Abstract
Background
The purpose of this prospective semi-randomised comparative study was to compare fusion rates, course of fusion, and occurrence of collapse and subsidence of autologous and allogenic bone grafts in instrumented anterior cervical fusion. The number of fused levels and the smoking status were investigated as potential factors influencing the bone-healing process. No similar prospective study on instrumented anterior cervical discectomy and fusion was found in the literature.
Methods
Seventy-nine consecutive patients were operated on using the Smith–Robinson technique with a single instrumentation system at one or two levels. Seventy-six cadaverous fibular bone grafts and 37 autologous iliac-crest bone grafts were inserted. All patients were followed up for at least 2 years.
Results
The radiographs obtained during the follow-up were analysed, and showed no statistical difference in fusion and collapse rate between autografts and allografts. Allografts showed significantly longer time to union. No case of graft migration was observed. No difference was found between fusion and collapse rate with respect to the number of fused levels in general, but greater time to union was seen in two-level fusions. When one- and two-level subgroups were compared, there was no evidence of any significant difference in fusion or collapse rates between autografts and allografts, and the healing process took longer in allogenic grafts. Smoking status did not alter any of the fusion or collapse rates, or the course of bone fusion.
Conclusions
This study demonstrates that allografts are suitable substitutes for autografts in instrumented ACDF. Prolonged time to union observed in allogenic bone grafts does not seem to be an important factor in instrumented procedures. Two-level grafting does not imply a significantly lower fusion rate, but longer time to union can be expected than with single-level instrumented procedures in both allograft and autograft subgroups. Our relatively small number of patients may not have been sufficient to decipher significant differences between smokers and non-smokers in the rate or course of fusion as previously reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cervical discectomy and fusion (ACDF) in the subaxial cervical spine as originally suggested by Badgley in 1939 and described by Robinson and Smith in 1955 [28] and Cloward in 1958 [8] has established itself as a method of treatment for patients with neural compression by disc material or osteophytes.
Although the interbody location of applied bone graft is a favourable healing environment, because of the relatively large surface area of subchondral cancellous bone combined with the compressive axial loads [19], the most common cause of technical failure of this procedure is non-union. Numerous published studies have shown considerable variation in the incidence of non-union. The lowest rates (3–20%) are reported in patients after single-level procedures [2, 8, 12, 16, 19, 22, 26, 27, 29, 30], while the incidence of pseudoarthrosis increases to 44% with a greater number of levels fused [4, 12, 16, 22, 26, 27, 30, 34, 35, 36, 38, 39].
The lower fusion rates have been attributed to the increased amount of graft material and number of interfaces that must be consolidated with multi-level surgery, as well as the increased compressive loads on the multiple graft sites resulting in micro-motions [35]. Although non-fusion is not typically associated with poor clinical outcomes, its significant role in postoperative morbidity is well documented [4, 5, 10, 14, 23].
Furthermore, a high complication rate (10–19%) associated with iliac crest bone graft harvesting, even in the hands of experienced spine surgeons [16, 37], has heightened interest in the use of alternatives to autologous bone grafting. The most common alternative materials with the longest experience in anterior cervical spine surgery are allogenic bone grafts, first employed by Cloward in the 1950s [9].
Allografts are considered to be highly osteoconductive [20], weakly osteoinductive and non-osteogenic [13, 21]. Therefore, fusion rates in allogenic grafting can be expected to be inferior to that of autologous bones. Indeed, some of the published studies report lower fusion rates in allografts, while others have shown healing rates similar to those of autografts. As several studies have been considered either inconclusive or contradictory [15], the present study was performed to determine the comparative fusion success with allogenic freeze-dried fibular bone graft and autologous iliac crest grafts in instrumented ACDF.
Little has been written on the influence of potential risk factors (e.g. cigarette smoking and the number of fused segments) on bone fusion or the capacity to achieve a solid osseous union. Hence, the second goal of this study was to determine whether smoking and the number of levels fused had any effect on the bone healing process of the aforementioned grafts.
Materials and methods
From February 1998 to March 2000, a total of 80 consecutive patients underwent instrumented ACDF in our department. The indications for surgery in all patients were spondylosis, cervical disc protrusion/prolapse, or both. Patients with a history of previous cervical spine surgery, severe osteoporosis or disease and/or medication potentially affecting the process of bone healing were excluded from the study.
All operations were carried out by six neurosurgeons from our department in a similar fashion. Anterolateral retropharyngeal approach through a right-sided horizontal skin crease incision was used, followed by a one- or two-level discectomy. Subsequent steps to prepare the disc space for grafting included osteophyte removal, posterior longitudinal ligament resection, removal of all endplate cartilage with a curette and, finally, perforation of the subchondral bone of the endplates.
These steps were carried out under direct visualisation through an operating microscope. Following distraction, bone grafts were inserted into the intervertebral space and the appropriate segments were fixed using monocortical non-locked screws and trapezoid plates (Aesculap, Tuttlingen, Germany). This technique of static plating was described in detail by Sonntag in 2001 [32] and Pitzen in 1999 [25] and was not significantly altered in this study.
Autologous grafts were harvested from the left anterior iliac crest using a low-speed oscillating saw and/or chisels. Tricortical grafts obtained by this technique were then adjusted to an appropriate size and shape and implanted into the disc space, spongious side first.
The allografts used were freeze-dried cadaverous fibular bone pieces that were supplied vacuum-sealed. When unpacked, they were soaked in normal saline with antibiotics and then fashioned with a high-speed drill before being inserted into the intervertebral space.
It was the patient who, following appropriate consultation with the surgeon, made an informed decision regarding the choice of graft. Both autologous and allogenic grafts were offered to all patients, and possible complications were explained. Patients were particularly warned about the possibility of local complications associated with autograft harvesting, including local pain, haematoma, fracture of the ilium, infection, hernia, iliohypogastric and ilioinguinal nerve injury, peritoneal perforation and cosmetic deformity. With regards to allogenic grafts, all patients were informed about the possible higher rates of non-union, delayed union, immunological reaction, infective disease transmission, graft fracture and subsidence.
All information was based on our long-term results at that time and on the evidence found in the literature. In cases where the patient did not express any preference, a coin was tossed to determine the method of fusion. This system of method selection does not represent true randomisation but can in our opinion increase the validity of the results.
Postoperatively, all patients wore a rigid cervical collar (Philadelphia) for 6 weeks. Collar-free periods were then progressively increased to achieve a complete removal by 8 weeks. By this stage, there was no restriction on patients’ everyday physical activity.
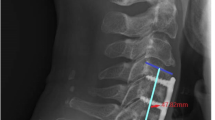
All preoperative, intraoperative and postoperative radiographs were assembled as well as clinical records in the form of a prospective protocol. Patients were seen at 10 days, 6 weeks, 3 months and 6 months after surgery. They were then seen annually for a minimum of 2 years. Bone fusion on the anteroposterior and lateral radiographs was defined according to the criteria described by Brown et al. [7], and graft collapse was assessed according to Zdeblick and Ducker’s proposal [41]. The graft subsidence was defined as any migration of the graft into the superior or inferior vertebral body [22] (Table 1, Fig. 1).
A case of solid bone fusion in allogenic bone graft 6 months after the C5/6 surgery. Bone trabeculae are bridging the intervertebral space. b A case of pseudoarthrosis in a patient 2 years after instrumented ACDF at the C5/6 level where allograft was inserted. A radiolucent gap persists between the bone graft and the end plate of the upper vertebra
The evaluation of postoperative radiographs was carried out together by the treating surgeon and the independent radiologist. The data were statistically analysed using the M-L chi-square test, and the level of statistical significance was set at 0.05 (a=0.05).
Prior to surgery and during the follow-up, all patients were questioned about their smoking status. Smokers were defined as patients with a smoking history or those describing occasional or habitual cigarette use. All the remaining patients were considered non-smokers as there were no cigar or pipe smokers.
Results
One out of 80 patients was lost during the 2-year follow-up as he decided not to attend the clinic. The remaining 79 patients included 30 women and 49 men (mean age 47.8 years, range 37–73 years). A total of 113 disc levels were operated upon using 76 allogenic bone grafts and 37 autologous bone grafts. The average duration of follow-up was 39.4 months (range 24–48 months). Three other patients operated on for degenerative disease of the cervical spine during the trial period were excluded from the study because of previously treated severe osteoporosis. Bicortical screw fixation was used in these patients.
In 35 out of 37 autologous grafts (94.6%) and 71 out of 76 allogenic grafts (93.4%) the radiological criteria for union were achieved. Three autologous grafts (8.1%) and three allogenic grafts (3.9%) significantly collapsed during the 2-year follow-up. There was no statistically significant difference in either the non-union rate or the collapse rate between the two groups (p=0.806 and p=0.369, respectively).
The allograft group had a significantly longer time to union (p<0.001). Six months after the operation, bone fusion was observed in 89.2% of autografts and 63.1% of allografts. At 3 months after surgery, this difference was even more pronounced: 64.9% of autologous bone grafts versus 25% of allografts. One year after surgery, the fusion rate difference was no longer statistically significant (94.6% of autografts and 85.5% of allografts) (Fig. 2). In this prospective study, no bone graft subsidence into the end plates of adjacent vertebrae was observed. There was also no case of graft migration.
The number of operated levels was evaluated as a potential factor influencing the bone union. Overall, no significant difference was observed in achieving solid bone fusion 2 years after surgery in one- and two-level procedures (95.6% vs. 92.6%, p=0.522). The graft collapse rate showed no significant difference (2.2% vs 7.6%, p=0.208) either. In the one-level group, the time to bone fusion was significantly shorter (p<0.001) (Fig. 3a).
General comparison of the fusion course between one- and two-level procedures. One-level procedures demonstrated shorter time to fusion. b Comparison of autologous and allogenic bone grafts that were inserted in one level. A trend towards earlier fusion can be seen in the autografts. c Comparison of the autograft and allograft fusion course in two-level procedures. Autologous bone grafts demonstrate a shorter time to bone union
The autograft and allograft groups were also pooled to compare one- and two-level operations. In single-level procedures, there was no significant difference in fusion rates (100% vs 93.3%, p=0.197) and graft collapse rates (0% vs 3.4%, p=0.365) between autografts and allografts. Similarly, in two-level procedures the differences were also insignificant: fusion rates of 90.9% vs 93.5% (p=0.709) and graft collapse incidence of 13.6% vs 4.3% (p=0.187) when comparing autografts and allografts, respectively. In both one- and two-level groups, the autologous bone grafts fused more readily (p<0.001) (Fig. 3b, c).
Altogether, 48 smoking patients with 66 operated levels and 31 non-smokers with 47 grafts finished the 2-year follow-up. When we compared the morphological results in these two subgroups of patients, we found that smoking did not significantly affect the fusion rate (95.7% in non-smokers vs 92.4% in smokers, p=0.461), the collapse incidence (8.5% vs 3%, p=0.203) or the readiness of the graft to produce the fusion (p=0.079). The fusion rate was not significantly changed by the ‘nicotinism’ in either the autologous grafts (5.3% in non-smokers vs 5.6% in smokers p=0.969), or the allografts (3.6% vs 8.3%, p=0.399) (Fig. 4).
Discussion
In their meta-analysis, Floyd and Ohnmeiss [15] found four published studies comparing autograft and allograft using the technique of Robinson and Smith from 1966 to 1997. All the studies reported a statistically insignificant difference between the fusion rates in these two groups of grafts. Zdeblick and Ducker’s study [41] was the only study to report a higher fusion rate in two-level autografts than in two-level allograft operations. Bishop et al. [3] were the only authors to report a significantly longer time to union in allografts. Both these studies documented significantly higher collapse rates in allografts. Brown et al. [7] reported a higher rate of multi-level allograft collapses as the only difference. Conversely, only An et al. [1] did not demonstrate any significant difference in fusion and collapse rates between autologous and allogenic bone grafts.
All these studies analysed the non-instrumented ACDFs, and there were no other comparative studies performed later with the Smith–Robinson technique. The results of our study mainly agree with the results of these studies. Like the other authors, we did not find any significant difference between autograft and allograft fusion rates. As far as we are aware, this is the first prospective comparative study on this topic in instrumented ACDFs.
This series was designed to be semi-randomised, and our system of graft selection certainly carries a risk of selection bias. True randomisation would have definitely brought more statistical significance to our study—but at that time it was not acceptable to our ethics committee. Hence, the chosen method of patient selection led to unequal numbers in the cohorts. Nonetheless, we felt that the study could proceed and provide valuable data for statistical analysis.
A review of the literature reveals a wide disparity in fusion rates. One reason for this discrepancy could be the way in which fusion is determined. It is clear that no gold-standard method of assessment exists for determining arthrodesis in the cervical spine. Radiological modalities such as MRI, reconstructed CT images, classical tomography or bone scans may be reliable enough to determine the degree of fusion. However, these methods are either quite expensive, impose a high degree of unnecessary ionising radiation on the patient or are simply impossible due to implanted metals in instrumented fusions. It would therefore be desirable to establish plain radiological criteria for the assessment of fusion after instrumented ACDF. The adoption of uniform criteria is critical for designing prospective studies and also for routine patient follow-up.
The static plating system used in our study precludes a segmental motion in flexion–extension views [25]. This renders dynamic radiological evaluation inappropriate in this study, despite the fact that it is widely accepted as a very useful form of evaluation of the degree of intervertebral fusion.
There seems to be increasing evidence in the literature suggesting that cigarette smoking has an adverse effect on bone metabolism and fusion. It is well documented in laboratory studies that nicotine both inhibits re-vascularisation of bone grafts [11] and impairs osteoblast function [33]. These mechanisms are thought to be the pathophysiological mechanisms responsible for defective bone healing.
A significantly higher incidence of non-union after lumbar postero-lateral intertransverse fusion was documented by an in vivo experiment in rabbits that received nicotine [31]. Results of clinical observations attempting to demonstrate such a correlation between smoking and non-union are variable. Some of the studies even describe a three- to fourfold relative risk of pseudoarthrosis in smokers [6, 17, 18], while others failed to find any statistically significant difference [5].
In our study, the morphological outcome was not significantly poorer in smokers. In agreement with Hilibrand et al. [18], we believe that the negative impact of smoking on graft healing is additive with other factors, such as multi-level interbody grafting, the use of allograft bone, interbody grafting adjacent to a solid fusion and alcohol consumption. We did not have sufficient clinical material to prove this theory statistically.
Accurate evidence of risk factors and their relative risk to the bone healing process is, in our opinion, important information since modalities that accelerate and stimulate bone fusion could be used. These adjuvant approaches include growth factors from the BMP family, physical therapy, i.e. low-intensity pulsed ultrasound [24] and electrical stimulation, or (in the near future) gene therapy [40] or bone stem cell transplantation.
Conclusions
This study demonstrates no statistically significant difference in fusion and collapse rate between autologous and allogenic bone grafts in instrumented ACDF. In our relatively small number of patients we found a significantly greater time to union in allografts. At 3-month follow-up, the radiological criteria of bone fusion were observed in 64.9% of autologous bone grafts and 25% of allografts. At 6 months, the figures had increased to 89.2% versus 63.1%. One year after surgery, the difference in fusion rates was no longer statistically significant (94.6% of autografts and 85.5% of allografts).
The number of levels fused altered neither the rates of solid bone fusion nor those of collapse. A significantly longer time to fusion was seen in two-level procedures than in one-level discectomies. In one- and two-level subgroups, no significant difference was seen between patients who received autografts and those with allografts with respect to their fusion and collapse rates. In both subgroups the healing process took longer in allogenic bone grafts. Neither the autografts nor the allografts were significantly affected by smoking, and all analysed results were similar for both smokers and non-smokers.
In instrumented ACDFs, allogenic bone grafts can be expected to result in fusion rates similar to those seen in autografts. The significantly greater time to union observed in patients receiving allografts does not seem to be of significant importance in instrumented cervical fusion. Nonetheless, many factors must be considered in the decision-making process prior to surgery. These include risks of infectious agent transmission (in fact very low), donor site morbidity, previous autograft harvests, osteoporosis and, last but not least, the preferences of a well-informed patient.
References
An HS, Simpson JM, Glover JM, Stephany J (1995) Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine 20:2211–2216
Aronson N, Fitzer DL, Bagan M (1968) Anterior cervical fusion by the Smith–Robinson approach. J Neurosurg 29:396–404
Bishop RC, Moore KA, Hadley MN (1996) Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg 85:206–210
Bohlman HH, Emery SE, Goodfellow DB, Jones PK (1993) Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 75:1298–1307
Brodsky AE, Khalil MA, Sassard WR, Newman BP (1992) Repair of symptomatic pseudoarthrosis of anterior cervical fusion. Posterior versus anterior repair. Spine 17:1137–1143
Brown CW, Orme TJ, Richardson HD (1986) The rate of pseudoarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine 11:942–943
Brown MD, Malinin TI, Davis PB (1976) A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop 119:231–236
Cloward RB (1958) The anterior approach for removal of ruptured cervical disc. J Neurosurg 15:602–617
Cloward RB (1980) Gas-sterilized cadaver bone grafts for spinal fusion operations. A simplified bone bank. Spine 5:4-10
Conolly ES, Seymour R, Adams JE (1965) Clinical evaluation of anterior cervical fusion for degenerative disc disease. J Neurosurg 23:431–437
Daftari TK, Whitesides TE Jr, Heller JG, Goodrich AC, McCarey BE, Hutton WC (1994) Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine 19:904–911
DePalma AF, Rothman RH, Lewinnek GE, Canale ST (1972) Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet 134:755–758
Erbe EM, Marx JG, Clineff TD, Bellincampi LD (2001) Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur Spine J 10:S141–146
Farey ID, McAfee PC, Davis RF, Long DM (1990) Pseudoarthrosis of the cervical spine after anterior arthrodesis. Treatment by posterior nerve-root decompression, stabilization and arthrodesis. J Bone Joint Surg Am 72:1171–1177
Floyd T, Ohnmeiss D (2000) A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J 9:398–403
Gore DR, Sepic SB (1984) Anterior cervical fusion for degenerated or protruded disc. A review of one hundred forty-six patients. Spine 9:667–671
Hanley EN Jr, Levy JA (1989) Surgical treatment of isthmic lumbosacral spondylolisthesis. Analysis of variables influencing results. Spine 14:48–50
Hilibrand AS, Fye SE, Emery SE, Palumbo MA, Bohlman HH (2001) Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg Am 83-A:668–673
Khan SN, Sama A, Sandhu HS (2001) Bone graft substitutes in spine surgery. Curr Opin Orthop 12:216–222
Lind M, Bunger C (2001) Factors stimulating bone formation. Eur Spine J 10:S102–109
Marchesi DG (2000) Spinal fusion: bone and bone substitutes. Eur Spine J 9:372–378
Martin GJ Jr, Haid RW Jr, MacMillan M, Rodts GE Jr, Berkman R (1999) Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine 24:852–858
Newman M (1993) The outcome of pseudoarthrosis after cervical anterior fusion. Spine 18:2380–2382
Nolte PA, van der Krans, Patka P, Janssen IM, Ryaby JP, Albers GH (2001) Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma 51:693–702
Pitzen T, Wilke HJ, Caspar W, Claes L, Steudel WI (1999) Evaluation of a new monocortical screw for anterior cervical fusion and plating by a combined biomechanical and clinical study. Eur Spine J 8:382–387
Riley LH Jr, Robinson RA, Johnson KA, Walker AE (1969) The results of anterior interbody fusion of the cervical spine: review of ninety-three consecutive cases. J Neurosurg 30:127–133
Rish BL, McFadden JT, Penix JO (1976) Anterior cervical fusion using homologous bone grafts: a comparative study. Surg Neurol 5:119–121
Robinson RA, Smith GW (1955) Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull John Hopkins Hospital 96:223–224
Robinson RA, Walker AE, Ferlic DC, Wiecking DK (1962) The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg Am 44:1569–1587
Shapiro S (1996) Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg 84:161–165
Silcox DH, Daftari T, Boden SD, Schimandle JH, Hutton WC, Whitesides TE Jr (1995) The effect of nicotine on spinal fusion. Spine 20:1549–1553
Sonntag VK, Han PP, Vishteh AG (2001) Anterior cervical discectomy. Neurosurgery 49(4):909–912
Vernejoul MC de, Bielakoff J, Herve M, Gueris J, Hott M, Modrowski D, Kuntz D, Miravet L, Ryckewaert A (1983) Evidence for defective osteoblastic function. A role for alcohol and tobacco consumption in osteoporosis in middle-aged men. Clin Orthop 179:107–115
Wang JC, McDonough PW, Endow KK, Delamarter RB (2001) A comparison of fusion rates between single level cervical corpectomy and two level discectomy and fusion. J Spinal Disord 14:222–225
Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB (2001) Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine 15; 26(6):643–646
White AA, Southwick WO, Deponte RJ, Gainor JW, Hardy R (1973) Relief of pain by anterior cervical spine fusion for spondylosis. A report of sixty-five patients. J Bone Joint Surg Am 55:525–534
Whitecloud TS, LaRocca SH (1976) Fibular strut graft in reconstructive surgery of the cervical spine. Spine 1:33–43
Williams JL, Allen MB Jr, Harkess JW (1968) Late results of cervical discectomy and interbody fusion: some factors influencing the results. J Bone Joint Surg Am 50:277–286
Yonenobu K, Fuji T, Ono K, Okada K, Yamomoto T, Harada N (1985) Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine 10:710–716
Yoon ST (2001) Cytokines and gene therapy in spine fusion and its potential in cervical fusion. Curr Opin Orthop 12:251–256
Zdeblick TA, Ducker TB (1991) The use of freeze-dried allograft bone for anterior cervical fusions. Spine 16:726–727
Acknowledgement
The study was supported by the Czech Ministry of Health, grant no. ND/6892-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suchomel, P., Barsa, P., Buchvald, P. et al. Autologous versus allogenic bone grafts in instrumented anterior cervical discectomy and fusion: a prospective study with respect to bone union pattern. Eur Spine J 13, 510–515 (2004). https://doi.org/10.1007/s00586-003-0667-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-003-0667-z