Abstract
Monosodium glutamate (MSG) is a food additive that can be toxic to multiple organs of the body. It has been demonstrated that Garcinia kola seed has enormous health benefits and protects against exposure to toxicants. This study aimed to investigate the effect of Garcinia kola seed extracts on multi-organ toxicity in MSG-administered rats. Methanol and alkaloid-rich extracts of Garcinia kola seeds (GKME and GKAE, respectively) were prepared and phytochemically characterized using HPLC–DAD. Adult male Wistar rats received 2 g/kg MSG orally for 21 days with or without co-treatment with GKME, GKAE, quercetin, or nicotinic acid. Biochemical analyses were carried out on the plasma, kidney, heart, liver, and the striatum, hippocampus, and cortex of the brain of the rats. Histopathological analysis was also carried out on the tissues and organs. Intoxication with MSG caused marked negative alterations to the levels of liver function markers, kidney function markers, and the lipid profile. MSG administration also produced deleterious effects on biomarkers related to cardiac and neurologic function as echoed by increased atherogenic and coronary risk indices, and altered levels of lactate dehydrogenase, acetylcholinesterase and dopamine. Also, MSG-intoxication evoked oxidative damage in the kidney, heart, liver, and discrete brain regions. These biochemical findings were corroborated by histopathological observation which revealed abnormalities in the tissues of MSG-administered rats. The multiorgan deteriorative and oxidative damage, and the histopathological aberrations of MSG toxicity were mitigated by GKME and GKAE. These findings suggest that Garcinia kola is beneficial in ameliorating MSG-induced toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food additives are organoleptic seasoning and flavoring agents used globally to improve or enhance the taste of food. However, their safety is a major concern. An example is monosodium glutamate (MSG) which has been linked to toxic effects on multiple organs (Niaz et al. 2018; Nnadozie et al. 2019). (Niaz et al. 2018; Nnadozie et al. 2019).
Monosodium glutamate (MSG) is a salt of glutamic acid salt which plays important roles in many physiological processes in the body It is an excitatory amino acid (neurotransmitter), a source of energy for some tissues, and a precursor for glutathione synthesis (Freeman 2006). The daily average intake of MSG as a food additive has been assessed to be 0.3–1.0 g/day in the USA (Beyreuther et al. 2007), 0.6–2.0 g/day in the UK, and 0.3–1.0 in other parts of Europe (Rhodes et al. 1991); 1.5–3.0 g/day in Taiwan, 1.1–1.6 g/day in Japan, and 1.6–2.3 g/day in South Korea (Lee and Lee 1976); and 0.56–1.0 g/day in Nigeria (Unaeze 2010). The intake of MSG as a food additive is between 5 and 10% of the total daily glutamate intake from various dietary sources (Brosnan et al. 2014). However, there is an elevated plasma concentration of the absorbed free unbound glutamate after intestinal absorption of MSG, which does not happen when glutamate is gradually released from dietary protein and other food sources (Nnadozie et al. 2019), hence, toxicity ensues.
The use of MSG has reportedly been linked to neurotoxicity (Hazzaa et al. 2020a), nephrotoxicity (Ortiz et al. 2006; Sharma 2015), hepatoxicity (Sharma 2015), male and female reproductive malfunctions (Dong and Robbins 2015; Mondal et al. 2017), obesity (Araujo et al. 2017), and neoplastic cell growth and differentiation (Zhang et al. 2003). Several reports have demonstrated oxidative stress and altered activity of glutamate receptors as a major culprit in the etiology of metabolic and toxic effects of MSG (Farombi and Onyema 2006; Sharma 2015; Khalil and Khedr 2016; Niaz et al. 2018). Uncontrolled formation of free radicals (including generation of reactive oxygen species) and the altered endogenous antioxidant system results in oxidative stress, leading to deregulation of cellular functions and the development of various disease conditions.
Medicinal plants are rich in phytochemical compounds that act as antioxidants and can safely interact with altered redox reactions that cause the damage of biomolecules (Cock 2015), such as in MSG toxicity. Garcinia kola Heckel, also known as bitter kola, is a member of the Clusiaceae family found throughout West and Central Africa. All the parts of the plant have been reported to be useful ethnomedicinally. The seed, leaves, and bark are used in folklore medicine for the treatment of fever, gastric, inflammatory, brain, and liver disorders (Maňourová et al. 2019). The most studied part of the plant is its seed because of its numerous medicinal and biological activities. The most abundant phytochemicals detected in G. kola seeds are flavonoids, saponins, tannins, phenols, glycosides, and alkaloids (Maňourová et al. 2019). Kolaviron (KV), a biflavonoid complex, is the most studied flavonoid in G. kola seeds. KV has been demonstrated to possess antioxidant, anti-inflammatory, anti-ischemic, anti-hepatotoxic, and anti-diabetic properties (Abarikwu 2014; Oyenihi et al. 2015; Ojo et al. 2019). Alkaloids, which are among phytochemicals found in G. kola seeds, have a wide range of pharmacological activities including antioxidant, anti-inflammatory, anti-cholinergic, anti-tumor, anti-microbial, anticancer, analgesic, anti-ulcerative, and antihyperglycemic activities (Pradeep and Kuttan 2004; De Almeida et al. 2017). This study evaluated the protective effect of methanol crude and alkaloid-rich extract of G. kola seeds on MSG-induced tissue toxicity in rats.
Materials and methods
Chemicals
Reduced glutathione (GSH), ammonium molybdate, glutamic acid, sodium potassium tartrate, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB), sodium pyruvate, bovine serum albumin (BSA), adenosine triphosphate (ATP), perchloric acid (PCA), reduced nicotinamide adenine dinucleotide (NADH), β-nicotinamide adenine dinucleotide phosphate reduced (NADPH), ethylenediaminetetraacetic acid (EDTA), 2,4-dinitrophenyl hydrazine (DNPH), epinephrine, sulfosalicylic acid (SSA), acetylcholine iodide, and 2,4,5-tripyridyl-s-triazine (TPTZ) were obtained from Sigma-Aldrich (St-Louis, MO, USA). All other chemicals and reagents used were of analytical grade. Monosodium glutamate (C5H9NO4-Na, 99% purity) was obtained from a store in Akure, Nigeria under the brand name VEDAN© by VEDAN International (Holdings) Limited, Vietnam. Commercially available assay kits used in this study were obtained from Agappe Diagnostics Ltd, (Switzerland), and Randox Laboratories Ltd, (Antrim, UK).
Preparation of extracts
Preparation of methanol extract of Garcinia kola seeds
Fresh seeds of bitter kola (Garcia kola Heckel) were obtained from Oja Oba, Akure, Nigeria. The seeds were identified at the Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria, and a sample was deposited at the herbarium (IFE 17,348). The seeds were peeled, air-dried, and pulverized with an electric machine. The powdered sample was macerated in 80% methanol for 48 h, sieved with mesh cloth and filtered using Whatman filter paper (No. 1). The filtrate was concentrated using a rotary evaporator and freeze-dried to obtain the crude methanol extract of Garcinia kola (GKME) which was preserved in a refrigerator for further use.
Preparation of alkaloid-rich extract of Garcinia kola seeds
Alkaloid-rich extract was prepared from GKME according to the method described by Manuwa et al. (2017). Briefly, 2 g of GKME was dissolved in distilled water (500 ml) acidified with concentrated sulfuric acid. Then, it was poured into a separating funnel and made alkaline by adding aqueous ammonia. Thereafter, chloroform was added with vigorous shaking, and it was allowed to stand for a few minutes. The chloroform layer containing the alkaloid fraction was collected. The procedure was repeated severally, and the chloroform layers collected were concentrated using a rotary evaporator and air-dried to obtain the alkaloid-rich extract of Garcinia kola seed (GKAE) which was preserved in a refrigerator for further use.
HPLC–DAD fingerprinting of GKAE and GKME
HPLC–DAD fingerprinting of GKAE and GKME was carried out according to a previously described method (Saliu et al. 2021). The analysis was performed on a Shimadzu (NexeraMX) HPLC system fitted with uBONDAPAK C18 column (length 100 mm, diameter 4.6 mm, and thickness 7 μm). The mobile phase consisted of a mixture of aqueous acetonitrile (acetonitrile/water, 80:20). Briefly, GKAE and GKME were dissolved in aqueous acetonitrile and were injected into the machine at a volume of 5 μl, and the flow rate was set at 0.08 ml/min for water and 2 ml/min for acetonitrile at a pressure of 15 MPa. Compounds were detected by a UV detector at 254 nm (Diode Array Detector, DAD), and the retention times of the identified compounds of interest were measured. The extract was injected into the high-performance liquid chromatographic machine to obtain a curve providing peak area and retention time in a chromatogram. Then, the peak area of the sample is compared with that of the standard relative to the concentration of the standard to obtain the concentration of the sample.
Animal handling and experimental design
Eighty male Wistar rats weighing 180 ± 20 g were obtained from the animal colony of Science Technology Department, Federal Polytechnic, Ado Ekiti, Nigeria, and housed at the animal house of the Department of Biochemistry, the Federal University of Technology, Akure, Nigeria. The animals were kept under standard laboratory conditions and fed with laboratory chow (Vital Feed Lagos, Nigeria) and water. The rats were allowed to acclimatize for two weeks before the commencement of the experiment. All animals were handled and used according to the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, 1985). The experiments were approved by the institutional Committee for the Ethical Use of Research Animals of the Federal University of Technology, Akure. The animals were divided into 10 groups (n = 10) as follows.
Group I: rats received distilled water only (control).
Group II: rats were orally administered 2 g/kg MSG only for 21 days (MSG).
Group III: rats were orally co-administered MSG and 10 mg/kg GKAE for 21 days (MSG + 10 mg/kg GKAE).
Group IV: rats were orally co-administered MSG and 20 mg/kg GKAE for 21 days (MSG + 20 mg/kg GKAE).
Group V: rats were orally co-administered MSG and 100 mg/kg GKME for 21 days (MSG + 100 mg/kg GKME).
Group VI: rats were orally co-administered MSG and 200 mg/kg GKME for 21 days (MSG + 200 mg/kg GKME).
Group VII: rats were orally co-administered MSG and 10 mg/kg quercetin for 21 days (MSG + 10 mg/kg QUE).
Group VIII: rats were orally co-administered MSG and 100 mg/kg nicotinic acid for 21 days (MSG + 100 mg/kg NA).
The works of Ikeuba et al. (2013) and Ajayi et al. (2011) informed the choice of the doses of GKAE and GKME, respectively, used in this study. Rats were administered orally with 2 g/kg (body weight) of MSG daily for 21 consecutive days (Calis et al. 2016). Twenty-four hours after the last administration, animals were sacrificed through cervical dislocation, and blood was collected from the animals by cardiac puncture into EDTA bottles. The blood was centrifuged for 10 min at 3500 rpm to obtain plasma samples used for biochemical analyses. The liver, kidney, heart, and brain were excised, rinsed in ice-cold 1.15% (w/v) potassium chloride solution, blotted with filter paper, and weighed. The brain was dissected into the hippocampus, striatum, and cortex.
Plasma biochemical analyses
Evaluation of markers of the liver, and kidney toxicity
The activity of alkaline phosphatase (ALP), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), as well as plasma concentration of creatinine, glucose, and electrolytes (potassium, sodium, and calcium ions) were estimated using kits obtained from Agappe Diagnostics Ltd. following the manufacturer’s instruction.
Determination of plasma lipid profiles and cardiovascular risk indices
The concentrations of total cholesterol (CHOL), triacylglycerol (TRIG), and high-density lipoprotein-cholesterol (HDL-c) were determined using kits obtained from Agappe Diagnostics Ltd. following the manufacturer’s instructions. The concentration of low-density lipoprotein-cholesterol (LDL-c) and very low-density lipoprotein-cholesterol (VLDL-c) in the plasma were calculated using the formula of Friedewald et al. (1972). The activity of creatine kinase (CK-MB) in the plasma was evaluated using kits obtained from Agappe Diagnostics Ltd. following the manufacturer’s instructions. The atherogenic index (AI) and coronary risk index (CRI) were calculated as described by Wilson et al. (1998) and Liu et al. (1999).
Tissue biochemical analyses
Liver, heart, kidney, and discrete brain regions (hippocampus, striatum, and cortex) were homogenized separately in 10% (w/v) phosphate-buffered saline (PBS, pH 7.4) using a Teflon homogenizer. The resulting homogenate was centrifuged at 10,000 × g at 4 °C for 25 min to obtain the supernatant which was used for biochemical analyses.
Evaluation of cardiac lactate dehydrogenase activity
Lactate (LDH) dehydrogenase activity was evaluated in the heart sample using the method described by McKee et al. (1972).
Evaluation of dopamine level and acetylcholinesterase activity in discrete brain region
The level of dopamine (Guo et al. 2009) and activity of acetylcholinesterase (Ellman et al. 1961) were evaluated in the hippocampus, striatum, and cortex of the brain of rats using the cited methods. Briefly, to evaluate the level of dopamine, supernatant from brain samples (0.1 ml) was added to 0.1 ml of 5 mM FeCl3 and 0.1 ml of 5 mM potassium ferricyanide. The resultant solution was made up to 2.5 ml with phosphate buffer (pH 4.0). The mixture was incubated for 35 min at room temperature, and the absorbance was read against a reagent blank. The concentration of dopamine was extrapolated from a calibration curve of dopamine hydrochloride prepared by plotting the absorbance reading at 735 nm against varying concentrations of dopamine hydrochloride. AChE activity was estimated based on the principle of the thiol group in the samples reacting with DTNB to form yellow-colored thionitrobenzoic acid which was read at 412 nm.
Evaluation of oxidative stress markers in the liver, kidney, heart, and discrete brain regions
The extent of lipid peroxidation was assessed using the method described by Varshney and Kale (2009) by measuring the formation of thiobarbituric acid reactive substances (TBARS) and expressed as the concentration of malondialdehyde (MDA) per mg. The sample supernatant (0.4 ml) was mixed with 1.6 ml of Tris-KCl buffer, TCA (0.5 ml, 30% (w/v)) and 0.5 ml of 0.75% (w/v) TBA were added followed by incubation in a water bath for 45 min at 90 °C. It was then cooled in ice and centrifuged at 3000 g. The clear supernatant was collected and absorbance was read against a reference blank of distilled water at 532 nm. Lipid peroxidation was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1 a t 532 nm. Protein carbonyl (PC) contents were determined by the method of Levine et al. (1990). Supernatant from each tissue was incubated with DNPH for 60 min at room temperature and allowed to precipitate, before the addition of 20% trichloroacetic acid. The pellet was washed with acetone and dissolved in 1 ml of Tris buffer containing sodium dodecyl sulfate (8% w/v, pH 7.4). The absorbance was measured at 360 nm and expressed as nmol carbonyls/mg protein. Reduced glutathione (GSH) concentration was determined according to a previously described method (Beutler et al. 1985). This was based on the development of a relatively stable yellow color after the reaction of the sample with DTNB. The concentration of GSH was proportional to the absorbance at 412 nm and estimated from a standard curve of GSH. Superoxide dismutase (SOD) activity was evaluated using the method described by Kakkar et al. (1984) by measuring the absorbance of the mixture of the sample supernatant, carbonate buffer (0.05 M, pH 10.2), and epinephrine (0.3 mM) for 150-s at 30-s interval at 480 nm. Ferric reducing antioxidant power (FRAP) was determined by the method described by Benzie and Strain (1996). A serial dilution of 0–2 mM FeSO4.7H2O was prepared as the standard solution. 0.1 ml of the supernatant from the samples and 2.8 ml of FRAP reagent (10:1:1 v/v/v; 300 mM, pH 3.6, sodium acetate buffer: 10 mM tripyridyltriazine in 0.1 M HCl: 20 mM FeCl3) were added to 0.15 ml of standard solution in test tubes. This was vortexed, incubated at 37 °C for 30 min in the dark, and the absorbance was read at 593 nm. FRAP was deduced using a standard curve, and the result obtained was expressed as µmol/mg protein. These estimations were performed on the liver, kidney, heart, and discrete brain regions (hippocampal, striatal, and cortical regions) separately.
Determination of total protein in the tissues
Total protein concentration was determined in the liver, kidney, heart, and discrete brain regions using Randox assay kits following the manufacturer’s instructions.
Histopathology
Three out of the animals were sacrificed by cervical dislocation, and the liver, kidney, heart, and brain tissues were excised and fixed by immersion method in 10% formalin for histopathological examination. Histopathological assessment was carried out on the liver, kidney, heart and hippocampus and cortex of the brain according to the method described by Frank et al. (1997). Freshly excised tissues were fixed in 10% formalin solution for 12 h after which they were embedded in paraffin wax. The wax block was cut on a microtome to yield a 4-µM thick slice of paraffin containing the tissues. The specimen slices were placed on a microscope slide, air-dried, and heated. Residual paraffin was dissolved by rinsing with an acid-alcohol followed by rinsing with water to remove the acid-alcohol. Slides were stained with hematoxylin, and the excess hematoxylin (bluing solution) was removed by rinsing with water. Other cytoplasmic elements were stained with an alcoholic solution of eosin Y, a red stain, and light green or fast green. Excess stain and water were removed by a series of sequential washes in a dehydrating reagent, and slides were rinsed with a chemical-clearing agent (xylene) to remove residual dehydrating reagent remaining from the washing step; coverslip mountants were applied after removing slides from the chemical-clearing agent. Analyses of the prepared slides were performed using an Acuscope® (China) microscope with a TSView® Software (China) for imaging. The brain tissue morphometry was performed using the Motic Image Plus 2000® software (China). A minimum of 5 fields for each tissue section were examined and assigned for histological changes.
Statistical analysis
The results were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. In all the tests, p < 0.05 was taken as the criterion for statistical significance. The statistical software used for the analysis was GraphPad Prism 6.01 (GraphPad Software Inc., CA, USA).
Results
HPLC–DAD fingerprinting analysis of GKME and GKAE
The HPLC–DAD chromatograms in Supplementary Fig. 1a showed the presence of various classes of phytochemicals such as alkaloids (theobromine, caffeine, and theophylline), flavonoids (catechin, epicatechin, kolaflavonones, kolaviron, apigenin, amentoflavone, fisetin and garcinoic acid), coumarin, garcifuran-A, and xanthone in GKME. Supplementary Fig. 1b shows the alkaloids detected in GKAE. Appreciable amount of these phytochemicals were analyzed from the chromatograms and were presented in Table 1. Kolaviron (8.28 mg/g) was the most abundant phytochemical in GKME, followed by quercetin (7.25 mg/g), caffeine (3.53), and catechin (3.48 mg/g) and the least abundant compound was fisetin (0.38 mg/g). Theobromine (4.96 mg/g) was the most abundant alkaloid in GKAE, followed by caffeine (3.52 mg/g), and theophylline (1.38 mg/g). This showed that GKAE is an alkaloid-rich extract compared to the GKME.
Effect of GKME and GKAE on plasma biomarkers of hepatic and renal injury in MSG-administered rats
The effect of GKME and GKAE on altered plasma biomarkers and electrolyte levels in MSG-administered rats is presented in Table 2. Liver toxicity was significantly evident (p < 0.05) in MSG-administered rats. This was shown by increased activities of ALP, AST, and GGT, as well as an increase in glucose concentration in the plasma compared with control rats (Table 2). GKME and GKAE significantly ameliorated (p < 0.05) MSG-induced hepatotoxicity. However, GKAE did not affect the activity of GGT compared to MSG-toxified rats. There was a significant increase (p < 0.05) in creatinine level in the plasma of MSG-administered rats which signifies kidney damage compared with control rats. Also, MSG-administered rats showed a significant increase in the level of Na+, as well as a decreased level of K+ in their plasma compared with control (p < 0.05); however, there was no significant difference in Ca2+ levels of MSG-administered and control animals. G. kola extracts (GKME and GKAE) modulated creatinine levels and the electrolyte imbalances elicited by MSG intoxication in rats. GKAE (20 mg/kg) showed the best protection against renal malfunction in MSG-induced organ damage. In addition, the control compounds (QUE and NA) also significantly mitigated (p < 0.05) the altered plasma biomarkers in MSG-administered rats.
Effect of GKME and GKAE on plasma lipid profile and cardiac injury indices of MSG-administered rats
The plasma lipid profile and the cardiac injury indices presented in Tables 3 and 4, respectively, showed that the administration of MSG to rats caused cardiotoxicity. Table 3 shows that MSG caused a significant increase (p <0.05) in plasma CHOL, TRIG, LDL-c, and VLDL-c levels, while treatment with GKME, GKAE, QUE, and NA reversed this effect of MSG. Conversely, the reduced HDL-c concentration in the plasma of MSG-toxified rats was ameliorated by treatment with GKME and GKAE. An increase in the values of the cardiovascular risk indices (AI and CRI) and increased activity of plasma CK-MB were recorded in MSG-administered rats and were significantly reversed (p < 0.05) in groups co-administered with GKME, GKAE, quercetin, and nicotinic acid (Table 4). The LDH activity in the heart of MSG-administered rats is also presented in Table 4. There was a significant reduction (p < 0.05) in LDH activity in the heart of MSG-administered rats compared with the control, whereas rats co-treated with GKME or GKAE showed a significant increase (p < 0.05) (Table 4). Treatment with 20 mg/kg GKAE had the most pronounced effect in ameliorating the decrease in LDH activity. The reference compounds were also effective (p < 0.05) in ameliorating the MSG-induced decrease in LDH activity.
Effect of GKME and GKAE on dopamine level and acetylcholinesterase activity in discrete brain regions of MSG-administered rats
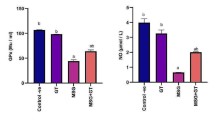
The effect of GKME and GKAE on dopamine concentration and acetylcholinesterase activity in the striatum, cortex, and hippocampus of the brain of MSG-administered rats is presented in Fig. 1. MSG intoxication caused a significant increase (p < 0.05) in the striatal, cortical, and hippocampal dopamine concentration compared with the control (Fig. 1a). Co-administration of MSG with GKME, GKAE, QUE, and NA ameliorated the altered level of striatal and hippocampal dopamine concentration. However, there was no change observed in cortical dopamine concentration after GKME, GKAE, or NA co-treatment.
Effect of Garcinia kola seed extracts on neurotransmitter dysregulation in the striatal, cortical, and hippocampal regions of the brain of MSG-toxified rats. a Dopamine level. b Acetylcholinesterase activity. Each bar represent mean ± SD (n = 7). MSG, monosodium glutamate; GKME, methanol extract of Garcinia kola seed; GKAE, alkaloid-rich extract of Garcinia kola seed; QUE, quercetin; NA, nicotinic acid
The activity of AChE in the striatum, hippocampus, and cortex of the brain of rats administered with MSG and treated with G. kola extracts is presented in Fig. 1b. A significant increase (p < 0.05) was observed in the striatum, cortex, and hippocampus of the brain of MSG-administered rats compared with control, while co-administration with GKME or GKAE attenuated the activity of striatal and hippocampal AChE compared with MSG-toxified rats. Co-administration of MSG with GKME did not have a significant effect in the cortical AChE activity. The activity of AChE was also significantly attenuated (p < 0.05) in different brain regions by QUE and NA compared with MSG-toxified rats (except NA in the cortex).
GKME and GKAE ameliorate MSG-induced oxidative stress in the tissues rats
Administration of rats with MSG provoked the generation of oxidative stress in the striatum, hippocampus, and cortex, as well as in the kidney, liver, and heart tissues. Significant peroxidation of lipids which was measured as the level of malondialdehyde (MDA) produced and carbonylation of protein (Table 5), reduction in GSH level, and decreased SOD activity (Table 6), as well as decreased FRAP (Table 7), were observed (p < 0.05) in all tissues of MSG-administered rats compared with the control. Treatment with GKAE or GKME, as well as QUE and NA, significantly ameliorated (p < 0.05) oxidative stress parameters that were evaluated in the striatal, hippocampal, and cortical regions of brain, kidney, liver, and heart tissues of MSG-administered rats. However, treatment with GKAE did not have any significant ameliorative effect on hippocampal lipid peroxidation and GKME did not have any significant ameliorative effect on cortical lipid peroxidation. Also, treatment with the highest dose of GKAE or GKME (20 mg/kg GKAE or 200 mg/kg GKME) produced the best results.
Effects of GKAE or GKME on histopathological changes in MSG-administered rats
MSG intoxication caused histopathological changes and provoked varying degrees of loss/damage of neurons in the hippocampus and cortex regions of the brain of rats (Figs. 2 and 3, respectively). MSG intoxication caused altered histological features characterized by severe vacuolation and spongiosis, vascular/capillary engorgement, and neuronal degeneration. However, treatment with GKAE or GKME as well as QUE and NA significantly preserved histological features with evidence of recovery from damage to the brain tissues of rats administered with MSG. Also, the administration of MSG caused severe damage to the heart, kidney, and liver tissues of rats (Figs. 4, 5 and 6) but treatment with GKAE or GKME as well as QUE and NA slightly protect the histoarchitectural integrity of these tissues as evident by mild recovery seen in the tissue sections of the treated rats. Indeed, MSG toxicity triggered the deformation of many histological features of these organs.
Representative photomicrographs showing hematoxylin and eosin-stained sections of the hippocampus of rat brain (× 100). A Control: normal histological features with stratified layers. B MSG: vacuolation (blue arrows); early neuronal degeneration and vascular/capillary engorgement (red arrow). C MSG + 10 mg/kg GKAE: vacuolation (blue arrow); neuronal degeneration and vascular/capillary engorgement (red arrow). D MSG + 20 mg/kg GKAE: mild gliosis and pyknotic changes (red arrow). There is evidence of cellular recovery in the granular layer. E MSG + 100 mg/kg GKME: vacuolation (red arrows) with signs of early neuronal degeneration (red circle). F MSG + 200 mg/kg GKME: normal histological features with signs of mild vacuolation (red arrows). G MSG + 10 mg/kg QUE: mild pyknosis with gliosis (red arrow) with preserved hippocampal cell layers. H MSG + 100 mg/kg NA: mild neuronal loss (red arrow) and interstitial congestion with signs of recovery. MSG, monosodium glutamate; GKME, methanol extract of Garcinia kola seed; GKAE, alkaloid-rich extract of Garcinia kola seed; QUE, quercetin; NA, nicotinic acid
Representative photomicrographs showing hematoxylin and eosin-stained sections of the cortex of rat brain (× 100). A Control: normal histological features in stratified layers including molecular, granular, and pyramidal. B MSG: neuronal loss, neuronal degeneration (red arrow), apoptotic changes (red circle), and mild spongiosis. C MSG + 10 mg/kg GKAE: mild spongiosis with pyknosis and apoptotic changes (red arrows) with traces of neuronal recovery. D MSG + 20 mg/kg GKAE: mild spongiosis (red arrow) with few loss of normal cerebral histoarchitecture. E MSG + 100 mg/kg GKME: moderate vacuolation (blue arrows) with early neuronal degeneration and vascular congestion (red circle). F MSG + 200 mg/kg GKME: neuronal tissues interspersed within the submeningeal milieu; no visible lesion seen. G MSG + 10 mg/kg QUE: vascular congestion (red circle). The neuronal tissues are mildly pyknotic with gliosis showing evidence of recovery from cerebral injury. H MSG + 100 mg/kg NA: normal neuronal tissue with very mild vacuolation with spongiosis. MSG, monosodium glutamate; GKME, methanol extract of Garcinia kola seed; GKAE, alkaloid-rich extract of Garcinia kola seed; QUE, quercetin; NA, nicotinic acid
Representative photomicrographs showing hematoxylin and eosin-stained sections of the cardiac tissues of rats (× 100). A Control: normal myocardial histoarchitecture. B MSG: complete loss of normal myocardial fiber histoarchitecture, evidence of interstitial and vascular congestion due to edema. There is moderate myofiber degeneration. C MSG + 10 mg/kg GKAE: loss of normal myocardial fiber histoarchitecture, evidence of interstitial and vascular congestion due to edema. There is mild myofiber degeneration. D MSG + 20 mg/kg GKAE: moderate interstitial congestion and mild neutrophilic infiltration (red arrow). However, myocardiac histoarchitecture was preserved. E MSG + 100 mg/kg GKME: loss of myocardiac fiber orientation with dysplastic changes. F MSG + 200 mg/kg GKME: reduced (shrunken) myocardial fibers with prominent gap junctions; myocardiac histoarchitecture was preserved showing a sign of tissue recovery. G MSG + 10 mg/kg QUE: normal myocardiac histoarchitecture with mildly enlarged myocardial fibers (red arrow). H MSG + 100 mg/kg NA: reduced (shshrunken myocardial fibers with prominent gap junctions (red arrow); there are signs of recovery of the myocardial fibers. MSG, monosodium glutamate; GKME, methanol extract of Garcinia kola seed; GKAE, alkaloid-rich extract of Garcinia kola seed; QUE, quercetin; NA, nicotinic acid
Representative photomicrographs showing hematoxylin and eosin-stained sections of the renal tissues of rats (× 100). A Control: normal renal histoarchitecture. No visible lesion seen. B MSG: evidence of foci of interstitial hemorrhages and congestion characterized by severe tubular degeneration and necrosis. C MSG + 10 mg/kg GKAE: moderately distorted histoarchitecture with loss of interstitial tubules (tubular degeneration — Td). D MSG + 20 mg/kg GKAE: distorted histoarchitecture with interstitial tubular degeneration (Td) and necrosis. The renal cells show sign of recovery. E MSG + 100 mg/kg GKME: severe interstitial congestion and mild tubular degeneration (Td). F MSG + 200 mg/kg GKME: normal renal histoarchitecture with mild tubular degeneration (Td). G MSG + 10 mg/kg QUE: interstitial congestion (red arrows), diffuse tubular degeneration (Td), and vascular engorgement. H MSG + 100 mg/kg NA: interstitial congestion, glomerular and tubular degeneration with ongoing necrosis. MSG, monosodium glutamate; GKME, methanol extract of Garcinia kola seed; GKAE, alkaloid-rich extract of Garcinia kola seed; QUE, quercetin; NA, nicotinic acid
Representative photomicrographs showing hematoxylin and eosin-stained sections of the hepatic tissues of rat (× 100). A Control: normal hepatocellular histoarchitecture. B MSG: loss of normal hepatic histoarchitecture with severe portal congestion, bile ductal hyperplasia, and hydropic degeneration of hepatocytes. C MSG + 10 mg/kg GKAE: severe extensive hepatic vacuolar degeneration and necrosis. There are evident pyknotic changes in the peripheral hepatocytes. D MSG + 20 mg/kg GKAE: preserved hepatic histoarchitecture with signs of recovery from moderate periportal hepatic degeneration and necrosis (central vein — Cv). E MSG + 100 mg/kg GKME: severe interstitial and central venous congestion. There is also a moderate periportal cellular infiltration with necrosis of hepatocytes. F MSG + 200 mg/kg GKME: moderate extensive hepatic vacuolar degeneration and necrosis. There are evident pyknotic changes in the peripheral hepatocytes. G MSG + 10 mg/kg QUE: moderate interstitial and central venous congestion (central vein — Cv). H MSG + 100 mg/kg NA: evidence of extensive diffuse hemosiderosis. MSG, monosodium glutamate; GKME, methanol extract of Garcinia kola seed; GKAE, alkaloid-rich extract of Garcinia kola seed; QUE, quercetin; NA, nicotinic acid
Discussion
Uncontrolled usage of food additives may portend nutritional inadequacy, deficiency, and toxicity. MSG is considered one of the most used food enhancers, and its toxic effect has been attributed to the generation of ROS which results in oxidative stress and decreased mitochondrial membrane fluidity leading to change of cellular events in multiple organs (Dong and Robbins 2015; Maňourová et al. 2019). Search for bioactive entities from medicinal plants is logical due to their fewer side effects and affordability, especially in developing countries where the cost of healthcare is high. In this study, MSG administration caused multiorgan toxicity involving the brain, kidney, liver, and heart as shown by the alteration of biochemical markers. Therefore, its use as a food additive should be reviewed. Conversely, in this study, co-administration with Garcinia kola extracts (GKAE and GKME) abrogated the altered biochemical indices in multiple organs through its antioxidant, neuroprotective, nephroprotective, hepatoprotective, and cardioprotective properties.
The HPLC–DAD characterization of GKAE and GKME revealed the presence of bioactive phytochemicals which have been previously demonstrated in various works as pharmacologically active compounds. Kolaviron, a biflavonoid complex of Garcinia kola seeds, modulates apoptosis by suppressing redo-inflammation in the brain of rats with ischemic injury and in diabetes-induced nephrotoxic rats (Ojo et al. 2019; Farombi et al. 2019). Caffeine preferentially protects against oxygen-induced retinopathy and promotes angiogenesis through modulating endothelial mitochondrial dynamics (Wang et al. 2021). Cardiomyocyte injury was mitigated by catechin via reduction of oxidative stress and epicatechin-mediated ischemia/reperfusion cardiovascular injury through inhibition of arginase activity (Ortiz-Vilchis et al. 2018). Theophylline showed neuroprotective effect as an add-on to thrombolytic therapy in acute ischemic stroke (Modrau et al. 2020). Theobromine alleviated oxidative stress, and inflammation and improves neurobehavioral performance in transient cerebral ischemia/reperfusion injury, and also improves mood and cognition via peripheral physiological changes (Bhat et al. 2021). Quercetin attenuated hepatic and renal injury by suppressing inflammation and oxidative stress (Chaudhary et al. 2015; Tang et al. 2016), and garcinoic acid from Garcinia kola acts as a powerful antioxidative agent (Terashima et al. 2002).
Toxicity of the liver is characterized by an increase in the activities of plasma enzymes and non-enzyme molecules such as ALP, AST, GGT, bilirubin, and glucose as shown in this study. The increase in the level of these molecules in the plasma is indicative of hepatocellular damage which can be ascribed to MSG intoxication in the liver. This supports the findings of Abdulsalam et al. (2018). Likewise, the high plasma concentration of creatinine, Na+, and Ca2+, coupled with the low plasma concentration of K+, indicate kidney dysfunction. Monosodium glutamate consumption is associated with urolithiasis and urinary tract obstruction in rats, which may arise as a result of deposition of mineral and disturbance of electrolyte homeostasis. The hepatoprotective and nephroprotective effect of Garcinia kola has previously been demonstrated (Gomina et al. 2020). The ameliorative effect shown by the methanol and alkaloid extracts of Garcinia kola against MSG-induced liver and kidney injury in this study can be ascribed to the active phytochemicals present in the extracts (Costa et al. 2016; Ortiz-Vilchis et al. 2018; Nilnumkhum et al. 2019).
Disturbance in lipid metabolism which can cause hyperlipidemia has been demonstrated to be one of the several consequences of MSG toxicity (Collison et al. 2009). Dyslipidemia has been reported as a risk factor for cardiovascular disease and fatty liver disease. MSG-administered animals in the present study, showed altered lipid profile and cardiac injury indices (Collison et al. 2009). The altered cardiac injury indices (CK-MB, LDH, atherogenic index, and coronary risk index) indicate cardiac dysfunction. GKAE and GKME ameliorated the MSG-induced deleterious changes in the lipid profile and cardiac injury markers in treated rats, revealing the cardioprotective property of Garcinia kola which may be attributed to the presence of polyphenolic compounds.
MSG is a derivative sodium salt of glutamic acid and biological systems are sensitive to its high dosage. The brain is a primary target of MSG toxicity due to the abundance of glutamate receptors, polyunsaturated fatty acids, and high metabolic activities (Chakraborty 2018). The presence of a high amount of MSG in the biological system may cause an increase in glutamate levels which has been linked to several neurological dysfunctions (Niaz et al. 2018; Araujo et al. 2017). In this study, MSG causes a change in the level of dopamine, as well as in the activities of acetylcholinesterase and LDH in striatal, hippocampal, and cortical regions of the brain. These brain region controls the body’s cognitive, motor, and metabolic functions. This may inadvertently lead to an increase in intracellular calcium concentration and the disturbance in cellular redox potential secondary to the activation of the Krebs Cycle (Sharma 2015) which are the major contributors to the acceleration of cell death caused by MSG. Alkaloids and flavonoids are known phytochemicals that have been previously shown to be neuroprotective owing to their neurotransmitter modulatory activity and metabolic regulatory actions (Rosa et al. 2018; Ojo et al. 2019) This may be responsible for the neuroprotective effect shown by GKAE and GKME in this study.
Several studies including those on animals have reported oxidative stress as major pathophysiology mechanism that contributes to the toxic effect of MSG in multiple organs (Farombi and Onyema 2006; Sharma 2015; Rosa et al. 2018). Endogenous antioxidants are abundantly expressed in various organs of the body and they function to terminate the damaging actions of ROS that can lead to oxidative stress if uncontrolled. Oxidative stress which arises as a result of uncontrollable generation of ROS reduced the level/activity of GSH, FRAP, and SOD as well as caused lipid peroxidation and protein carbonylation. This was evident in the brain, liver, kidney, and heart of MSG-administered rats in this study. The impaired antioxidative system in multiple organs was repaired by treatment with GKAE and GKME. The pharmacological activity of polyphenols and alkaloids have widely been reported to include restoration of the antioxidant system and improved membrane fluidity (Terashima et al. 2002; Costa et al. 2016; Nilnumkhum et al. 2019; Firgany and Sarhan 2020; Hazzaa et al. 2020a, b; Bhat et al. 2021; Kushida et al. 2021).
The histopathological changes observed in the MSG-administered rats validate the observed biochemical alterations. The neuronal loss in the hippocampus and cortex of the brain of rats as well as hepato-renal and cardiac histopathological changes observed in this study may be attributed to redox imbalance. Reports have demonstrated that oxidative stress caused mitochondrial dysfunction with the release of an apoptotic marker, which is the main culprit that leads to neuronal cell loss and degeneration in MSG-toxified rats (Sadek et al. 2015; Hazzaa et al. 2020b). Also, other workers have reported that oxidative stress as a result of MSG intoxication caused histomorphological changes in multiple organs of rodents such as hepato-renal (Othman and Bin-Jumah 2019) and cardiac tissue (Hassan et al. 2020). GKME and GKAE ameliorated the histopathological changes and reestablished the histopathological patterns of the tissues by restoring the tissue redox system. The synergistic effect of the phytochemical compounds present in these extracts may be responsible for their pharmacological and modulatory action.
Conclusion
The results of this study highlight the ameliorative effect of Garcinia kola seed extracts on MSG-related anomalies in multiple organs. This study has demonstrated the protective effect of the methanol and alkaloid-rich extracts of Garcinia kola seed against renal, cardiac, hepatic, and neurologic deterioration associated with MSG intake through its antioxidative effect. There is no distinct comparative difference between the protective action of GKAE and GKME on the organs although each extract was more effective at the highest dose.
Availability of data and material
All data generated or analyzed in this study are included in this article and are also available from the corresponding author on reasonable request.
References
Abarikwu SO (2014) Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK. Biochim Biophys Acta BBA Gen Subj 1840:2373–2381. https://doi.org/10.1016/j.bbagen.2014.03.006
Abdulsalam H, Adamu S, Sambo SJ, Chiroma MA, Gadzama JJ, Mohzo DL, Atata JA (2018) Monosodium glutamate-induced changes on plasma markers of pancreatic function in adult male Wistar rats. Sokoto J Vet Sci 16(2): 21–27–27. https://doi.org/10.4314/sokjvs.v16i2.3
Ajayi SA, Ofusori DA, Ojo GB, Ayoka OA, Abayomi TA, Tijani AA (2011) The microstructural effects of aqueous extract of Garcinia kola (Linn) on the hippocampus and cerebellum of malnourished mice. Asian Pac J Trop Biomed 1(4):261–265. https://doi.org/10.1016/S2221-1691(11)60039-7
Araujo TR, Freitas IN, Vettorazzi JF, Batista TM, Santos-Silva JC, Bonfleur ML, Balbo SL, Boschero AC, Carneiro EM, Ribeiro RA (2017) Benefits of L-alanine or L-arginine supplementation against adiposity and glucose intolerance in monosodium glutamate-induced obesity. Eur J Nutr 56:2069–2080. https://doi.org/10.1007/s00394-016-1245-6
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the frap assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Beutler E, Duwn O, Kelly, (1985) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R (2007) Consensus meeting: monosodium glutamate-an update. Eur J Clin Nutr 61:304–313. https://doi.org/10.1038/sj.ejcn.1602526
Bhat JA, Gupta S, Kumar M (2021) Neuroprotective effects of theobromine in transient global cerebral ischemia-reperfusion rat model. Biochem Biophys Res Commun 571:74–80. https://doi.org/10.1016/j.bbrc.2021.07.051
Brosnan JT, Drewnowski A, Friedman MI (2014) Is there a relationship between dietary MSG and obesity in animals? Amino Acids 46:2075–2087. https://doi.org/10.1007/s00726-014-1799-7
Calis IU, Cosan DT, Saydam F, Kolac UK, Soyocak A, Kurt H, Gunes HV, Sahinturk V, Mutlu FS, Koroglu ZO, Degirmenci I (2016) The effects of monosodium glutamate and tannic acid on adult rats. Iran Red Crescent Med J 18(10). https://doi.org/10.5812/ircmj.37912
Chakraborty SP (2018) Patho-physiological and toxicological aspects of monosodium glutamate. Toxicol Mech Met. https://doi.org/10.1080/15376516.2018.1528649
Chaudhary S, Ganjoo P, Raiusddin S, Parvez S (2015) Nephroprotective activities of quercetin with potential relevance to oxidative stress induced by valproic acid. Protoplasma 252(1):209–217. https://doi.org/10.1007/s00709-014-0670-8
Cock IE (2015) The medical properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacol 23:203–229. https://doi.org/10.1007/s10787-015-0246-z
Collison KS, Maqbool Z, Saleh SM, Inglis A, Makhoul NJ, Bakheet R, Al-Johi M, Al-Rabiah R, Zaidi MZ, Al-Mohanna FA (2009) Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J Lipid Res 50(8):1521–1537. https://doi.org/10.1194/jlr.M800418-JLR200
Costa LG, Garrick JM, Roquè PJ, Pellacani C (2016) Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. https://doi.org/10.1155/2016/2986796
De Almeida ACA, de-Faia FM, Dunde RJ, Manzo LPB, Souza-Brito ARM, Luiz-Ferreir A, (2017) Recent trends in pharmacological activity of alkaloids in animal colitis: potential use for inflammatory bowel disease. Evid Based Complement Alternat Med. https://doi.org/10.1155/2017/8528210
Dong HV, Robbins WA (2015) Ingestion of monosodium glutamate (MSG) in adult male rats reduces sperm count, testosterone, and disrupts testicular histology. Nutr Bytes 19(1)
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Farombi EO, Onyema OO (2006) Monosodium glutamate induced oxidative damage and genotoxicity in rat: modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol 25(5):251–259. https://doi.org/10.1191/0960327106ht621oa
Farombi EO, Awogbindin IO, Farombi TH, Oladele JO, Izomoh ER, Aladelokun OB, Ezekiel IO, Adebambo OI, Abah VO (2019) Neuroprotective role of kolaviron in striatal redo-inflammation associated with rotenone model of Parkinson’s disease. Neurotoxicology 73:132–141. https://doi.org/10.1016/j.neuro.2019.03.005
Firgany AEL, Sarhan NR (2020) Quercetin mitigates monosodium glutamate-induced excitotoxicity of the spinal cord motoneurons in aged rats via p38 MAPK inhibition. Acta Histochem 122(5):151554. https://doi.org/10.1016/j.acthis.2020.151554
Frank RJ, Damasio H, Grabowski TJ (1997) Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage 5(1):13–30. https://doi.org/10.1006/nimg.1996.0250
Freeman M (2006) Reconsidering the effects of monosodium glutamate: a literature review. J Am Acad Nurse Pract 18(10):482–486. https://doi.org/10.1111/j.1745-7599.2006.00160.x
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Gomina M, Salifou T, Djidonou G, Zinsou S (2020) Effect of the Garcinia kola seed on glycemia, creatininemia and aminotransferases in adult subjects. Int J Biochem Res Rev 29(2):25–32. https://doi.org/10.9734/ijbcrr/2020/v29i230169
Guo L, Zhang Y, Li Q (2009) Spectrophotometric determination of dopamine hydrochloride in pharmaceutical, banana, urine and serum samples by potassium ferricyanide-Fe(III). Anal Sci 25(12):1451–1455. https://doi.org/10.2116/analsci.25.1451
Hassan DA, Alim MA, Sharkawi SM, Nabil S (2020) Detection of cardiac tissue toxicity caused by monosodium glutamate and the protective role of vitamin C by immunohistochemical method, herat tissue oxidative stress biomarkers and cardiac dysfunction biomarkers. Egypt J Forensic Sci Appli Toxicol 20(3):1–9
Hazzaa SM, Abdelaziz SAM, Mabrouk AE, Abdel-Daim MM, Elgarawany GE (2020a) Neuroprotective potential of Allium sativum against monosodium glutamate-Induced excitotoxicity: impact on short-term memory, gliosis, and oxidative stress. Nutrients 12:1028. https://doi.org/10.3390/nu12041028
Hazzaa SM, El-Roghy ES, Abd Eldaim MA, Elgarawany GE (2020b) Monosodium glutamate induces cardiac toxicity via oxidative stress, fibrosis, and P53 proapoptotic protein expression in rats. Environ Sci Pollut Res Int 27(16):20014–20024. https://doi.org/10.1007/s11356-020-08436-6
Ikeuba AI, Okafor PC, Ekpe UJ, Ebenso EE (2013) Alkaloid and non-alkaloid ethanolic extract from seeds of Garcinia kola as green corrosion inhibitors of mild steel in H2SO4 solution. Int J Electrochem Sci 8:7455–7467
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. IJBB 21(2). Retrieved from http://nopr.niscair.res.in/handle/123456789/19932
Khalil RM, Khedr NF (2016) Curcumin protects against monosodium glutamate neurotoxicity and decreasing NMDA2B and mGluR5 expression in rat hippocampus. Neurosignals 24:81–87. https://doi.org/10.1159/000442614
Kushida H, Matsumoto T, Ikarashi Y (2021) Properties, pharmacology, and pharmacokinetics of active indole and oxindole alkaloids in Uncaria Hook. Front Pharmacol 12:688670. https://doi.org/10.3389/fphar.2021.688670
Lee EH, Lee DI (1976) A study of intake of monosodium glutamate in Korea. Korean J Environ Health Soc 12:75–85
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-H
Liu CS, Lin CC, Li TC (1999) The relation of white blood cell count and atherogenic index ratio of LDL-cholesterol to HDL-cholesterol in Taiwan school children. Acta Paediatr Taiwan 40:319–324
Maňourová A, Leuner O, Tchoundjeu Z, Van Damme P, Verner V, Přibyl O, Lojka B (2019) Medicinal potential, utilization and domestication status of bitter kola (Garcinia kola Heckel) in West and Central Africa. Forests 10:124. https://doi.org/10.3390/f10020124
Manuwa TR, Akinmoladun AC, Crown OO, Komolafe K, Olaleye MT (2017) Toxicological assessment and ameliorative effects of Parinari curatellifolia alkaloids on triton-induced hyperlipidemia and atherogenicity in rats. PNAS India Section b: Biol Sci 87(2):611–623. https://doi.org/10.1007/s40011-015-0630-x
McKee RW, Longstaff E (1972) Latner AL (1972) Lactate dehydrogenase in human testes. Clin Chim Acta 39(1):221–227. https://doi.org/10.1016/0009-8981(72)90319-1
Modrau B, Andersen G, Mikkelsen IK, Nielsen A, Hansen MB, Johansen MB, Eskildsen HW, Povlsen JP, Yavarian Y, Mouridsen K, Østergaard L, Bach FW, Hjort N (2020) Theophylline as an add-on to thrombolytic therapy in acute ischemic stroke: a randomized placebo-controlled trial. Stroke 51(7):1983–1990. https://doi.org/10.1161/strokeaha.119.027446
Mondal M, Sarkar K, Nath PP, Paul G (2017) Monosodium glutamate suppresses the female reproductive function by impairing the functions of ovary and uterus in rat. Environ Toxicol 33:198–208. https://doi.org/10.1002/tox.22508
Niaz K, Zaplatic E, Spoor J (2018) Extensive use of monosodium glutamate: a threat to public health? EXCLI J 17: 273–278. https://doi.org/10.17179/excli2018-1092
Nilnumkhum A, Kanlaya R, Yoodee S, Thongboonkerd V (2019) Caffeine inhibits hypoxia-induced renal fibroblast activation by antioxidant mechanism. Cell Adh Migr 13(1):259–271. https://doi.org/10.1080/19336918.2019.163.8691
Nnadozie JO, Chijioke UO, Okafor OC, Olusina DB, Oli AN, Nwonu PC, Mbagwu HO, Chijioke CP (2019) Chronic toxicity of low dose monosodium glutamate in albino Wistar rats. BMC Res Notes 12:593. https://doi.org/10.1186/s13104-019-4611-7
Ojo OB, Amoo ZA, Saliu IO, Olaleye MT, Farombi EO, Akinmoladun AC (2019) Neurotherapeutic potential of kolaviron on neurotransmitter dysregulation, excitotoxicity, mitochondrial electron transport chain dysfunction and redox imbalance in 2-VO brain ischemia/reperfusion injury. Biomed Pharmacother 111:859–872. https://doi.org/10.1016/J.BIOPHA.2018.12.144
Ortiz GG, Bitzer Quintero OK, Zarate CB, Rodriguez Reynoso S, Larios Arceo F (2006) Monosodium glutamate induced damage in liver and kidney: a morphological and biochemical approach. Biomed Pharmacother 60: 86–91. http://hdl.handle.net/20.500.12104/66309
Ortiz-Vilchis P, Ortiz-Flores M, Pacheco M, Ramirez-Sanchez I, Moreno-Ulloa A, Vega L, Ortiz A, Villarreal F, Rubio-Gayosso I, Najera N, Meaney E, Ceballos G (2018) The cardioprotective effects of (-)-Epicatechin are mediated through arginase activity inhibition in a murine model of ischemia/reperfusion. Eur J Pharmacol 818:335–342. https://doi.org/10.1016/j.ejphar.2017.11.007
Othman SI, Bin-Jumah M (2019) Histomorphological changes in mono-sodium glutamate induced hepato-renal toxicity in mice. Int J Pharmacol 15:449–456. https://doi.org/10.3923/ijp.2019.449.456
Oyenihi OR, Brooks NL, Oguntibeju OO (2015) Effects of kolaviron on hepatic oxidative stress in streptozotocin induced diabetes. BMC Complement Altern Med 15:236. https://doi.org/10.1186/s12906-015-0760-y
Pradeep CR, Kuttan G (2004) Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory gene expression in B16F–10 melanoma cells. Int Immunopharmacol 4(14):1795–1803
Rhodes J, Titherley AC, Norman JA, Wood R, Lord DW (1991) A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutamate. Food Addit Contam 8(5):663–672. https://doi.org/10.1080/02652039109374021
Rosa SG, Chagas PM, Pesarico AP, Nogueira CW (2018) Monosodium glutamate induced nociception and oxidative stress dependent on time of administration, age of rats and susceptibility of spinal cord and brain regions. Toxicol Appl Pharmacol 351:64–73. https://doi.org/10.1016/j.taap.2018.05.019
Sadek K, Abouzed T, Nasr S (2015) Lycopene modulated cholinergic dysfunction, Bcl-2/Bax balance and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in rat model. Can J Physiol Pharmacol 94:394–401
Saliu IO, Amoo ZA, Khan MF, Olaleye MT, Rema V, Akinmoladun AC (2021) Abatement of neurobehavioral and neurochemical dysfunctions in cerebral ischemia/reperfusion injury by Tetrapleura tetraptera fruit extract. J Ethnopharmacol 264:113284. https://doi.org/10.1016/j.jep.2020.113284
Sharma A (2015) Monosodium glutamate induced oxidative kidney damage and possible mechanisms: a mini-review. J Biomed Sci 2015(22):93. https://doi.org/10.1186/s12929-015-0192-5
Tang Y, Li J, Gao C, Xu Y, Li Y, Yu X, Wang J, Liu L, Yao P (2016) Hepatoprotective effect of quercetin on endoplasmic reticulum stress and inflammation after intense exercise in mice through Phosphoinositide 3-Kinase and Nuclear Factor-Kappa B. Oxid Med Cell Longev. https://doi.org/10.1155/2016/8696587
Terashima K, Takawa Y, Niwa M (2002) Powerful antioxidative agents based on garcinoic acid from Garcinia kola. Bioorg Med Chem 10:1619–1625. https://doi.org/10.1016/s0968-0896(01)00428-x
Unaeze HN (2010) Consumer knowledge attitude and practice towards the use of monosodium glutamate and food grade bouillon cubes as dietary constituents. Pak J Nutr 9(1):76–80. https://doi.org/10.3923/pjn.2010.76.80
Varshney R, Kale RK (2009) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Rad Biol 58(5):733–743. https://doi.org/10.1080/09553009014552121
Wang LT, He PC, Li AQ, Cao KX, Yan JW, Guo S, Jiang L, Yao L, Dai XY, Feng D, Xu YM, Tan N (2021) Caffeine promotes angiogenesis through modulating endothelial mitochondrial dynamics. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-021-00623-6
Wilson TA, Meservey CM (1998) Nicolosi RJ (1998) Soy lecithin reduces plasma lipoprotein cholesterol and early atherogenesis in hypercholesterolemic monkeys and hamsters: beyond linoleate. Atherosclerosis 140(1):147–153. https://doi.org/10.1016/S0021-9150(98)00132-4
Zhang Y, Lu X, Bhavnani BR (2003) Equine estrogens differentially inhibit DNA fragmentation induced by glutamate in neuronal cells by modulation of regulatory proteins involved in programmed cell death. BMC Neurosci 4:32. https://doi.org/10.1186/1471-2202-4-32
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare no competing interests.
Ethics approval
This experiment was approved by the animal research ethical committee of the Federal University of Technology, Akure. Animals were handled and used following the ethical principles established by the National Institute of Health Guide for the Care and Use of Laboratory Animals (National Institute of Health, NIH Publication No. 8523, 1978, revised 2011).
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kareem, A., Josiah, S.S., Saliu, I.O. et al. Ameliorative influence of Garcinia kola seed extracts against multiple organ toxicity in monosodium glutamate-administered Wistar rats. Comp Clin Pathol 31, 987–1004 (2022). https://doi.org/10.1007/s00580-022-03406-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03406-5