Abstract
The relationship of the esophagus to the cervical vertebral body (CVB), disc space and longus colli (LC) muscles, to our knowledge, has not been previously studied. The purpose of this study was to quantify the relationship of the esophagus to the CVB, disc space and LC. 30 patients were selected for a retrospective review of computed tomography (CT) scans. Measurements between the esophagus and the C5, C6, and C7 vertebral bodies as well as the C5/6 and C6/7 disc spaces were performed in the midline, 3 mm right and left of midline, and at the edge of the LC on both sides. The closest distance of the esophagus to the CVB and disc space occurs at the midline (range 1.02–1.31 mm at each level). The furthest distance occurred at the edge of the right LC (range 2.67–3.30 mm at each level). The mean distance from the edge of the right LC to the midline was significantly greater (P < 0.01) than mean distance from the edge of the left LC to the midline. No statistical significant differences were observed when comparing measurements at the individual vertebral bodies and disc spaces. The results of the study demonstrate that the esophagus lies in closest proximity to the CVB and disc space in the midline. A larger potential space exists between the esophagus and the CVB and disc space at the edge of the LC. These results may provide insight into a potential cause of post-operative dysphagia. Furthermore, it may help guide the future design of cervical plates to better utilize the potential space between the esophagus and the CVB and disc space at the edge of the LC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of cervical spine disorders with anterior cervical discectomy and fusion (ACDF) was first popularized by Smith and Robinson in 1958. Since then, it has evolved into the gold standard for the treatment of cervical radiculopathy and myelopathy [1, 5, 6, 13]. In the literature, the results of ACDF have been reported to produce good clinical results with high patient satisfaction scores [4, 9].
Despite the reliability of this procedure, dysphagia has been reported as a common complication of ACDF [2, 3, 14, 15]. Riley et al. [12] found a 21.3% incidence of dysphagia at 2-year follow-up. Other authors have even documented abnormal swallowing studies and videolaryngoendoscopy following ACDF [8].
The etiology of dysphagia following ACDF is multifactorial. Hematoma, pharyngeal plexus denervation, vocal cord paralysis, adhesion formation and hardware have all been implicated as possible causes of post-operative dysphagia. The hardware used in ACDF procedures is at the discretion of the operating surgeon. Therefore, it may be one of the few modifiable factors over which the surgeon has direct control. Fogel and McDonnell [7] have shown that the removal of cervical instrumentation improves dysphagia in the majority of patients. They reported finding extensive adhesions between the esophagus, the prevertebral fascia, the hardware and the anterior cervical spine at the time of removal. In another study, Lee et al. [11] demonstrated that a smoother, thinner plate reduces the incidence of post-operative dysphagia following ACDF.
Despite these findings, there is little literature about the anatomy of the esophagus in relation to the anterior cervical spine. A search of the English literature resulted in only one relevant article. Karaikovic et al. [10] performed CT scans on ten cadavers to assess the safety zone anterior to the cervical spine when placing pedicle screws posteriorly. They found no safety zone anterior to the cervical spine below C2.
The purpose of this study is to describe the anatomy of the esophagus in relation to the anterior cervical spine. By carefully defining the anatomy, it is hoped that implant designs can be modified to decrease contact between the instrumentation and the esophagus, and thereby reducing the incidence of post-operative dysphagia.
Materials and methods
Thirty patients who had undergone cervical CT scans were randomly selected and the CT images reviewed (Fig. 1). Patients were excluded from the study if they had previous anterior cervical surgery, previous esophageal surgery, or significant anterior osteophyte formation. The study group consisted of 13 females and 17 males. The mean age of the patients was 51 years (range 32 to 70 years old).
The CT scans were performed on a GE high speed CT/I. The image thickness was set at 1 mm with an interslice gap of 0.5 mm. Settings were: FOV—12 cm; kV—120; and mA—approx. 100 to 200.
Measurements were performed by a spine surgery fellow. Utilizing the Phillips/PACS system, measurements were made on the CT images and rounded to the nearest 0.01 mm. All measurements were confirmed by an orthopedic radiologist or a senior orthopedic spine surgeon. Measurements were taken at the midpoint of the C5, C6 and C7 vertebral bodies and at the level of the C5/6 and C6/7 disc spaces. These levels were in the study because they represent the most common sites of symptomatic cervical spondylosis.
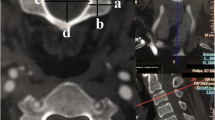
The measurements included anterior–posterior (AP) measurements between the esophagus and vertebral body in the midline, 3 mm left and right of the midline and at the medial edge of the longus colli muscles bilaterally (Fig. 2a–c). Horizontal measurements were taken from the midline of the vertebral body or disc space to the medial edge of the longus colli muscles bilaterally (Fig. 3).
a Anterior–posterior measurements. Measurements were performed by measuring the distance from the anterior aspect of the vertebral body or disc space to the posterior aspect of the esophagus in the midline of the CVB or disc space (space between arrowheads). b Anterior–posterior measurements. Measurements were performed by measuring the distance from the anterior aspect of the vertebral body or disc space 3 mm from the midline to the posterior aspect of the esophagus (space between arrowheads). c Anterior–posterior measurements. Measurements were performed by measuring the distance from the anterior aspect of the vertebral body or disc space at the medial edge of the longus colli muscle bilaterally to the posterior aspect of the esophagus (space between arrowheads)
The mean with standard deviations were calculated for AP as well as for horizontal distances at all five levels (C5, C5/6, C6, C6/7, C7). Statistical differences between distances from the right and left of the esophagus to the midline were calculated using a two-tailed Student’s t test. Statistical significance was determined at an alpha level of 0.05.
Results
The closest distance of the esophagus to the cervical spine was located in the midline of the vertebral body or disc space (mean from 1.02 to 1.31 mm; see Table 1 and Figs. 4, 5, 6, 7, 8). The farthest distance between the esophagus and the cervical spine occurred at the medial edge of the right longus colli muscle (mean 2.67–3.30 mm). Significance was found when comparing the mean distances from the edge of the longus colli to the midline of the vertebral body or disc space, with a greater distance on the right (P < 0.01). No statistical significance was found in the AP measurements when comparing values taken at the vertebral bodies to values taken at the disc spaces. Horizontal measurements showed no statistically significant difference between the right and left side (see Table 2).
Discussion
This study serves as the first to explicitly describe the anatomy of the esophagus relative to the cervical spine. It illustrates that the least quantity of potential space between the esophagus and the cervical spine occurs in the midline. In terms of laterality, there is significantly more usable space adjacent to the medial edge of the right longus colli. The relationship between the esophagus and spine does not change significantly from the level of the C5 vertebral body to the C7 vertebral body.
Given the relatively high incidence of dysphagia following ACDF any factor which may limit this complication could be potentially beneficial for patients [2, 3, 8, 12, 14, 15]. Hardware is known to be a contributor to this problem [7, 11]. Now, with a clear description of the normal anatomy in this region, implants can be designed which will place limited pressure on crucial anterior structures such as the esophagus. With an average distance in the midline of 1.02 to 1.31 mm between the cervical spine and the esophagus, the vast majority of cervical plates available today cause resting pressure on the esophagus. This could potentially contribute to dysphagia. It is anticipated that future hardware designs can incorporate these findings and limit hardware profiles in the midline. By preserving the natural course of the esophagus, it would be anticipated that the incidence of dysphagia could be limited. Much like anterior cervical fusion plates, cervical disc replacements that incorporate anterior flanges or screw fixation may benefit from similar considerations. Given the maintenance of motion with cervical disc replacement and potential frictional effects of hardware on the esophagus, our findings may be even more pertinent to these devices.
The limitations of our study include the fact that we used “normal” patients for analysis. Surgical candidates can often have significant anterior cervical osteophytes and may even present with antero- or retrolisthesis at one or more levels. The osteophytes are typically removed at surgery in order to fully decompress the disc space and to accommodate hardware. These osteophytes are produced over years of the degenerative process, and given that extended time period the surrounding structures have time to accommodate to the mass effect that is created. Hence these osteophytes may not contribute to dysphagia in the un-operated cervical spine. Only after there has been surgical trauma and the edition of a plate do the symptoms of dysphagia manifest. Since osteophyte removal is necessary for plate application, they should not contribute to the ultimate relationship that would be found between the esophagus and the cervical spine following ACDF. Rather our findings, recorded from “normal” patients, would be applicable.
The CT scans were obtained in the supine position. It remains unknown if the position of the esophagus relative to the cervical spine changes in an upright position. This appears unlikely as the supine position via gravity, directs more of a posterior force on the esophagus. Lastly, our study was limited to the levels C5 through C7. Findings at other levels will require further study and cannot be directly extrapolated from this report.
One interesting finding observed in this study, although not specifically measured quantitatively, was the anatomic variability of the esophagus as it descends anterior to the cervical spine. In most cases, the esophagus proper becomes well defined at the level of C6. Above, the cricopharyngeus muscle marks the transition from the hypopharynx to the esophagus. There was some anatomic variance as to the exact level where this transition occurred. This too may contribute to the potential risk or predisposition that any particular patient has in developing dysphagia following ACDF.
Conclusions
In summary, the relationship between the esophagus and anterior cervical spine has been described from the level of the C5 vertebral body to the C7 vertebral body. The esophagus lies closest to the vertebral bodies and disc spaces in the midline. A potentially usable space exists between the esophagus and cervical spine at the medial edge of the longus colli muscle bilaterally, right greater than left. Future implant designs will hopefully utilize the relationship of the esophagus to the anterior cervical spine to reduce the incidence of post-operative dysphagia.
References
Bailey RW, Badgley CE (1960) Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg Am 42-A:565–594
Baron EM, Soliman AM, Gaughan JP et al (2003) Dysphagia, hoarseness, and unilateral true vocal cord impairment following anterior cervical diskectomy and fusion. Ann Otol Rhinol Laryngol 112(11):921–926
Bazaz R, Lee MJ, Yoo JU (2002) Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine 27(22):2453–2458
Bohlman HH, Emery SE, Goodfellow DB et al (1993) Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 75-A:1298–1307
Cloward RB (1959) Vertebral body fusion for ruptured cervical discs. Am J Surg 98:722–727
Drompp BW, Siebert WE, Fulgenzi WR (1964) Early stabilization of certain fractures and fracture-dislocations of the cervical spine. Experiences with interbody fusion via the anterior approach. Clin Orthop Relat Res 34:42–52
Fogel GR, McDonnell MF (2005) Surgical treatment of dysphagia after anterior cervical interbody fusion. Spine J 5(2):140–144
Frempong-Boadu A, Houten JK, Osborn B et al (2002) Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech 15(5):362–368
Gore DR, Sepic SB (1998) Anterior discectomy and fusion for painful cervical disease. A report of 50 patients with an average follow-up of 21 years. Spine 23:2047–2051
Karaikovic EE, Yingsakmongkol W, Griffiths HJ et al (2002) Possible complications of anterior perforation of the vertebral body using cervical pedicle screws. J Spinal Disord Tech 15(1):75–78
Lee MJ, Bazaz R, Furey CG et al (2005) Influence of anterior cervical plate design on dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 18(5):406–409
Riley LH 3rd, Skolasky RL, Albert TJ et al (2005) Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine 30(22):2564–2569
Smith GW, Robinson RA (1958) The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 40-A(3):607–624
Smith-Hammond CA, New KC, Pietrobon R et al (2004) Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine 29(13):1441–1446
Yue WM, Brodner W, Highland TR (2005) Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5 to 11 year follow-up study. Eur Spine J 14(7):677–682
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhyne , A.L., Spector, L.R., Schmidt, G.L. et al. Anatomic mapping and evaluation of the esophagus in relation to the cervical vertebral body. Eur Spine J 16, 1267–1272 (2007). https://doi.org/10.1007/s00586-007-0339-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-007-0339-5