Abstract
Thoracic ossification of ligamentum flavum (OLF) caused by skeletal fluorosis is rare. Only six patients had been reported in the English literature. This study reports findings from the first clinical series of this disease. This was a retrospective study of patients with thoracic OLF due to skeletal fluorosis who underwent surgical management at the authors’ hospital between 1993 and 2003. Diagnosis of skeletal fluorosis was made based on the epidemic history, clinical symptoms, radiographic findings, and urinalysis. En bloc laminectomy decompression of the involved thoracic levels was performed in all cases. Cervical open door decompression or lumbar laminectomy decompression was performed if relevant stenosis was present. Neurological status was evaluated preoperatively, at the third day postoperatively, and at the end point of follow-up using the Japanese Orthopaedic Association (JOA) scoring system of motor function of the lower extremities. A total of 23 cases were enrolled, 16 (69.6%) males and 7 (30.4%) females, age ranging from 42 to 72 years (mean 54.8 years). All patients came from a high-fluoride area, and 22 (95.7%) had dental fluorosis. Medical imaging showed OLF together with ossification of many ligaments and interosseous membranes, including interosseous membranes of the forearm (18/23 patients 78.3%), leg (14/23 patients 60.9%), and ribs (11/23 patients 47.8%). OLF was classified into five types based on MRI findings: localized (4/23 patients 17.4%), continued (12/23 patients 52.2%), skip (3/23 patients 13.0%), combining with anterior pressure (2/23 patients 8.7%), and combining with cervical and/or lumbar stenosis (2/23 patients, 8.7%). Urinalysis showed a markedly high urinary fluoride level in 14 of 23 patients (60.9%). Patients were followed up for an average duration of 4 years, 5 months. Paired t-test showed that the JOA score was slightly but nonsignificantly increased relative to preoperative measurement 3 days after surgery (P = 0.0829) and significantly increased at the end of follow-up (P = 0.0001). In conclusion, Fluorosis can cause ossification of thoracic ligamentum flavum, as well as other ligaments. Comparing with other OLF series, a larger number of spinal segments were involved. The diagnosis of skeletal fluorosis was made by the epidemic history, clinical symptom, imaging study findings, and urinalysis. En bloc laminectomy decompression was an effective method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thoracic spinal stenosis, in contrast to stenosis of the cervical and lumbar spine, is rare. The condition may be congenital or acquired. Congenital thoracic spinal stenosis resulting in myelopathy has been demonstrated to a limited extent in patients with skeletal disorders. Acquired thoracic spinal canal stenosis related to disc degeneration, osteophytes, or hypertrophy of the posterior spinal elements has been reported in numerous case reports [1, 2, 7–8, 13, 22, 25, 28, 33, 35, 43] and clinical series [3, 5, 10, 15, 17–18, 20–21, 23–24, 27, 30–32, 34, 36–39, 45]. Of the posterior spinal elements that might be implicated in acquired thoracic spinal canal stenosis, ossification of the ligamentum flavum (OLF) has been suggested as the most common contributing factor [30].
Fluoride is an important factor in bone mineralization. Fluoride stimulates the osteogenetic process, resulting in an increase in bone mass [6]. However, excessive fluoride intake may cause fluoride intoxication—fluorosis [4, 9, 11–12, 16, 19, 29, 40–42, 44]. Typical clinical features of fluoride intoxication include dental fluorosis, diffuse densification of bone, and calcification of bony insertions of many ligaments, discs, and interosseous membranes (i.e., interosseous membranes of the ribs, forearm, and leg; posterior longitudinal ligament; transverse atlantal ligament; ligamentum flavum; and membrana obturatoria) [40–42]. Rarely, skeletal fluorosis may cause thoracic OLF; six cases have been reported in the English literature [9, 22]. No large-sample clinical series of such cases has been reported in the English literature.
The purpose of this study was to describe the clinical features of thoracic OLF caused by skeletal fluorosis and evaluate the en bloc decompression method, and prognosis.
Materials and methods
Study population
Seventy-four thoracic OLF patients who underwent surgical management in the authors’ hospital between 1993 and 2003 were retrospectively studied. The diagnostic criteria for thoracic OLF were: numbness in the lower limbs and below the relative spinal cord segment, motor weakness in the lower extremities and difficulty walking, and increased patellar and Achilles reflexes and lower limb muscle tension upon physical examination. X-ray, Computed Tomography (CT), and magnetic resonance imaging (MRI) were used to confirm the diagnosis in each patient.
Based on the following diagnostic criteria, 23 patients were determined to have OLF caused by fluorosis. There were 16 (69.6%) male and 7 (30.4%) female patients, mean age 54.8 years (range 42–72 years). The diagnostic criteria for fluorosis were [44]: (1) habitation in an endemic fluorosis region since birth, (2) dental fluorosis, (3) regular consumption of water with fluoride levels above 1.2 ppm, and (4) urine fluoride level above 1.5 mg/l. Other clues used for diagnosis were X-ray findings of diffuse densification of bone and calcification of bony insertions of many ligaments, discs, and interosseous membranes (i.e., interosseous membranes of the ribs, forearm, and leg).
Imaging study
To better describe the imaging feature of thoracic OLF caused by fluorosis, serial imaging study were performed in every patient. A–P- and lateral-view X-rays of the thoracic spine were taken, followed by thoracic MRI to verify the diagnosis and identify the involved segments, and CT scan of the involved segments. An A–P- and lateral-view X-ray of the forearms and legs and an A–P-view X-ray of the chest were also taken.
Surgical procedure
En bloc decompression was performed for each patient, at the segments shown in Table 2. In the cases of thoracic OLF combined with cervical ossification of the posterior longitudinal ligament, cervical open door decompression was additionally performed. In the cases of thoracic OLF combined with lumbar stenosis, lumbar laminectomy decompression was additionally performed.
Preoperative radiographic localization with a Kirschner wire was used to confirm the operative level on the morning of the operation. After induction of general anesthesia, the patient was placed prone with an indwelling bladder catheter. The abdomen was decompressed to avoid excessive epidural bleeding. A midline incision was made at the appropriate level (based on the X-ray localization) and extended to the fascia. Subperiosteal dissection of the paraspinal muscles was performed using electrocautery. Rongeurs were used to shorten (not totally remove) the spinous processes. The laminectomy was performed with a high-speed drill (Fig. 1a). The width of the laminectomy was approximately one third the size of the inside of the facet. After the laminae were floated entirely, they were removed en bloc by grasping the residual spinous processes (Fig. 1b, c).
a Anteroposterior view radiograph of both forearms showed significant calcifications of interosseous membranes of forearm. b Anteroposterior view radiograph of both legs showed significant calcifications of interosseous membranes of leg (highlighted by the arrows). c Thoracic anteroposterior view radiograph showed diffuse densification of thoracic vertebral bodies and calcifications of interosseous membranes of ribs (highlighted by the arrows)
Follow-up evaluation
Patients were followed up for a mean duration of 4 years, 5 months (range 12 months–9 years, 3 months). Neurological status was evaluated preoperatively, at the third day postoperatively, and at the end point of follow-up using the Japanese Orthopaedic Association (JOA) scoring system of motor function of the lower extremities (Table 1) [24]. X-ray and CT scan were performed 3 days after the operation to confirm the decompression levels and decompression area. X-ray was performed at the end of the follow-up to investigate the presence of spinal instability.
Statistical analysis
Differences in results before and after surgery were analyzed by paired t-test using statistical analysis system (SAS) software. Significance was set at P < 0.05.
Results
Clinical presentation
In 17 of the 23 patients (73.9%) the most common initial symptom was numbness in the lower limbs and below the relative spinal segment. In six patients (26.1%) the initial symptoms were motor weakness in the lower extremities and difficulty walking. Physical examination showed increased muscle tension in 20 patients (87.0%), numbness in the lower limbs in 21 patients (91.3%), motor weakness in 8 patients (34.8%), increased patellar reflex in 3 patients (13.0%), increased patellar and Achilles reflexes in 18 patients (78.3%), and decreased patellar and Achilles reflexes in 2 patients (8.7%). All 23 patients had lived in a high-fluoride area for a long period of time. Excess fluoride intake from water was verified for 21 patients (91.3%) and excess fluoride intake from coal smoke was found for 2 cases (8.7%). Twenty-two of 23 patients (95.7%) had some level of dental fluorosis. Urinalysis showed markedly high urinary fluoride in 14 of 23 patients (60.9%).
Preoperative imaging results
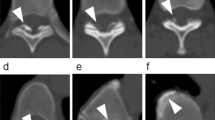
In 8 of 23 patients (34.8%) the OLF showed obscuring of the laminal margin on the A–P-view X-ray. In 12 of 23 patients (52.2%) high density projection into the spinal canal at the level of compression was found using lateral-view X-ray (Fig. 2). CT scans revealed that every patient’s OLF had the density of cortical bone; sometimes there was a thin gap between the laminae (Fig. 4b). T2-weighted MR images demonstrated every patient’s OLF as triangular protrusion, with a low signal intensity resembling cortical bone (Figs. 3, 4a, 5, 6, 7a, 8).
The 23 patients were classified into 5 types based on the MRI findings. Four patients (17.4%) were classified as having localized type, only one single segment was involved (Fig. 3). Twelve patients (52.2%) were classified as having a continuous type, more than one continuous segments were involved (Fig. 4a). Three patients (13.0%) were classified as having skip type, there are normal segments between two parts of involved segments (Fig. 5). Two patients (8.7%) were classified as having OLF combining with anterior pressure (Fig. 6). An additional two patients (8.7%) were classified as having OLF combining with cervical and/or lumbar stenosis (Figs. 7a, b, 8).
X-ray of the forearms, legs, and chest showed ossification of the interosseous membranes of the forearm in 18 of 23 patients (78.3%) (Fig. 9a), ossification of the interosseous membranes of the leg in 14 of 23 patients (60.9%) (Fig. 9b), and ossification of the interosseous membranes of the ribs in 11 of 23 patients (47.8%) (Fig. 9c).
a Inter operation picture showed en bloc method for laminectomy with a high speed drill. b Inter operation picture showed the dura mater was totally decompressed. c The en bloc removed lamina, note the nodular ossified ligamentum flavum. d Postoperative CT scan showed sufficient decompression, note the lateral two thirds of the facet joint was preserved and the ossified dura mater was partly left
Surgery and prognosis
Surgical duration ranged from 2.5 to 4.3 h (mean 3.2 h). Blood loss ranged from 400 to 2,800 ml (mean 850 ml). Dura mater rupture occurred in four patients. Deep infection occurred in one patient.
No patient experienced postoperative neurological deterioration. The JOA score was increased slightly 3 days after the operation relative to before surgery, but the increase did not reach statistical significance (P = 0.0829). However, the JOA score at the end of follow-up was increased significantly compared to the preoperative determination (P = 0.0001). The detail was shown in Table 2. There was no postoperative instability (Fig. 10a, b).
Discussion
Etiology
Thoracic OLF was first reported by Polgar [28] in 1920 based on lateral radiographs. Since then, hundreds of cases of thoracic OLF have been reported from several clinical series and numerous case reports [1–3, 5, 10, 15, 17–18, 20–25, 27–28, 30–39, 43, 45]. However the etiology of OLF is unclear. As most OLF cases had involved T9 and T12, Barnett et al. [2] suggested that hypermobility of the lower thoracic spine might promote degeneration and canal stenosis. Liao [18] found high prevalence of anterior osteophytes co-occuring with herniated intervertebral disc at the symptomatic OLF segments and concluded that OLF might be a degenerative response to microinjury of the ligamentum flavum. This hypothesis is histologically supported by Okada and colleagues [24] who found that OLF formed in the hypertrophic ligamentum flavum with fibrocartilage proliferation. This was thought to be a form of mechanical injury; therefore, it was theorized that OLF might develop secondary to the specific fiber reconstruction of the ligamentum flavum in response to mechanical stress. However, Muthukumar [22] recently reported two cases of OLF caused by fluorosis, and Wang et al. [40–42] reported that fluorosis could cause ossification of numerous ligaments. These reports provide evidence that fluorosis could play a role in OLF.
Fluoride is one of the necessary minor elements in humans, and the daily requirement is 0.05–0.07 mg/kg body weight per day [4, 16]. The benefits of water fluoridation in controlling dental caries are well documented. Fluoride was first added to water for this purpose in 1945 and 1946, in the United States [6] and Canada [11], respectively. However, excess fluoride intake causes fluorosis [4, 9, 11–12, 16, 19, 29, 41–42, 44]. Fluorosis caused by fluoride intoxication was first reported by Feil in 1930, and skeletal fluorosis was reported by Short in 1937 [29]. There are two common sources of excess fluoride intake: water and coal smoke. In high-fluoride areas, where the density of fluoride in water is greater than 5–8 mg/l and people drink directly from a well, dental fluorosis, skeletal fluorosis, and even systemic fluorosis develop.
It has been reported that neurological complications occur in approximately 10% of patients with skeletal fluorosis, usually in the later stages of the disease [29]. Myeloradiculopathy caused by skeletal fluorosis was thought to be a result of compression of the spinal cord by osteophytes and vertebral osteosclerosis [12, 29]. However, myelopathy caused by OLF in patients with skeletal fluorosis has recently been recognized [9, 22]. Thus, fluorosis should be considered a potential contributing factor for OLF, especially in patients from high-fluoride areas.
The pathogenesis of ossification of the ligaments in OLF remains speculative. High expression of transforming growth factor beta-1 (TGF-β1) by fibroblasts was found in the ossified matrix within ossified ligaments and in chondrocytes within cartilaginous areas adjacent to the ossified ligaments [26]. TGF-β1 may play a role in chondroid metaplasia and ectopic ossification in OLF. Recent experimental evidence suggests the involvement of proto-oncogenes c-fos and c-jun in skeletal fluorosis. Zhang et al. [46] have demonstrated that exposure to excessive fluoride can stimulate the activation and proliferation of osteoblast-like cells, enhancing expression of messenger ribonucleic acid and c-fos and c-jun proteins.
Distinctive features of OLF caused by fluorosis
Thoracic OLF is rare and usually asymptomatic [14]. The disease usually has an insidious onset and very slow progression. Epidemiological data show that thoracic OLF most commonly involves the vertebrae between T9 and T12, where greater mobility and vulnerability (due to spinal motion) may result in frequent mechanical injury. Although the neurological findings in our series are similar to other authors’ findings, we observed unique features of OLF caused by fluorosis. First, all patients in our series had the characteristic features of fluorosis, including dental fluorosis; diffuse densification of bone; and calcification of bony insertions of many ligaments, discs, and interosseous membranes [40–42]. Second, a larger number of spinal segments were involved. In Shiokawa’s series [32], OLF was located between T9 and T12 in 27 of 31 patients (87%); in only 4 patients was OLF located outside of this range. In two patients coexistent OLF was also located between T9 and T12. In 26 of 31 patients the OLF was located in one or two levels. OLF involved three segments in one patient and four segments in another patient. Three of 31 patients had skip type; 2 of these cases involved single segments, and one involved multiple segments. However, in our series, only four cases involved single segments; one involved two segments, six involved three segments, two involved four segments, two involved five segments, and one involved six segments. All three cases of skip-type OLF involved multiple segments: one involved T3–7 and T10–L1, one involved T1–T5 and T9–L1, and one involved T1−6 and T9–L1. The two cases of combining-type OLF also involved multiple segments: C7–T9 and T8–T12. Two of the cases even involved the cervical and lumbar region.
Operation and prognosis
A nonoperative treatment method is not effective for symptomatic patients with OLF [3, 5, 10, 15, 17–18, 20–21, 23–24, 27, 30–31, 32, 34, 36–39, 45]. Rather, early diagnosis and operative intervention are recommended. As thoracic OLF compresses the spinal cord posteriorly, several posterior decompression methods have been developed. These operative techniques include open-door laminectomy, en bloc laminectomy, fenestration, total decompression, and others [23–24, 37–39]. In our series, all patients underwent en bloc decompression, which our results show to be effective. Blood loss was much greater than that for our non-fluorosis cases (unpublished data). This is partly due to the fact that fluorosis makes the soft tissue vulnerable to bleeding, and partly because there were a greater number of decompression segments.
In four cases, ossification of the dura mater occurred and required repair. Other investigators have also reported ossification of the dura mater together with ossification of the thoracic ligamentum flavum [23]. In such cases, severe adhesion between the OLF and dura mater may occur. Great attention must be paid to avoid rupturing the dura mater. However, we did not always remove the ossified ligament completely, but rather floated it and abraded it as thinly as possible with a high-speed drill (Fig. 1d).
Okada et al. [24] reported that the en bloc method may induce postoperative spinal instability and expressed a preference for an open-door method. However, many authors have reported the en bloc method to be safe and effective, with no postoperative spinal instability [27, 32]. All patients in the present study underwent posterior thoracic laminectomy to remove the intruding ossified lesion. Efforts were made to preserve the lateral two thirds of the facet joints in order to maintain segmental stability, and no postoperative instability was observed. Thus, the key factor seems to be preservation of the lateral half of the facet. In addition, fluorosis makes the spine more rigid, decreases movement, and lessens the possibility of postoperative instability.
In conclusion, fluorosis can cause ossification of the thoracic ligamentum flavum, as well as other ligaments. Comparing with other OLF series, a larger number of spinal segments were involved. In this patient series, skeletal fluorosis was diagnosed based on epidemic history, clinical symptoms, medical imaging, and urinalysis. We found en bloc laminectomy decompression an effective method for treating OLF caused by fluorosis.
References
Akhaddar A, Mansouri A, Zrara I, Gazzaz M, Maftah M, Mostarchid B, Benomar S, Boucetta M (2002) Thoracic spinal cord compression by ligamentum flavum ossifications. Joint Bone Spine 69:319–323
Barnett GH, Hardy RW, Little JR, Bay JW, Sypert GW (1987) Thoracic spinal canal stenosis. J Neurosurg 66:338–344
Ben Hamouda K, Jemel H, Haouet S, Khaldi M (2003) Thoracic myelopathy caused by ossification of the ligamentum flavum: a report of 18 cases. J Neurosurg 99:157–161
Browne D, Whelton H, O’Mullane D (2005) Fluoride metabolism and fluorosis. J Dent 33:177–186
Chang UK, Choe WJ, Chung CK, Kim HJ (2001) Surgical treatment for thoracic spinal stenosis. Spinal Cord 39:362–369
Dean HT, Arnold FA, Jay P, Knutson JW (1950) Studies on mass control of dental caries through fluoridation of the public water supply. Public Health Rep 65:1403–1408
Enomoto H, Kuwayama N, Katsumata T , Doi T (1988) Ossification of the ligamentum flavum. A case report and its MRI finding. Neuroradiology 30:571–573
Farooq MT, Chao T, Bennett M (1996) Paraplegia following post-traumatic thoracic spinal stenosis: a case report. Arch Phys Med Rehabil 77:84–85
Gupta RK, Agarwal P, Kumar S, Surana PK, Lal JH, Misra UK (1996) Compressive myelopathy in fluorosis: MRI. Neuroradiology 38:338–342
Hanakita J, Suwa H, Ohta F, Nishi S, Sakaida H, Iihara K (1990) Neuroradiological examination of thoracic radiculomyelopathy due to ossification of the ligamentum flavum. Neuroradiology 32:38–42
Hutton WL, Linscott BW, Williams DB (1951) The Brantford fluorine experiment: interim report after five years of water fluoridation. Can J Public Health 42:81–87
Jain AP, Jajoo UN, Bhalla A, Chauhan NJ (1999) Cervical myelopathy due to fluorosis in non-endemic area of Vidarbha. J Assoc Physicians India 47:939
Kruse JJ, Awasthi D, Harris M, Waguespack A (2000) Ossification of the ligamentum flavum as a cause of myelopathy in North America: report of three cases. J Spinal Disord 13:22–25
Kudo S, Ono M, Russell WJ (1983) Ossification of thoracic ligamenta flava. AJR Am J Roentgenol 141:117–121
Kuh SU, Kim YS, Cho YE, Jin BH, Kim KS, Yoon YS, Chin DK (2005) Contributing factors affecting the prognosis surgical outcome for thoracic OLF. Eur Spine J (Epub ahead of print)
Levy SM, Kohout FJ, Guha-Chowdhury N, Kiritsy MC, Heilman JR, Wefel JS (1995) Infant’ s fluoride intake from drinking water alone, and from water added to formula, beverages and food. J Dent Res 74:1399–1407
Li KK, Chung OM, Chang YP, So YC (2002) Myelopathy caused by ossification of ligamentum flavum. Spine 27:E308–E312
Liao CC, Chen TY, Jung SM, Chen LR (2005) Surgical experience with symptomatic thoracic ossification of the ligamentum flavum. J Neurosurg Spine 2:34–39
Littleton J (1999) Paleopathology of skeletal fluorosis. Am J Phys Anthropol 109:465–483
Miyakoshi N, Shimada Y, Suzuki T, Hongo M, Kasukawa Y, Okada K, Itoi E (2003) Factors related to long-term outcome after decompressive surgery for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg 99:251–256
Miyasaka K, Kaneda K, Sato S, Iwasaki Y, Abe S, Takei H, Tsuru M, Tashiro K, Abe H, Fujioka Y (1983) Myelopathy due to ossification or calcification of the ligamentum flavum: radiologic and histologic evaluations. AJNR Am J Neuroradiol 4:629–632
Muthukumar N (2005) Ossification of the ligamentum flavum as a result of fluorosis causing myelopathy: report of two cases. Neurosurgery 56:E622
Nishiura I, Isozumi T, Nishihara K, Handa H, Koyama T (1999) Surgical approach to ossification of the thoracic yellow ligament. Surg Neurol 51:368–372
Okada K, Oka S, Tohge K, Ono K, Yonenobu K, Hosoya T (1991) Thoracic myelopathy caused by ossification of the ligamentum flavum. Clinicopathologic study and surgical treatment. Spine 16:280–287
Omojola MF, Cardoso ER, Fox AJ, Drake CG, Durward QJ (1982) Thoracic myelopathy secondary to ossified ligamentum flavum. J Neurosurg 56:448–450
Park JB, Chang H, Lee JK (2001) Quantitative analysis of transforming growth factor beta-1 in ligamentum flavum of lumbar spinal stenosis and disc herniation. Spine 26:E492–E495
Pascal-Moussellard H, Cabre P, Smadja D, Catonne Y (2005) Symptomatic ossification of the ligamentum flavum: a clinical series from the French Antilles. Spine 30:E400–E405
Polgar F (1920) Uber interakuelle wirbelverkalkung. Fortschr Geb Rontgenstrahlen Nuklearmed Erganzungsbd 40:292–298
Reddy DR (1996) Fluorosis. In: Textbook of neurosurgery. Churchill Livingstone, New Delhi pp 798–803
Sato T, Kokubun S, Tanaka Y, Ishii Y (1998) Thoracic myelopathy in the Japanese: epidemiological and clinical observations on the cases in Miyagi Prefecture. Tohoku J Exp Med 184:1–11
Seichi A, Nakajima S, Takeshita K, Kitagawa T, Akune T, Kawaguchi H, Nakamura K (2003) Image-guided resection for thoracic ossification of the ligamentum flavum. J Neurosurg 99(1 Suppl):60–63
Shiokawa K, Hanakita J, Suwa H, Saiki M, Oda M, Kajiwara M (2001) Clinical analysis and prognostic study of ossified ligamentum flavum of the thoracic spine. J Neurosurg 94:221–226
Shiraishi T, Crock HV, Lewis P (1995) Thoracic myelopathy due to isolated ossification of the ligamentum flavum. J Bone Joint Surg Br 77:131–133
Stollman A, Pinto R, Benjamin V, Kricheff I (1987) Radiologic imaging of symptomatic ligamentum flavum thickening with and without ossification. AJNR Am J Neuroradiol 8:991–994
Storey GR, Ridley L, Van Der Wall H (2001) Ossification of the ligamentum flavum demonstrated by Tc-99 m MDP SPECT imaging of the thoracic spine. Clin Nucl Med 26:538–540
Sugimura H, Kakitsubata Y, Suzuki Y, Kakitsubata S, Tamura S, Uwada O, Kodama T, Yano T, Watanabe K (1992) MRI of ossification of ligamentum flavum. J Comput Assist Tomogr 16:73–76
Tomita K (1990) Total decompression of the spinal cord for combined ossification of posterior longitudinal ligament and yellow ligament in the thoracic spine. Arch Orthop Trauma Surg 109:57–62
Tomita K, Baba H, Takahashi K (1989) Total (anterior and posterior) decompression of the spinal cord: surgical treatment for combined ossification of the posterior longitudinal ligament and yellow ligament of the thoracic spine. Nippon Seikeigeka Gakkai Zasshi 63:501–506
Tomita K, Kawahara N, Baba H, Kikuchi Y, Nishimura H (1990) Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine 15:1114–1120
Wang W, Jiang F, Zhao H, Dong R, Zhou J, Jia Z, Hu Y (2004) Ossification of the transverse atlantal ligament, diagnosis and therapy. Zhong Hua Gu Ke Zha Zhi 24:442–444
Wang W, Kong L, Zhao H, Jia Z (2004) Ossification of the transverse atlantal ligament associated with fluorosis. A report of two cases and review of the literature. Spine 29:E75–E78
Wang Y, Yin Y, Gilula LA, Wilson AJ (1994) Endemic fluorosis of the skeleton: Radiographic features in 127 patients. AJR Am J Roentgenol 162:93–98
Williams DM, Gabrielsen TO, Latack JT (1982) Ossification in the caudal attachments of the ligamentum flavum. An anatomic and computed tomographic study. Radiology 145:693–697
Yildiz M, Akdogan M, Tamer N, Oral B (2003) Bone mineral density of the spine and femur in early postmenopausal Turkish women with endemic skeletal fluorosis. Calcif Tissue Int 72:689–693
Yonenobu K, Ebara S, Fujiwara K, Yamashita K, Ono K, Yamamoto T, Harada N, Ogino H, Ojima S (1987) Thoracic myelopathy secondary to ossification of the spinal ligament. J Neurosurg 66:511–518
Zhang WL, Cui YN, Gao S, Zhang XY, Li GS (2003) Expression of proto-oncogenes c-fos and c-jun in osteoblasts activated by excessive fluoride. Zhonghua Yu Fang Yi Xue Za Zhi 37:246–250
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Kong, L., Zhao, H. et al. Thoracic ossification of ligamentum flavum caused by skeletal fluorosis. Eur Spine J 16, 1119–1128 (2007). https://doi.org/10.1007/s00586-006-0242-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-006-0242-5