Abstract
18F-fluoro-d-deoxyglucose positron emission tomography ([18F]-FDG PET) is successfully employed as a molecular imaging technique in oncology, and has become a promising imaging modality in the field of infection. The non-invasive diagnosis of spinal infections (SI) has been a challenge for physicians for many years. Morphological imaging modalities such as conventional radiography, computed tomography (CT), and magnetic resonance imaging (MRI) are techniques frequently used in patients with SI. However, these methods are sometimes non-specific, and difficulties in differentiating infectious from degenerative end-plate abnormalities or postoperative changes can occur. Moreover, in contrast to CT and MRI, FDG uptake in PET is not hampered by metallic implant-associated artifacts. Conventional radionuclide imaging tests, such as bone scintigraphy, labeled leukocyte, and gallium scanning, suffer from relatively poor spatial resolution and lack sensitivity, specificity, or both. Initial data show that [18F]-FDG PET is an emerging imaging technique for diagnosing SI. [18F]-FDG PET appears to be especially helpful in those cases in which MRI cannot be performed or is non-diagnostic, and as an adjunct in patients in whom the diagnosis is inconclusive. The article reviews the currently available literature on [18F]-FDG PET and PET/CT in the diagnosis of SI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal infection (SI) accounts for 2–4% of cases of skeletal infection. Despite advances in antibiotic treatment regimens, the incidence is still increasing. This might be due to an increased number of patients at risk, e.g, immunocompromised patients, an increasing use of spinal-fusion surgery, and also due to a heightened awareness and improved diagnostic procedures [2, 18, 50, 84]. Prompt diagnosis is facilitated by early and appropriate imaging techniques together with histopathology or bacteriological culture [2, 84]. In addition, delineation of the extent of SI is crucial for the assessment of appropriate therapy. Although superficial infection can be diagnosed with relative ease, the diagnosis of a deep-seated musculoskeletal infection—such as SI—is more difficult to establish and often requires a multidisciplinary approach with close collaboration between orthopedic surgeons, clinicians, and imaging specialists [3, 38, 48, 83]. Numerous imaging techniques including conventional radiography, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and radionuclide studies have been used to diagnose and monitor SI [2, 81, 83].
Nuclear medicine imaging of infection is nowadays molecular imaging of different pathophysiological steps in infectious or inflammatory processes. The increased uptake and localization of various diagnostic radiopharmaceuticals at the sites of infection and inflammation can be explained by both non-specific and specific mechanisms, covering the two main steps of the inflammatory cascade, including first the migration of white blood cells (WBC) and second the increased vascular permeability at the site of bacterial infection [27]. For this purpose, since more than three decades, commonly used conventional nuclear medicine procedures—both planar scintigraphy and Single-Photon Emission Computed Tomography (SPECT)—are bone scintigraphy, gallium-67 citrate, and technetium- and indium-labeled leukocyte scintigraphy [22, 27, 63]. On the other hand, since more than one decade, fluorine-18 fluorodexoyglucose ([18F]-FDG)-PET has entered the field of clinical infectious and inflammatory diseases [17, 44]. In the following chapters, various SPECT tracers (with also a few investigational tracers) and FDG as PET tracer will be extensively reviewed and compared regarding their usefulness in detecting SI. First, several morphological imaging techniques used for spine imaging will be shortly addressed.
Morphological spine imaging
In the early course of SI conventional radiography is normal and it may take up to 4–8 weeks, after the initial symptoms, until loss of definition and irregularity of the vertebral end-plate become evident [2, 28, 81]. CT- or fluoroscopy-guided biopsy may be of value for obtaining diagnostic specimens for microbiology and histopathology.
Gadolinium-enhanced MRI, demonstrating pathologic changes of the disc and adjacent bone marrow, is the imaging modality of choice for diagnosing SI [49, 58, 60, 88]. SI commonly demonstrate typical signal intensity on T1- and T2-weighted images and enhancement within the affected bone marrow after the administration of gadolinium-based contrast material [49]. Sensitivity, specificity, and accuracy are 96, 92, and 94%, respectively [60]. Moreover, epidural, subdural, intramedullary, and paraspinal soft tissue involvement are clearly delineated on contrast-enhanced MRI [49, 54, 58, 81]. There are, however, limitations to MR imaging, such as atypical findings of early SI, age-related changes in the signal intensity caused by the marrow composition, and issues more related to technical MRI parameters [8, 26, 37]. In the so-called atypical MR imaging patterns of SI, it may be difficult to differentiate infectious spondylitis from neoplastic conditions and sometimes benign compression fractures [37]. Furthermore, known disorders that can mimic SI are degenerative and inflammatory spinal diseases, such as ankylosing spondylitis, the neuropathic spine and several metabolic conditions [2, 19, 37, 71, 72, 75, 80, 81, 88]. Furthermore, the role of MR imaging in postoperative SI remains controversial because signal characteristics are not specific, and therefore it can be more difficult to differentiate active infection from ongoing reparative and/or fibrotic tissue [2, 19, 29, 45, 80, 87]. Moreover, both CT and MRI are hampered by artifacts induced by spinal implants or hardware. The characteristic features of postoperative discitis develop only slowly. Although absence of vertebral edema has a high negative predictive value for SI [86], MRI is less reliable for distinguishing septic from aseptic discitis in the early postoperative period because scar tissue behaves very much like SI [29]. Even as a late examination, MRI is sometimes inadequate for diagnosing discitis because it is not always possible to differentiate postoperative changes from infection [45]. Nevertheless, using special MR imaging sequences such as fat suppression technique may help to differentiate SI from other conditions [4, 37]. Finally, MRI is contraindicated in patients with pacemakers and cardiac valves.
Functional spine imaging
Conventional radionuclide techniques (Table 1)
Bone scintigraphy is widely available, easily performed, and rapidly completed. The test can be positive within 2 days after onset of symptoms [1]. Modic et al. reported a sensitivity and specificity of 90 and 78% for bone scintigraphy and 96 and 92% for MRI, respectively [60]. Gratz et al. reported that planar bone imaging was 86% sensitive for diagnosing spinal osteomyelitis; single-photon emission computed tomography (SPECT) increased the sensitivity to 92% [30]. Love et al. reported sensitivities of 73 and 82%, respectively, and specificities of 31 and 23%, respectively, for planar and planar plus SPECT bone imaging [53]. Three-phase bone scintigraphy and analysis of uptake patterns have been used to enhance the accuracy of the test to diagnose spinal osteomyelitis [55]. Gratz et al. found that three-phase bone imaging was positive in patients with severe infection, but not in patients with mild or moderate infection [30]. Love et al. reported that although specificity improved with the three-phase technique, it did so at the expense of sensitivity, which fell from 92 to 36% [53]. These investigators also studied uptake patterns and found that abnormal uptake in two contiguous vertebrae on SPECT images was the single most accurate criterion (71%) for detecting spinal osteomyelitis [53].
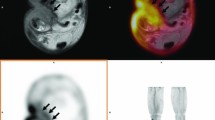
There are other limitations to bone scintigraphy. Sometimes disc space infection can present as decreased, rather than increased uptake. Although the explanation for this is not well understood, it is thought that inadequate blood supply (induced by pus or vasospasm), or destructive bone lesion, as well as the involvement of non-pyogenic microorganisms, such as mycobacteria may be responsible. False-negative results have been reported in elderly patients with spinal osteomyelitis, possibly due to regional ischemia secondary to arteriosclerotic disease [70]. Abnormalities may persist even after the infection has resolved, due to ongoing bony remodeling as part of the healing process. Finally, because it is not especially sensitive for detecting adjacent soft tissue infections, bone scintigraphy cannot be the only imaging test performed when SI is suspected (Fig. 1a).
Infected spinal hardware. A 53-year-old woman, who underwent spinal fusion with spinal implants 6 years earlier, was admitted to the hospital with an inflammatory syndrome of unknown origin. Posterior planar bone (a) and WBC images (c), demonstrate normal distribution of activity in the lumbar spine. Posterior planar gallium (b), and ciprofloxacin images (d), however, both demonstrate intense uptake in the lower lumbar spine and upper sacrum. Infected orthopedic hardware was surgically confirmed
Gallium-67 citrate (67Ga) imaging often is used as a complement to bone scintigraphy. Gallium-67 scintigraphy improves the specificity of the bone scan, detects infection earlier than the bone scan, and identifies the presence of accompanying soft tissue infection [1, 35, 51, 53, 60, 64] (Fig. 1b). This dual tracer technique, however, has several disadvantages [24, 53]. The procedure requires two different tracers as well as multiple, sometimes lengthy, imaging sessions on different days.
Although recent data suggest that single 67Ga-SPECT is as accurate for diagnosing spinal osteomyelitis as combined planar bone/gallium imaging [30, 53]. However, even when used alone, gallium is not an ideal tracer. The physical characteristics and normal biodistribution of the agent degrade image quality. Although positive results have been reported as early as 4 h after injection, imaging for infection typically is performed 24–72 h after injection, and therefore more than one patient visit to the Nuclear Medicine Department is required. Finally, there are few data about the role of gallium imaging in postoperative SI. Only 3 of 22 patients reported on by Love et al. had undergone spinal surgery [53]. Only one series evaluated the role of gallium imaging in postoperative SI, and these investigators concluded that this modality could not differentiate infection from postoperative changes [35].
Radiolabeled leukocyte (WBC) imaging
In contrast to other locations in the skeleton, imaging with in vitro, indium (111In) or technetium (99mTc) labeled, WBCs is of limited value for diagnosing SI [92]. Although increased uptake is virtually diagnostic, 50% or more of all cases of SI present as areas of normal, decreased, or even absent activity on WBC imaging [62] (Fig. 1c).
Unfortunately, photopenia is not specific for vertebral osteomyelitis and is associated with a variety of non-infectious conditions including degenerative disease, fracture, tumor, infarction, post-radiation therapy changes, Paget’s disease, vertebral hemangioma, and also in previously treated SI [56, 66]. Equally disappointing results have been reported with radiolabeled antigranulocyte antibodies, which essentially are an in vivo WBC labeling technique [31, 34]. In a subgroup analysis of a recent review concerning the role of several radiological and nuclear medicine techniques, including FDG PET, Termaat et al. reported that the pooled sensitivity of WBC imaging for detection of chronic osteomyelitis in the axial skeleton was only 21% [82].
Streptavidin/111–indium-biotin complex
Streptavidin accumulates at sites of inflammation and infection as a result of increased capillary permeability. In addition to being utilized by bacteria for their own growth, biotin forms a stable, high-affinity non-covalent complex with avidin. Lazzeri et al. investigated a two-step streptavidin/111In-biotin imaging in 55 consecutive patients with suspected SI within 2 weeks after onset of symptoms [47]. Streptavidin/111In-biotin was positive in 32/34 patients with SI (94% sensitivity) and negative in 19/21 patients without infection (95% specificity). These investigators concluded that streptavidin/111In-biotin scintigraphy is highly sensitive and specific for detecting vertebral osteomyelitis in the first 2 weeks after the onset of clinical symptoms [47].
Although these results were encouraging, the use of a heterologous protein, such as streptavidin potentially could incite an immunogenic reaction. Recently, Lazzeri et al. reported on the use of 111In-biotin alone for diagnosing SI in 110 patients, including 71 with suspected hematogenous infection and 39 with suspected postoperative infection [46]. 111In-biotin scintigraphy had a sensitivity of 84% and a specificity of 98% in the hematogenous infection group and a sensitivity of 100% and a specificity of 84% in the postoperative infection group. In order to achieve these results, however, patients with focal tracer uptake in the spine had to undergo additional radionuclide imaging with 99mTc-nanocolloid, which helped to confirm both the presence and location of the infection [46].
Radiolabeled ciprofloxacin
Radiolabeled antibiotics have been investigated for their potential as “infection specific” tracers [14, 89, 96]. The most extensively investigated of these compounds is the 4-fluoroquinolone antibiotic, ciprofloxacin, a broad spectrum antibiotic that inhibits the actions of DNA gyrase in gram-negative bacteria and type IV topoisomerase in gram-positive bacteria, resulting in rapid bacterial death. 99mTc-ciprofloxacin (99mTc-cipro) has been investigated extensively for diagnosing a variety of infections, and the results are inconclusive [6, 20, 68, 74, 91]. The limited data on its value in SI also are inconclusive [11, 23]. Falagas et al. retrospectively reviewed 17 99mTc-cipro scans performed in 11 patients [21]. Thirteen of these studies were performed during active infection and all were positive. Four studies were negative, and none of the patients had SI. de Winter et al. evaluated 99mTc-cipro in 48 patients, including 30 with spinal implants, with suspected postoperative SI [11] (Fig. 1d). The median interval between surgery and imaging was 7 months. Thirteen patients were treated with antibiotics at the time of the test. Imaging was performed at multiple time points up to 24 h postinjection. The final diagnosis was confirmed by microbiological culture in 22 patients and by clinical follow-up for more than 1 year in 26 patients. There were 13 cases of deep SI. Sensitivity, specificity, and accuracy of the test were, respectively, 54, 71, and 67% at 1 h, 62, 77, and 73% at 3 h, and 42, 91, and 77% at 24 h for planar imaging and 100, 74, and 81% for SPECT imaging. When recently operated patients were excluded, the specificity of SPECT rose to 81%. This rather low specificity of the test could be explained by non-specific accumulation of the radiopharmaceutical in the spinal tissues and around the implants [74].
Positron emission tomography with [18F]-fluorine-fluoro-d-deoxyglucose
One of the most powerful radiopharmaceutical is [18F]-fluorine-fluoro-d-deoxyglucose (FDG), a fluorinated glucose analog. [18F]-Fluorine is a positron-emitting radionuclide and a cyclotron product, with a physical half-life of 110 min. Positron emission tomography uses a completely different physical principle of imaging than does the SPECT technique, derived from conventional radionuclides (like technetium, indium, and gallium), in that after annihilation of a positron with a nearby electron, two gamma rays instead of one are emitted at the same time [17].
Fluoro-d-deoxyglucose is transported into cells via glucose transporters, and phosphorylated by hexokinase to 18F-2′-FDG-6 phosphate, but is not metabolized. The degree of cellular FDG uptake is related to the cellular metabolic rate and to the number of glucose transporters [65, 94]. Kubota et al. demonstrated that FDG uptake in tumors was, in fact, partly the result of newly formed granulation tissue and activated macrophages associated with tumor necrosis and growth [43]. These investigators demonstrated autoradiographically that FDG uptake by non-neoplastic cells was even higher than its accumulation in viable tumor cells [93]. Detailed histopathologic and autoradiographic analysis of an experimental soft tissue abscess model in rats showed that the highest FDG uptake was within areas of inflammatory cell infiltrate, which was composed primarily of neutrophils in the acute phase and macrophages in the chronic phase [39]. These same investigators also demonstrated that glucose utilization by the fibroblast-enriched granulation tissue does not substantially contribute to FDG uptake.
Increased FDG uptake in inflammation presumably is due to several factors [57]. Glucose transporters, such as GLUT-1 and GLUT-3 appear to be the targets of many cytokines and growth factors, especially after stimulation, as demonstrated in vitro in both murine and human WBCs. In activated inflammatory cells, such as neutrophils, lymphocytes, monocytes, and macrophages, there are both an increased number and increased expression of glucose transporters, as well an increased affinity of these transporters for deoxyglucose [59, 61, 65].
18F-fluoro-d-deoxyglucose positron emission tomography ([18F]-FDG PET) offers several potential advantages over conventional nuclear medicine tests in the evaluation of musculoskeletal and SI [15, 16, 33, 44, 78] (Table 2).
Positron emission tomography intrinsically is a high-resolution tomographic technique that enables precise localization, especially when performed as PET/CT, of sites of infection and inflammation. The procedure is completed in 1–2 h and has a relatively low radiation dose. Recent improvements in its production and distribution have made FDG imaging possible even in institutions without an on-site cyclotron. FDG is less expensive than the combinations of conventional radionuclide imaging techniques.
Semiquantitative analysis, readily available with PET, but less feasible with conventional gamma camera imaging, potentially could be useful for differentiating infectious from non-infectious conditions and for monitoring response to therapy.
Normal bone marrow has a low glucose metabolism and usually show only faintly increased FDG uptake, which may facilitate the differentiation of inflammatory cellular infiltrates from hematopoietic marrow and degenerative bone changes [17]. FDG uptake normalizes relatively rapidly, usually within 3–4 months, following non-union fracture or surgery, compared to conventional bone tracers and to a lesser degree gallium-citrate [16, 33, 77]. Schmitz et al. and Bredella et al. investigated the FDG avidity in various types of vertebral compression fractures and concluded that, in general, benign fractures demonstrated significantly less FDG uptake than malignant fractures or SI and furthermore that recent and older fractures demonstrated, respectively, moderate and no or only mildly increased FDG uptake [5, 72].
Published data indicate that [18F]-FDG PET is useful for diagnosing SI [24, 90] (Table 3; Fig. 2). In an early investigation including four patients with suspected SI, Guhlmann et al. reported that [18F]-FDG PET correctly diagnosed all three cases of infection and was true-negative in the one patient without infection [33]. Guhlmann et al. as part of a larger investigation, studied 15 patients with suspected chronic osteomyelitis of the central skeleton, including the spine, and compared [18F]-FDG PET to a radiolabeled antigranulocyte antibody (AGA) [34]. Two readers reviewed images independently. [18F]-FDG PET was significantly more accurate than the AGA for reader 1 (93 vs. 70%) and reader 2 (100 vs. 80%) [34]. Schiesser et al. evaluated [18F]-FDG PET in the diagnosis of metallic implant-associated infection in 22 patients (29 scans) with prior history of trauma [69]. In a subgroup of six patients with clinically suspected spinal implant infection, [18F]-FDG PET was true negative for implant infection in all six patients (including three patients with fracture non-union and one patient with osseous necrosis). One of the six patients had a soft tissue infection, correctly detected and localized with [18F]-FDG PET. In this study the degree of overall interobserver agreement was high [69].
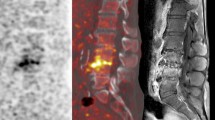
Chronic hematogenous spondylodiscitis of the thoracic spine. A 45-year-old man with multiple myeloma presents with severe back pain and prolonged fever in combination with increased inflammatory parameters. Sagittal T2-weighted MRI study (a) demonstrates abnormal signal at T11-12, but, nevertheless, the differential diagnosis with a vertebral compression fracture or even a spinal malignancy cannot reliably be made. Selected PET images (b transverse, c coronal, d sagittal view) demonstrate increased FDG uptake at the level of T11-12 vertebral disc, confirming a Staphylococcus aureus spondylodiscitis
Kalicke et al. evaluated the clinical usefulness of [18F]-FDG PET in acute and chronic osteomyelitis in 21 patients; including 7 with spondylitis and reported that [18F]-FDG PET yielded true-positive results in all 7 cases [40]. Zhuang et al. studied the accuracy of [18F]-FDG PET for the diagnosis of chronic osteomyelitis [95]. In five patients, the region of interest was the spine and [18F]-FDG PET correctly diagnosed the presence or absence of infection in all cases.
Schmitz et al. investigated 16 consecutive patients, suspected of SI. Twelve of the 16 patients had histopatologically confirmed SI [71]. [18F]-FDG PET was true positive in all 12 patients with infection and true negative in 3 of 4 patients without infection. The authors also noted that PET clearly delineated involvement of the paravertebral soft tissues [71].
Meller et al. prospectively investigated 30 patients with suspected active chronic osteomyelitis with a (limited resolution) coincidence detection [18F]-FDG PET device [56]. In eight patients (nine sites), the region of interest was the spine. [18F]-FDG PET findings were true positive in four cases and true negative in five cases (100% accuracy). As expected, the test was superior to radiolabeled leukocyte scintigraphy in the axial skeleton [56]. Gratz et al. also using a coincidence detection device, investigated the value of FDG imaging in 16 patients with suspected spondylitis [32]. They found that, even with its limited resolution, coincidence detection [18F]-FDG PET imaging was superior to MRI for detecting low-grade spondylitis or discitis. They also found that the test was superior to 67Ga imaging for identification of paraspinal soft tissue infection and superior to bone scintigraphy for differentiating advanced degenerative arthritis from infection [32].
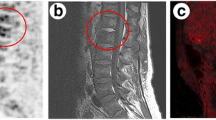
In one investigation of five patients, Stumpe et al. reported that [18F]-FDG PET correctly identified all three patients with, and both patients without, SI [77]. In a subsequent investigation, Stumpe et al. prospectively compared [18F]-FDG PET to MRI for diagnosing SI in 30 patients with substantial vertebral end-plate abnormalities of the lumbar spine detected on MRI [79]. A total of 38 sites were evaluated; there were 5 sites of infection in four patients. [18F]-FDG PET was true-positive in all 5 foci of infection and true negative in all 33 uninfected sites (100% sensitivity and 100% specificity). The sensitivity and specificity of MRI for detecting disc space infection were 50 and 96%, respectively. They found [18F]-FDG PET to be very helpful in the differentiation of severe degenerative changes of intervertebral discs from infective discitis (n = 5). These investigators concluded that [18F]-FDG PET may be useful for differentiating degenerative from infectious end-plate abnormalities detected on MRI [79].
In perhaps the largest series reported to date, de Winter et al. investigated [18F]-FDG PET in 73 patients [10]. The study population consisted of 57 patients who had undergone previous spinal surgery, including 27 with spinal implants. The median interval between surgery and imaging was 10 months (range 1.25–288 months). Sixteen patients with suspected hematogenous SI, served as a control group [10]. In six patients, follow-up [18F]-FDG PET was ordered for monitoring treatment response. Final diagnoses were based on histopathology, microbiology, and/or intraoperative visual assessment by the orthopedic surgeon in 26 (46%) patients, and by clinical follow-up of at least 12 months in 31 (54%) patients. Thirteen patients (23%) were under treatment with antibiotics at the time of imaging. Fifteen (26%) of 57 patients had SI, including 10 (37%) of 27 patients with, and 5 (17%) of 30 patients without, hardware. Using optimal cut-off values, sensitivity, specificity, and accuracy were 100, 81, and 86%, respectively. The positive predictive value of the test was 65%, and the negative predictive value was 100%. In the control group all 16 patients were correctly classified with [18F]-FDG PET (5 true positive and 11 true negative cases). In the investigational group, among the 30 patients without spinal implants, there were two false-positive studies; both occurred in patients who had undergone surgery within 6 months before imaging. Among 27 patients with spinal implants, there were six false-positive results, none of which were related to recent surgery. Unfortunately, the cause of false-positive results could be established in only one case, namely instability of the hardware. Sensitivity, specificity, and accuracy were not significantly different between the subgroup that had surgery within 6 months before the [18F]-FDG PET and the subgroup with surgery more than 6 months prior to [18F]-FDG PET. The specificity was 65% in the group with spinal implants and 92% in the group without. In 60% of patients (34/57) infection was excluded with [18F]-FDG PET. The overall accuracy of [18F]-FDG PET in this prospective study was good (86%), and the negative predictive value (100%) was excellent. The authors concluded that chronic postoperative SI can be excluded when the PET study is negative. In the patient with spinal hardware, however, a positive study must be interpreted cautiously [9] (Table 4).
Hartmann et al. investigated the diagnostic value of [18F]-FDG PET co-registered with in-line CT (PET/CT device) in patients with trauma and suspected chronic osteomyelitis in the axial and appendicular skeleton, including patients with metallic implants and prosthetic devices, at a minimum interval of 6 months after surgery [36]. For the subgroup analysis of nine spinal regions (all of them involving the lumbar spine), [18F]-FDG PET/CT was true positive in all seven cases of SI, and true negative in the two patients who did not have SI (100% accuracy). These investigators found that the precise anatomical localization provided by, and the extent of increased FDG uptake detected on, combined PET/CT was especially useful for planning surgical intervention, and, in the cases in which infection was limited to the soft tissues, for initiating antibiotic treatment [36].
Kim et al. performed dual time point [18F]-FDG PET/CT in 22 consecutive patients with a high suspicion of spondylitis (based on clinic-radiography and bone scan findings), both pyogenic as well as tuberculous [42]. They imaged the patients, at 1 h and at 2 h after injection of FDG. Although the test was very sensitive for detecting SI, neither visual nor semiquantitative analysis, using dual time point imaging, could reliably differentiate pyogenic from tuberculous infection [42].
Overall, the results of [18F]-FDG PET for diagnosing SI that have been reported by various investigators are very encouraging. PET studies, however, are dealing with a mixture of hematogeneous and postoperative SI. Therefore, it is quite difficult to give sensitivity and specificity figures for the different types of SI. There are, nevertheless, limitations to the test since FDG uptake reflects enhanced glucose metabolism in general, not infection specifically. While uptake is significantly higher in infection than in normal bone or benign compression fractures, there are few data comparing FDG uptake in SI to uptake in spinal tumors. Infection and malignancy are not mutually exclusive and it is likely that the test will be less reliable for differentiating infection from tumor and detecting infection superimposed on tumor. Also in spinal-fusion surgery, especially in the presence of metallic implants, the test suffers from a lower specificity. Increased FDG uptake, in the absence of infection, has been described in foreign body reactions [12, 93], and aseptic loosening of orthopedic hardware [13, 52, 85]. Nevertheless, it seems to be rather consistent that the PET technique is very sensitive for diagnosing SI. A negative PET study can, therefore, exclude SI with a high certainty [10]. Larger series including patients with spinal instrumentation, with intraoperative verification of the false positive cases, are needed [10].
Although FDG accumulation in degenerative changes has been reported to be low [79], this is not always the case. In a retrospective study of 150 patients, Rosen et al. were the only one describing significant focal FDG uptake (of varying degree) in more than half of the patients, corresponding to degenerative spinal disease, primarily in the lumbosacral spine [67].
A limitation of PET imaging in general is that despite a relatively high spatial resolution, the anatomic information available with PET images remains limited. To improve this, integrated in-line PET/CT systems that provide “hardware” co-registered PET and CT images have been introduced recently [41, 69] (Figs. 3, 4). These systems offer excellent anatomic localization of the actual site of uptake, minimizing misinterpretation of localization from areas of clear-cut arthritic bony disease and infection, such as demonstrating the uptake to be associated with an arthritic facet joint rather than with the vertebral body or interspace [63, 67].
Hematogenous spinal infection of the lumbar spine with secondary psoas abscess. A 60-year-old man, with known rheumatic disease (polychondritis/arthritis, episcleritis), treated with corticosteroids and methotrexate, presents with a grippal syndrome, malaise, chillings and fever up to 39°, and substantial weight loss. Laboratory results reveal increased inflammatory parameters (including a BSE of 56 and a CRP of 35 mg/dl). X-ray of the lumbar spine (a) revealed no pathological findings. Co-registered PET/enhanced CT (Biograph Siemens HiRez device) (selected sagittal CT, PET and fused PET/CT images, demonstrate high metabolic uptake at the L3–L4 level (b; dashed lines), extending in the soft prevertebral tissues
Same patient as in Fig. 3, high metabolic uptake localized in the right iliopsoas region (dashed lines, resp. a sagittal, b transverse, and c coronal CT, PET, and fused PET/CT images), indicating an occult spondylodiscitis complicated by a secondary psoas abscess; eventually the portal of entry was confirmed to be the urinary tract with a Staphylococcus aureus infection
Conclusions and future prospects
The diagnosis of SI has been a challenge to physicians for many years. The increasing frequency of these infections is becoming a global health concern [84]. Prompt and accurate diagnosis of SI requires a high index of suspicion in high-risk patients, especially in the elderly and immunocompromised, and the appropriate investigation to identify the organism and to determine the extent of infection [80, 84]. Despite the emerge of advanced imaging techniques, the diagnosis of this entity and its differentiation from degenerative disease, non-union or benign compression fractures, non-infective inflammatory processes, and spinal neoplasm can be difficult [2, 24, 37, 80]. Plain radiographs are generally insensitive in the early course of disease and negative results do not reliably exclude infection. MR imaging is especially helpful in the evaluation of patients with hematogeneous SI [49, 54]. However, it is not always easy to diagnose a SI, particularly when some of the classic MR imaging features are absent or when unusual patterns are present [32, 37, 75, 80]. Moreover, MRI is less useful for diagnosing SI in the presence of fractures (traumatic or iatrogenic) and spinal implants [48, 73]. Among the radionuclide procedures, the combination of 67Ga-SPECT with bone scintigraphy, despite the inherent limitations, is still considered the radionuclide gold standard for diagnosing SI [53]. [18F]-FDG PET, however, is a very promising alternative to combined bone/gallium imaging [10, 24]. Although most of the published series are small, [18F]-FDG PET appears to be superior in the detection of SI and in the differentiation of degenerative from infectious end-plate abnormalities, compared to gallium imaging or MRI [7, 32, 71, 76, 79]. The advantages of PET are obvious: The study is sensitive, is completed in a single session, and image resolution is superior to that obtained with SPECT tracers. As with gallium, however, specificity remains an issue. While FDG uptake in uninfected fractures may normalize more rapidly than gallium or diphosphonate uptake, differentiating infection from tumor may still be problematic [25]. Furthermore, inflammatory reactions incited by spinal implants also may adversely affect specificity [10]. Nevertheless, there is an expanding body of evidence that supports the use of [18F]-FDG PET and PET/CT for diagnosing SI, especially in patients with MRI contraindications and in the postoperative spine [10, 24]. With the rapid evolution and proliferation of in-line PET/CT and SPECT/CT systems, well-designed prospective comparisons of [18F]-FDG PET/CT and 67Ga-SPECT/CT should be undertaken to validate these initial impressions and to determine the potential of each of these tracers and techniques for monitoring response to treatment [24, 71].
References
Adatepe MH, Powell OM, Isaacs GH, Nichols K, Cefola R (1986) Hematogenous pyogenic vertebral osteomyelitis: diagnostic value of radionuclide bone imaging. J Nucl Med 27:1680–1685
An HS, Seldomridge JA (2006) Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res 444:27–33
Aydinli U, Karaeminogullari O, Tiskaya K (1999) Postoperative deep wound infection in instrumented spinal surgery. Acta Orthop Belg 65:182–187
Boden SD, Davis DO, Dina TS, Sunner JL, Wiesel SW (1992) Postoperative diskitis: distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology 184:765–771
Bredella MA, Essary B, Torriani M, Ouellette HA, Palmer WE (2008) Use of FDG-PET in differentiating benign from malignant compression fractures. Skeletal Radiol 37:405–413
Britton KE, Wareham DW, Das SS, Solanki KK, Amaral H, Bhatnagar A, Katamihardja AH, Malamitsi J, Moustafa HM, Soroa VE, Sundram FX, Padhy AK (2002) Imaging bacterial infection with (99 m)Tc-ciprofloxacin (Infecton). J Clin Pathol 55:817–823
Chacko TK, Zhuang H, Nakhoda KZ, Moussavian B, Alavi A (2003) Applications of fluorodeoxyglucose positron emission tomography in the diagnosis of infection. Nucl Med Commun 24:615–624
Dagirmanjian A, Schils J, McHenry MC (1999) MR imaging of spinal infections. Magn Reson Imaging Clin N Am 7:525–538
de Winter F, Dierckx R, de Bondt P, Vogelaers D, Verdonk R, Van de Wiele C (2000) FDG PET as a single technique is more accurate than the combination bone scan/white blood cell scan in chronic orthopedic infections (COI). J Nucl Med 41:16P
de Winter F, Gemmel F, Van de Wiele C, Poffijn B, Uyttendaele D, Dierckx R (2003) 18-fluorine fluorodeoxyglucose positron emission tomography for the diagnosis of infection in the postoperative spine. Spine 28:1314–1319
de Winter F, Gemmel F, Van Laere K, De Winter O, Poffijn B, Dierckx RA, Van de Wiele C (2004) 99mTc-ciprofloxacin planar and tomographic imaging for the diagnosis of infection in the postoperative spine: experience in 48 patients. Eur J Nucl Med Mol Imaging 31:233–239
de Winter F, Huysse W, De Paepe P, Lambert B, Poffyn B, Dierckx R (2002) High F-18FDG uptake in a paraspinal textiloma. Clin Nucl Med 27:132–133
de Winter F, Van de Wiele C, de Clercq D, Vogelaers D, de Bondt P, Dierckx RA (2000) Aseptic loosening of a knee prosthesis as imaged on FDG positron emission tomography. Clin Nucl Med 25:923
de Winter F, Van de Wiele C, Dumont F, Van Durme J, Solanki K, Britton K, Slegers G, Dierckx RA, Thierens H (2001) Biodistribution and dosimetry of 99mTc-ciprofloxacin, a promising agent for the diagnosis of bacterial infection. Eur J Nucl Med 28:570–574
de Winter F, Van de Wiele C, Vandenberghe S, de Bondt P, de Clercq D, D’Asseler Y, Dierckx R (2001) Coincidence camera FDG imaging for the diagnosis of chronic orthopedic infections: A feasibility study. J Comput Assist Tomogr 25:184–189
de Winter F, Van de Wiele C, Vogelaers D, de Smet K, Verdonk R, Dierckx RA (2001) Fluorine-18 fluorodeoxyglucose-position emission tomography: a highly accurate imaging modality for the diagnosis of chronic musculoskeletal infections. J Bone Joint Surg Am 83A:651–660
de Winter F, Vogelaers D, Gemmel F, Dierckx RA (2002) Promising role of 18-F-fluoro-d-deoxyglucose positron emission tomography in clinical infectious diseases. Eur J Clin Microbiol Infect Dis 21:247–257
Deyo RA, Nachemson A, Mirza SK (2004) Spinal-fusion surgery—the case for restraint. N Engl J Med 350:722–726
Dufour V, Feydy A, Rillardon L, Redondo A, Le Page L, Bert F, Belmatoug N, Fantin B (2005) Comparative study of postoperative and spontaneous pyogenic spondylodiscitis. Semin Arthritis Rheum 34:766–771
Dumarey N, Blocklet D, Appelboom T, Tant L, Schoutens A (2002) Infecton is not specific for bacterial osteo-articular infective pathology. Eur J Nucl Med 29:530–535
Falagas ME, Valotassiou VJ, Papadouli D, Papadopoulos A, Malamitsi J (2006) 99mTechnetium-ciprofloxacin scintigraphy for the evaluation of spinal infections: a preliminary report. Clin Orthop Relat Res 444:34–37
Gemmel F, Dumarey N, Palestro CJ (2008) Non-invasive imaging of complicated osteomyelitis: the added value of scintigraphic techniques. Curr Radiopharm 1:73–86
Gemmel F, de Winter F, Van Laere K, Vogelaers D, Uyttendaele D, Dierckx RA (2004) 99mTc ciprofloxacin imaging for the diagnosis of infection in the postoperative spine. Nucl Med Commun 25:277–283
Gemmel F, Dumarey N, Palestro CJ (2006) Radionuclide imaging of spinal infections. Eur J Nucl Med Mol Imaging 33:1226–1237
Gemmel F, Dumarey N, Welling M (2009) Future diagnostic agents. Semin Nucl Med 39:11–26
Gillams AR, Chaddha B, Carter AP (1996) MR appearances of the temporal evolution and resolution of infectious spondylitis. AJR Am J Roentgenol 166:903–907
Goldsmith SJ, Vallabhajosula S (2009) Clinically proven radiopharmaceuticals for infection imaging: mechanisms and applications. Semin Nucl Med 39:2–10
Govender S (2005) Spinal infections. J Bone Joint Surg Br 87:1454–1458
Grane P, Josephsson A, Seferlis A, Tullberg T (1998) Septic and aseptic post-operative discitis in the lumbar spine : evaluation by MR imaging. Acta Radiol 39:108–115
Gratz S, Dörner J, Oestmann JW, Opitz M, Behr T, Meller J, Grabbe E, Becker W (2000) 67 Ga-citrate and 99Tc-MDP for estimating the severity of vertebral osteomyelitis. Nucl Med Commun 21:111–120
Gratz S, Braun HG, Behr TM, Meller J, Herrmann A, Conrad M, Rathmann D, Bertagnoli R, Willert HG, Becker W (1997) Photopenia in chronic vertebral osteomyelitis with technetium-99 m-antigranulocyte antibody (BW 250/183). J Nucl Med 38:211–216
Gratz S, Dorner J, Fischer U, Behr TM, Behe M, Altenvoerde G, Meller J, Grabbe E, Becker W (2002) F-18-FDG hybrid PET in patients with suspected spondylitis. Eur J Nucl Med Mol Imaging 29:516–524
Guhlmann A, Brecht-Krauss D, Suger G, Glatting G, Kotzerke J, Kinzl L, Reske SN (1998) Chronic osteomyelitis: detection with FDG PET and correlation with histopathologic findings. Radiology 206:749–754
Guhlmann A, Brecht-Krauss D, Suger G, Glatting G, Kotzerke J, Kinzl L, Reske SN (1998) Fluorine-18-FDG PET and technetium-99 m antigranulocyte antibody scintigraphy in chronic osteomyelitis. J Nucl Med 39:2145–2152
Hadjipavlou AG, Cesani-Vazquez F, Villaneuva-Meyer J, Mader JT, Necessary JT, Crow W, Jensen RE, Chaljub G (1998) The effectiveness of gallium citrate Ga 67 radionuclide imaging in vertebral osteomyelitis revisited. Am J Orthop 27:179–183
Hartmann A, Eid K, Dora C, Trentz O, von Schulthess GK, Stumpe KD (2007) Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Mol Imaging 34:704–714
Hong SH, Choi JY, Lee JW, Kim NR, Choi JA, Kang HS (2009) MR imaging assessment of the spine: infection or an imitation? Radiographics 29:599–612
Kaim AH, Gross T, von Schulthess GK (2002) Imaging of chronic posttraumatic osteomyelitis. Eur Radiol 12:1193–1202
Kaim AH, Weber B, Kurrer MO, Gottschalk J, von Schulthess GK, Buck A (2002) Autoradiographic quantification of 18F-FDG uptake in experimental soft-tissue abscesses in rats. Radiology 223:446–451
Kalicke T, Schmitz A, Risse JH, Arens S, Keller E, Hansis M, Schmitt O, Biersack HJ, Grunwald F (2000) Fluorine-18 fluorodeoxyglucose PET in infectious bone diseases: results of histologically confirmed cases. Eur J Nucl Med 27:524–528
Keidar Z, Militianu D, Melamed E, Bar-Shalom R, Israel O (2005) The diabetic foot: initial experience with 18F-FDG PET/CT. J Nucl Med 46:444–449
Kim SJ, Lee JS, Suh KT, Kim IJ, Kim YK (2008) Differentiation of tuberculous and pyogenic spondylitis using double phase F-18 FDG PET. Open Med Imaging J 2:1–6
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T (1992) Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 33:1972–1980
Kumar R, Basu S, Torigian D, Anand V, Zhuang H, Alavi A (2008) Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev 21:209–224
Kylanpaa-Back ML, Suominen RA, Salo SA, Soiva M, Korkala OL, Mokka RE (1999) Postoperative discitis: outcome and late magnetic resonance image evaluation of ten patients. Ann Chir Gynaecol 88:61–64
Lazzeri E, Erba P, Perri M, Tascini C, Doria R, Giorgetti J, Mariani G (2008) Scintigraphic imaging of vertebral osteomyelitis with 111In-biotin. Spine 33:E198–E204
Lazzeri E, Pauwels EK, Erba PA, Volterrani D, Manca M, Bodei L, Trippi D, Bottoni A, Cristofani R, Consoli V, Palestro CJ, Mariani G (2004) Clinical feasibility of two-step streptavidin/111In-biotin scintigraphy in patients with suspected vertebral osteomyelitis. Eur J Nucl Med Mol Imaging 31:1505–1511
Ledermann HP, Kaim A, Bongartz G, Steinbrich W (2000) Pitfalls and limitations of magnetic resonance imaging in chronic posttraumatic osteomyelitis. Eur Radiol 10:1815–1823
Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA (2003) MR imaging findings in spinal infections: rules or myths? Radiology 228:506–514
Liebergall M, Chaimsky G, Lowe J, Robin GC, Floman Y (1991) Pyogenic vertebral osteomyelitis with paralysis. Prognosis and treatment. Clin Orthop Relat Res 269:142–150
Lisbona R, Derbekyan V, Novales-Diaz J, Veksler A (1993) Gallium-67 scintigraphy in tuberculous and nontuberculous infectious spondylitis. J Nucl Med 34:853–859
Love C, Marwin SE, Tomas MB, Krauss ES, Tronco GG, Bhargava KK, Nichols KJ, Palestro CJ (2004) Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99 mTc-sulfur colloid marrow imaging. J Nucl Med 45:1864–1871
Love C, Patel M, Lonner BS, Tomas MB, Palestro CJ (2000) Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med 25:963–977
Ma LD, Frassica FJ, Bluemke DA, Fishman EK (1997) CT and MRI evaluation of musculoskeletal infection. Crit Rev Diagn Imaging 38:535–568
Maurer AH, Chen DC, Camargo EE, Wong DF, Wagner HN Jr, Alderson PO (1981) Utility of three-phase skeletal scintigraphy in suspected osteomyelitis: concise communication. J Nucl Med 22:941–949
Meller J, Koster G, Liersch T, Siefker U, Lehmann K, Meyer I, Schreiber K, Altenvoerde G, Becker W (2002) Chronic bacterial osteomyelitis: prospective comparison of F-18-FDG imaging with a dual-head coincidence camera and In-111-labelled autologous leucocyte scintigraphy. Eur J Nucl Med Mol Imaging 29:53–60
Meller J, Sahlmann CO, Liersch T, Hao TP, Alavi A (2007) Nonprosthesis orthopedic applications of (18)F fluoro-2-deoxy-d-glucose PET in the detection of osteomyelitis. Radiol Clin North Am 45:719–733
Meyers SP, Wiener SN (1991) Diagnosis of hematogenous pyogenic vertebral osteomyelitis by magnetic resonance imaging. Arch Intern Med 151:683–687
Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N (2001) FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med 42:1551–1555
Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, Rehm S (1985) Vertebral osteomyelitis: assessment using MR. Radiology 157:157–166
Paik JY, Lee KH, Choe YS, Choi Y, Kim BT (2004) Augmented 18F-FDG uptake in activated monocytes occurs during the priming process and involves tyrosine kinases and protein kinase C. J Nucl Med 45:124–128
Palestro CJ, Kim CK, Swyer AJ, Vallabhajosula S, Goldsmith SJ (1991) Radionuclide diagnosis of vertebral osteomyelitis: indium-111-leukocyte and technetium-99 m-methylene diphosphonate bone scintigraphy. J Nucl Med 32:1861–1865
Palestro CJ, Love C, Miller TT (2007) Diagnostic imaging tests and microbial infections. Cell Microbiol 9:2323–2333
Palestro CJ, Torres MA (1997) Radionuclide imaging in orthopedic infections. Semin Nucl Med 27:334–345
Pauwels EKJ, Ribeiro MJ, Stoot JHMB, McCready VR, Bourguignon M, Maziere B (1998) FDG accumulation and tumor biology. Nucl Med Biol 25:317–322
Roelants V, Tang T, Ide C, Laloux P (2002) Cold vertebra on (111)In-white blood cell scintigraphy. Semin Nucl Med 32:236–237
Rosen RS, Fayad L, Wahl RL (2006) Increased 18F-FDG uptake in degenerative disease of the spine: characterization with 18F-FDG PET/CT. J Nucl Med 47:1274–1280
Sarda L, Cremieux AC, Lebellec Y, Meulemans A, Lebtahi R, Hayem G, Genin R, Delahaye N, Huten D, Le Guludec D (2003) Inability of 99 mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J Nucl Med 44:920–926
Schiesser M, Stumpe KDM, Trentz O, Kossmann T, von Schulthess GK (2003) Detection of metallic implant-associated infections with FDG PET in patients with trauma: correlation with microbiologic results. Radiology 226:391–398
Schlaeffer F, Mikolich DJ, Mates SM (1987) Technetium Tc 99m diphosphonate bone scan. False-normal findings in elderly patients with hematogenous vertebral osteomyelitis. Arch Intern Med 147:2024–2026
Schmitz A, Risse JH, Grunwald F, Gassel F, Biersack HJ, Schmitt O (2001) Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: preliminary results. Eur Spine J 10:534–539
Schmitz A, Risse JH, Textor J, Zander D, Biersack HJ, Schmitt O, Palmedo H (2002) FDG-PET findings of vertebral compression fractures in osteoporosis: preliminary results. Osteoporos Int 13:755–761
Seabold JE, Nepola JV (1999) Imaging techniques for evaluation of postoperative orthopedic infections. Q J Nucl Med 43:21–28
Siaens RH, Rennen HJ, Boerman OC, Dierckx R, Slegers G (2004) Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J Nucl Med 45:2088–2094
Stabler A, Reiser MF (2001) Imaging of spinal infection. Radiol Clin North Am 39:115–135
Strobel K, Stumpe KD (2007) PET/CT in musculoskeletal infection. Semin Musculoskelet Radiol 11:353–364
Stumpe KD, Dazzi H, Schaffner A, von Schulthess GK (2000) Infection imaging using whole-body FDG-PET. Eur J Nucl Med 27:822–832
Stumpe KD, Strobel K (2006) 18F FDG-PET imaging in musculoskeletal infection. Q J Nucl Med Mol Imaging 50:131–142
Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, von Schulthess GK (2002) FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol 179:1151–1157
Tali ET, Gultekin S (2005) Spinal infections. Eur Radiol 15:599–607
Tay BK, Deckey J, Hu SS (2002) Spinal infections. J Am Acad Orthop Surg 10:188–197
Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ (2005) The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am 87:2464–2471
Tsiodras S, Falagas ME (2006) Clinical assessment and medical treatment of spine infections. Clin Orthop Relat Res 444:38–50
Tyrrell PN, Cassar-Pullicino VN, McCall IW (1999) Spinal infection. Eur Radiol 9:1066–1077
Van Acker F, Nuyts J, Maes A, Vanquickenborne B, Stuyck J, Bellemans J, Vleugels S, Bormans G, Mortelmans L (2001) FDG-PET, Tc-99m-HMPAO white blood cell SPET and bone scintigraphy in the evaluation of painful total knee arthroplasties. Eur J Nucl Med 28:1496–1504
Van Goethem JW, Parizel PM, van den Hauwe L, Van de Kelft E, Verlooy J, De Schepper AM (2000) The value of MRI in the diagnosis of postoperative spondylodiscitis. Neuroradiology 42:580–585
Van Goethem JWM, Parizel PM, Jinkins JR (2002) Review article: MRI of the postoperative lumbar spine. Neuroradiology 44:723–739
Varma R, Lander P, Assaf A (2001) Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am 39:203–213
Vinjamuri S, Hall AV, Solanki KK, Bomanji J, Siraj Q, O’Shaughnessy E, Das SS, Britton KE (1996) Comparison of 99mTc infecton imaging with radiolabelled white-cell imaging in the evaluation of bacterial infection. Lancet 347:233–235
Walker RC, Jones-Jackson LB, Martin W, Habibian MR, Delbeke D (2007) New imaging tools for the diagnosis of infection. Future Microbiol 2:527–554
Welling M, Stokkel M, Balter J, Sarda-Mantel L, Meulemans A, Le Guludec D (2008) The many roads to infection imaging. Eur J Nucl Med Mol Imaging 35:848–849
Whalen JL, Brown ML, McLeod R, Fitzgerald R-HJ (1991) Limitations of indium leukocyte imaging for the diagnosis of spine infections. Spine 16:193–197
Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N (1995) High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med 36:1301–1306
Zhuang HM, Alavi A (2002) 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med 32:47–59
Zhuang HM, Duarte PS, Pourdehand M, Shnier D, Alavi A (2000) Exclusion of chronic osteomyelitis with F-18 fluorodeoxyglucose positron emission tomographic imaging. Clin Nucl Med 25:281–284
Zijlstra S, Gunawan J, Freytag C, Burchert W (2006) Synthesis and evaluation of fluorine-18 labelled compounds for imaging of bacterial infections with pet. Appl Radiat Isot 64:802–807
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gemmel, F., Rijk, P.C., Collins, J.M.P. et al. Expanding role of 18F-fluoro-d-deoxyglucose PET and PET/CT in spinal infections. Eur Spine J 19, 540–551 (2010). https://doi.org/10.1007/s00586-009-1251-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-009-1251-y