymak O, Utas C (2010) Loss of bone mineral density in renal transplantation recipients. Transplant Proc 42:3550–3553. https://doi.org/10.1016/j.transproceed.2010.07.106
Techawathanawanna N, Avihingsanon Y, Praditpornsilpa K, Kingpetch K, Suwanwalaikorn S, Kanjanabuch T, Eiam-Ong S, Tungsanga K (2005) The prevalence and risk factors of osteoporosis in Thai renal-transplant patients. J Med Assoc Thai 88(Suppl 4):S103-109
Durieux S, Mercadal L, Orcel P, Dao H, Rioux C, Bernard M, Rozenberg S, Barrou B, Bourgeois P, Deray G, Bagnis CI (2002) Bone mineral density and fracture prevalence in long-term kidney graft recipients. Transplantation 74:496–500. https://doi.org/10.1097/00007890-200208270-00011
Akaberi S, Simonsen O, Lindergard B, Nyberg G (2008) Can DXA predict fractures in renal transplant patients? Am J Transplant 8:2647–2651. https://doi.org/10.1111/j.1600-6143.2008.02423.x
Marcen R, Caballero C, Uriol O, Fernandez A, Villafruela JJ, Pascual J, Martins J, Rodriguez N, Burgos FJ, Ortuno J (2007) Prevalence of osteoporosis, osteopenia, and vertebral fractures in long-term renal transplant recipients. Transplant Proc 39:2256–2258. https://doi.org/10.1016/j.transproceed.2007.07.073
Pereira S, Pedroso S, Martins L, Santos P, Almeida M, Freitas C, Dias L, Dores J, Almeida R, Castro Henriques A, Teixeira M (2010) Bone mineral density after simultaneous kidney-pancreas transplantation: four years follow-up of 57 recipients. Transplant Proc 42:555–557. https://doi.org/10.1016/j.transproceed.2010.01.046
Falkiewicz K, Boratynska M, Zmonarski SC, Milewicz A, Patrzalek D, Biecek P, Klinger M (2009) Evolution of bone disease at 2 years after transplantation: a single-center study. Transplant Proc 41:3063–3066. https://doi.org/10.1016/j.transproceed.2009.09.041
Mikuls TR, Julian BA, Bartolucci A, Saag KG (2003) Bone mineral density changes within six months of renal transplantation. Transplantation 75:49–54. https://doi.org/10.1097/00007890-200301150-00009
van der Jagt-Willems HC, van Munster BC, Lems WF (2013) Vertebral fractures in elderly adults: atypical presentation rather than asymptomatic. J Am Geriatr Soc 61:2047–2048. https://doi.org/10.1111/jgs.12520
Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR (1998) The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 128:793–800. https://doi.org/10.7326/0003-4819-128-10-199805150-00001
Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J (2000) Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 15:1384–1392. https://doi.org/10.1359/jbmr.2000.15.7.1384
Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR (1999) Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 159:1215–1220. https://doi.org/10.1001/archinte.159.11.1215
Rajapakse CS, Leonard MB, Bhagat YA, Sun W, Magland JF, Wehrli FW (2012) Micro-MR imaging-based computational biomechanics demonstrates reduction in cortical and trabecular bone strength after renal transplantation. Radiology 262:912–920. https://doi.org/10.1148/radiol.11111044
Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD (1991) Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 325:544–550. https://doi.org/10.1056/NEJM199108223250804
Horber FF, Casez JP, Steiger U, Czerniak A, Montandon A, Jaeger P (1994) Changes in bone mass early after kidney transplantation. J Bone Miner Res 9:1–9. https://doi.org/10.1002/jbmr.5650090102
Pichette V, Bonnardeaux A, Prudhomme L, Gagne M, Cardinal J, Ouimet D (1996) Long-term bone loss in kidney transplant recipients: a cross-sectional and longitudinal study. Am J Kidney Dis 28:105–114. https://doi.org/10.1016/s0272-6386(96)90138-9
Patel S, Kwan JT, McCloskey E, McGee G, Thomas G, Johnson D, Wills R, Ogunremi L, Barron J (2001) Prevalence and causes of low bone density and fractures in kidney transplant patients. J Bone Miner Res 16:1863–1870. https://doi.org/10.1359/jbmr.2001.16.10.1863
Giannini S, D’Angelo A, Carraro G, Antonello A, Di Landro D, Marchini F, Plebani M, Zaninotto M, Rigotti P, Sartori L, Crepaldi G (2001) Persistently increased bone turnover and low bone density in long-term survivors to kidney transplantation. Clin Nephrol 56:353–363
Braga Junior JW, Neves RM, Pinheiro MM, Frisoli Junior A, Castro CH, Szejnfeld VL, Carvalho AB (2006) Prevalence of low trauma fractures in long-term kidney transplant patients with preserved renal function. Braz J Med Biol Res 39:137–147. https://doi.org/10.1590/s0100-879x2006000100016
Elmstedt E, Svahn T (1981) Skeletal complications following renal transplantation. Acta Orthop Scand 52:279–286. https://doi.org/10.3109/17453678109050104
Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N (2009) Risk of fractures after renal transplantation in the United States. Transplantation 87:1846–1851. https://doi.org/10.1097/TP.0b013e3181a6bbda
Nam JH, Moon JI, Chung SS, Kim SI, Park KI, Song YD, Kim KR, Lee HC, Huh K, Lim SK (2000) Prevalence and risk factors for vertebral fractures in renal transplants. Transplant Proc 32:1877. https://doi.org/10.1016/s0041-1345(00)01472-x
Vautour LM, Melton LJ 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT (2004) Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int 15:160–167. https://doi.org/10.1007/s00198-003-1532-y
O’Shaughnessy EA, Dahl DC, Smith CL, Kasiske BL (2002) Risk factors for fractures in kidney transplantation. Transplantation 74:362–366. https://doi.org/10.1097/00007890-200208150-00012
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Van der Klift M, De Laet CE, McCloskey EV, Hofman A, Pols HA (2002) The incidence of vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res 17:1051–1056. https://doi.org/10.1359/jbmr.2002.17.6.1051
Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C (2002) Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288:3014–3018. https://doi.org/10.1001/jama.288.23.3014
Grotz WH, Mundinger FA, Gugel B, Exner V, Kirste G, Schollmeyer PJ (1994) Bone fracture and osteodensitometry with dual energy X-ray absorptiometry in kidney transplant recipients. Transplantation 58:912–915. https://doi.org/10.1097/00007890-199410270-00009
Braga Júnior JW, Neves RM, Pinheiro MM, Frisoli Júnior A, Castro CH, Szejnfeld VL, Carvalho AB (2006) Prevalence of low trauma fractures in long-term kidney transplant patients with preserved renal function. Braz J Med Biol Res 39:137–147. https://doi.org/10.1590/s0100-879x2006000100016
Evenepoel P, Claes K, Meijers B, Laurent MR, Bammens B, Naesens M, Sprangers B, Pottel H, Cavalier E, Kuypers D (2019) Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients. Kidney Int 95:1461–1470. https://doi.org/10.1016/j.kint.2018.12.024
Schreiber PW, Bischoff-Ferrari HA, Boggian K, Bonani M, van Delden C, Enriquez N, Fehr T, Garzoni C, Hirsch HH, Hirzel C, Manuel O, Meylan P, Saleh L, Weisser M, Mueller NJ, Swiss Transplant Cohort S (2018) Bone metabolism dynamics in the early post-transplant period following kidney and liver transplantation. PLoS ONE 13:e0191167. https://doi.org/10.1371/journal.pone.0191167
Nisbeth U, Lindh E, Ljunghall S, Backman U, Fellstrom B (1999) Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation 67:1218–1222. https://doi.org/10.1097/00007890-199905150-00004
Giannini S, Sella S, Silva Netto F, Cattelan C, Dalle Carbonare L, Lazzarin R, Marchini F, Rigotti P, Marcocci C, Cetani F, Pardi E, D’Angelo A, Realdi G, Bonfante L (2010) Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: role of calcium-sensing receptor polymorphisms and vitamin D deficiency. J Bone Miner Res 25:841–848. https://doi.org/10.1359/jbmr.091025
Rolla D, Ballanti P, Marsano L, Bianchi G, Messa P, Paoletti E, Cannella G (2006) Bone disease in long-term renal transplant recipients with severe osteopenia: a cross-sectional study. Transplantation 81:915–921. https://doi.org/10.1097/01.tp.0000178376.02130.ca
Mazzaferro S, Diacinti D, Proietti E, Barresi G, Baldinelli M, Pisani D, D’Erasmo E, Pugliese F (2006) Morphometric X-ray absorptiometry in the assessment of vertebral fractures in renal transplant patients. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association—European Renal Association 21:466–471. https://doi.org/10.1093/ndt/gfi206
Heaf J, Tvedegaard E, Kanstrup IL, Fogh-Andersen N (2003) Hyperparathyroidism and long-term bone loss after renal transplantation. Clin Transplant 17:268–274. https://doi.org/10.1034/j.1399-0012.2003.00047.x
Vestergaard P, Mollerup CL, Frøkjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L (2000) Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ (Clinical Research Ed) 321:598–602. https://doi.org/10.1136/bmj.321.7261.598
De Geronimo S, Romagnoli E, Diacinti D, D’Erasmo E, Minisola S (2006) The risk of fractures in postmenopausal women with primary hyperparathyroidism. Eur J Endocrinol 155:415–420. https://doi.org/10.1530/eje.1.02225
Molnar MZ, Naser MS, Rhee CM, Kalantar-Zadeh K, Bunnapradist S (2014) Bone and mineral disorders after kidney transplantation: therapeutic strategies. Transplant Rev (Orlando) 28:56–62. https://doi.org/10.1016/j.trre.2013.12.003
Kidney Disease: Improving Global Outcomes Transplant Work G (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(Suppl 3):S1-155. https://doi.org/10.1111/j.1600-6143.2009.02834.x
Maalouf NM, Shane E (2005) Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab 90:2456–2465. https://doi.org/10.1210/jc.2004-1978
Acknowledgements
This study did not receive any specific grants from any funding agencies in public, commercial, or not-for-profit sector.
Funding
This study did not receive specific grants from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contribution statement
All authors made substantial contributions to conception and design, and/or acquisition of data, analysis and interpretation of data participated in drafting the article or revising it critically for important intellectual content, and gave final approval of the version to be submitted.
Conflict of interest
Dilek Gogas Yavuz, Kadriye Aydin, Tugce Apaydın, Arzu Velioglu, Meral Mert, Zafer Pekkolay, Ergun Parmaksiz, Meral Mese, Ayse Esen Pazir, Emre Aydın, Onur Bugdayci, Serhan Tuglular declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gogas Yavuz, D., Aydin, K., Apaydin, T. et al. Clinical predictors of incipient vertebral fractures and bone mineral density in kidney transplant patients. Eur Spine J 31, 2423–2430 (2022). https://doi.org/10.1007/s00586-022-07162-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07162-6