Abstract
The current study was designed to evaluate the therapeutic efficacy of Zingiberene (ZB) against high-fat-diet (HFD)–induced obesity and its associated non-alcoholic fatty liver disease (NAFLD). NAFLD model was developed in rats by supplementation of HFD (20 g daily) initially for 15 weeks and 16th week onwards treated with ZB (50 mg/kg BW) for the period of 45 days. We evaluated the effects of ZB on glucose homeostasis, lipid profiles in both blood and tissue, and lipogenesis along with oxidative stress markers in liver. HFD supplementation significantly caused hyperglycemia, and hyperinsulinemia and insulin resistance thereby altered both circulatory and tissue lipids. At the same time, lipogenesis and oxidative stress in liver was noted in obese rats. On the other hand, supplementation of ZB (50 mg/kg BW) to obese rats successfully attenuated hyperglycemia, hyperinsulinemia, and hyperlipidemia in both blood and liver. ZB also regulated the activities of lipid metabolic marker enzymes (carnitine palmitoyl transferase, acetyl-CoA carboxylase, fatty acid synthase, and HMG CoA reductase) and suppressed oxidative stress via enhancement of activities of SOD, catalase, and GPx along with GSH content elevation in the liver. In conclusion, this study suggests that ZB supplementation might ameliorate the obesity-associated NAFLD via attenuation of elevated lipogenesis and oxidative stress in liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a persistent disease caused by dysregulated energy homeostasis pathways that persuade the addition of adiposity, which in turn results in the improvement or exacerbation of weight-associated comorbidities (Tchang et al. 2021). Over the last few years, obesity has turn out to be a universal outbreak and an imperative communal health problem in several countries. This state is mainly due to extreme utilization of simple sugars and saturated fats, which, connected with sedentarism, symbolize the contemporary way of life (Shaunak et al. 2021). Obesity is documented as a menace factor for numerous disorders including type 2 diabetes (T2DM) (Uddandrao et al. 2020) and non-alcoholic fatty liver disease (NAFLD) (Shaunak et al. 2021). NAFLD includes a range of ever more severe clinicopathological conditions ranging from fatty liver to steatohepatitis without or with hepatic cirrhosis or fibrosis. Recent data recommends that NAFLD is also allied with chronic kidney disease (Byrne and Targher 2020) and cardiovascular (Kasper et al. 2020).
The primary deposition of fat followed by consequent oxidative stress is essential to the development of liver damage (Chen et al. 2020). The model of NAFLD is developed via “two-hit theory.” The primary one is the insulin resistance (IR) that augments nutritional influx and enhances hepatic lipogenesis, ensuing in the gathering of triglycerides (TGs) and free fatty acids (FFAs) in the liver (Hu et al. 2020), and the second is the mishmash of lipid peroxidation and oxidative stress, which is considered the most important contributor to hepatic injury and disease evolution in NAFLD (Chen et al. 2020). It has been extensively documented that NAFLD is the hepatic materialization of obesity. Unwarranted exposure to a high-fat diet (HFD) has been initiated as a key characteristic in a growing number of individuals with NAFLD, which is designated by the fact that the pervasiveness of NAFLD is documented to be up to 90% in obese individuals (Wang et al. 2021). With the modernity of obesity and its associated metabolic syndrome, the occurrence of NAFLD will to be expected to mount needing imperative notice around the globe.
At present, everyday life intercession with exercise and diet is still the foundation in the supervision of individuals with NAFLD; the degree of liver histological perfection is directly relative to the quantity of weight lost. On the other hand, it can be hard to put into practice. Therapeutic drug modalities for NAFLD embattled toward some initiation factors such as IR, dyslipidemia, and oxidative stress might also alleviate the evolution of this disease (Than and Newsome 2015). In recent times, several natural compounds have been confirmed to show efficient effects on NAFLD. Therefore, the regular supplementation of natural bioactive substances may turn out to be a hopeful approach for NAFLD control while it exhibited effectual outcomes with no or less side effects (Bagherniya et al. 2018). Zingiberene (ZB) is a monocyclic sesquiterpene, which is the prime component of ginger (Zingiber officinale) and in addition, earlier reports revealed that ZB has diverse therapeutic effects such as a natural anticancer agent (Togar et al. 2015) and antioxidant (Yeh et al. 2014). On the other hand, there was no systematic evidence accessible on effect of ZB against NAFLD in animal models or any other clinical models. Therefore, the current study intended to find out therapeutic efficacy of ZB against HFD-induced NAFLD in rats.
Materials and methods
Chemicals
ZB was obtained from the Sigma-Aldrich, India, and the rest of the chemicals utilized for this study were analytical grade.
Animals
The male Wistar rats (weighing 120–140 g; aged at 8–10 weeks) were acquired from the Nandha College of Pharmacy, Erode, Tamil Nadu, India, and the animals were firstly acclimatized for the episode of 1 week with a 12-h day/night cycle, in a temperature of 22 ± 2 ºC and humidity of 45–64%. The rats were allowed to drink water ad libitum in polycarbonate bottles. The set of rules of this study was permitted by Institutional Animal Ethical Committee (IAEC), Nandha College of Pharmacy (Approval No: 688/PO/Re/S/02/CPCSEA).
HFD composition
HFD was commercially purchased (National Institute of Nutrition, Hyderabad, India) and composed of corn starch (15%), sugar (27.5%), lard oil (17.6%), vitamin mixture (1%), mineral mixture (3.5%), casein (20%), cellulose powder (5%), corn oil (9.9%), and choline bitartrate (0.2%).
Experimental design
The experimental rats were dived into four groups, and each group comprises six animals. NAFLD model was developed in rats by supplementation of HFD. The HFD (20 g daily) was supplemented to group II throughout the study without any drug treatment as a disease control model, and at the same time, group IV was supplemented with HFD for 15 weeks and 16th week onwards treated with ZB (50 mg/kg BW) for the period of 45 days orally once a day by using intragastric tube. On the other hand, groups I and III were fed with normal pellet diet as prepared according to AIN-93 guidelines with all the recommended macro and micronutrients as described earlier by Brahma Naidu et al. (2016). On the basis of the acute toxicity study, one-fifth (50 mg/kg body weight) maximum oral dose was selected for this study.
-
Group I: Normal control
-
Group II: HFD-induced NAFLD control
-
Group III: Normal control + ZB (50 mg/kg body weight)
-
Group IV: NAFLD + ZB (50 mg/kg body weight)
At the end of the experiments, all the rats were overnight fasted, and blood was collected by retro orbital sinus puncture method with mild anesthesia via intraperitoneal administration of pentobarbital sodium (40 mg/kg/BW) (Sangeethadevi et al. 2021). Then, the rats were sacrificed by the cervical decapitation, and liver was collected immediately and stored at –80 ºC until further use.
Assessment of body weight, blood glucose, insulin, and IR
At the end of the experimental treatment duration, body weights of the animals were recorded. On the other hand, plasma glucose (Sigma-Aldrich kits, India) and insulin (Bio-Merieux, France) were determined by using the respective kits. IR was measured by using the homeostasis model assessment (Rameshreddy et al. 2018).
Estimation of blood lipid profiles
Blood samples were centrifuged at 4000 rpm for 10 min to take apart plasma for analyzing lipid profile. The levels of low density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TGs), and total cholesterol (TC) were measured by using commercially available kits (Sigma-Aldrich kits, India).
Determination of liver tissue lipid profile
The liver tissues were collected from the sacrificed animals and washed with ice-cold physiological saline; dried out, homogenized in icy chloroform–methanol (2:1, v/v), and the contents were extracted after 24 h, and this was repeated for four times. The combined filtrate was washed with 0.7% KCl, and the aqueous layer was leftover. The organic layer was prepared up to a known volume with chloroform and used for tissue lipid analysis. The markers like TGs, TC, FFA, and phospholipids (PL) were assessed as described by enzymatic colorimetric methods using kits (Nicholas Piramal India Ltd., Mumbai, India).
Measurement of lipogenesis marker enzymes in liver
Carnitine palmitoyl transferase (CPT) (cat no: MBS2602676, Mybiosource, USA), total acetyl-CoA carboxylase (ACC) (cat. no. 7996C Cell Signaling Technology, USA), total fatty acid synthase (FAS) (cat. no. NBP2-82,490, Novus Biologicals, India), and HMG CoA reductase (HMGR) (cat. no. LS-F15758, LifeSpan BioSciences, USA) were measured by respective ELISA kits.
Determination of oxidative stress markers in liver
The levels of TBARS (thiobarbituric acid reactive substances) and glutathione (GSH) and the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in liver tissue were quantified using commercially obtainable kits, procured from Cayman Chemical Company, USA, following the manufacturer’s instructions.
Statistical analysis
The results of the study were articulated as the mean ± SD and n = 6. All the data in the group were evaluated statistically with SPSS 19.0 software. Statistical analysis was performed by one-way analysis of variance and the least-significant difference test. P values < 0.05 were considered significant.
Results
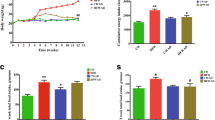
Figure 1 depicts that there was a noteworthy (P < 0.05) increase in the body weights, blood glucose, insulin, and IR in the experimental obese rats. Conversely, supplementation of ZB to the obese rats (group IV) significantly (P < 0.05) reduced the body weights (Fig. 1A), glucose (Fig. 1B), insulin (Fig. 1C), and IR (Fig. 1D) when compared to group II. On the other hand, group III did not show any changes in these parameters.
Table 1 demonstrates that HFD supplementation to rats notably elevated the levels of LDL, TGs, and TC and concomitant decrease in the levels of HDL when compared to group I. But the obese rats treated with ZB (group IV) exhibited noteworthy (P < 0.05) reduction in the levels of LDL, TGs, and TC and enhancement of HDL levels. However, group III rats have shown moderate changes in lipid profiles.
Figure 2 depicts the lipid profiles of liver in control and experimental obese rats. HFD supplementation to the rats significantly developed NAFLD which was confirmed by the elevated levels of TC (Fig. 2A), TGs (Fig. 2B), FFA (Fig. 2C), and PL (Fig. 2D) in the liver tissue. At the same time, obese rats treated with ZB (group IV) predominantly (P < 0.05) attenuated the hyperlipidemia in the liver. On the other hand, group III rats showed slight alterations in the lipid profile of liver.
Figure 3 represents those lipogenesis marker enzyme activities of liver in control and experimental obese rats. HFD supplementation to rats showed significant elevation in the activities of HMGR (Fig. 3A), FAS (Fig. 3B), ACC (Fig. 3C), and concomitant reduction in CPT-1 activity (Fig. 3D) in liver. Interestingly, obese rats supplemented with ZB (group IV) for the period of 45 days notably (P < 0.05) restored the altered activities of these enzymes to near normal when compared to group II.
Figure 4 demonstrates the oxidative stress status of the liver in control and experimental obese rats. This study revealed that there was a noteworthy (P < 0.05) elevation of TBARS levels (Fig. 4A) and concomitant reduction in the GSH (Fig. 4B), CAT (Fig. 4C), SOD (Fig. 4D), and GPx (Fig. 4E) in group II rats. At the same time, rats treated with ZB (group IV) successfully attenuated oxidative stress by increasing GSH content and activities of SOD, CAT, and GPx in obese rats. On the other hand, group III rats demonstrated notable (P < 0.05) antioxidant activity when compared to untreated normal control and obese rats.
Discussion
NAFLD develops over years as a consequence of the gathering of diverse threat factors such as amount and composition of diet, chronic stress, and sedentary lifestyle that are the decisive key factors for the materialization and cruelty of the disease (Kucera and Cervinkova 2014). On the other hand, HFD-treated animals attain obesity and also augmented epididymal fat, hyperglycemia and IR, because these linked comorbidities are also extensively used as a method of provoking hepatic steatosis, as they are the foundation to liver alterations like to that of human NAFLD (Jahn et al. 2019). For that reason, in the current study, we induced obesity by supplementation of HFD, and this model successfully progresses in to NAFLD model which was confirmed by the altered lipid metabolism in the liver. Then, we evaluated the therapeutic effects of ZB against NAFLD.
Progressively earlier numbers sustain a complex relationship between the metabolic condition T2DM and the pathologically defined NAFLD. NAFLD expects the development of T2DM and vice versa, and each state may hand out as a development feature for the other (Williams et al. 2013). NAFLD is an element of the metabolic syndrome and is robustly connected with IR. Mounting proofs point out that IR contributes to NAFLD in a straight line by increasing de novo lipogenesis and not directly by escalating FFA flux to the hepatic via decreased reticence of lipolysis. A number of rodent and clinical studies have established that IR is the initial step and physiopathological input towards NAFLD improvement and its brutality (Fargion et al. 2014). Similarly, in the current study, we found elevated levels of blood glucose, insulin, and IR in group II rats. Hyperglycemia, hyperinsulinemia, and elevated IR associated with HFD administration were restored to normal control levels after ZB treatment which might be due to that the ZB is able to increase the insulin access to the cells and thereby control the glucose homeostasis.
In addition, in the dietary pattern of Western countries, too much ingestion of high calories; affluence in fat, cholesterol, and sugar; and physical inactivity are risk factors for hepatic damage. Such features have a collision on lipid metabolism associated to liver diseases, which consist of decreased renovation of cholesterol to bile acids, augmented hydrolysis of cholesterol esters to liberated cholesterol (Min et al. 2013), improved endogenic cholesterol synthesis (Musso et al. 2013), increased assimilation of cholesterol-rich lipoproteins, and decreased cholesterol emission. Surplus cholesterol influences the flexibility of the cell membrane, distressing the function of its proteins (Ioannou 2016). At the same time, in the current study, we found that elevated levels of TC, TGs, LDL, and concomitant decrease in HDL in group II rats. On the other hand, ZB supplementation (group IV) notably attenuated the dyslipidemia in the blood. An individual with NAFLD in a slightly high TG and TC levels and in the case of HDL the minute level was autonomously connected with NAFLD incidence. The increase of serum HDL levels was connected with a roughly 40% diminish in NAFLD incident; quite the opposite, reduced serum HDL levels were allied with just about 70% augmentation in NAFLD, which established with NAFLD features of low HDL levels, as documented before (Souza et al. 2012). By multivariate investigation, NAFLD was found to be linked with the scale of dyslipidemia, corroborating an earlier documented perception that dyslipidemia is powerfully related with many predictors of cardiovascular disease, and in particular, NAFLD was amplified by more or less twofold with an enhancement in the amount of serum TG levels (Peng et al. 2017).
During the HFD-fed circumstances, an increase of chylomicrons is a usual aspect that causes a rise in the levels of TC, TGs, and endogenous VLDL production. The increased discharge of FFAs from fat tissue to different tissues increases, mainly to the liver in obesity (Uddandrao et al. 2020), and it might be because of severe leptin resistance. The increase in the level of TGs might be due to the dietary cholesterol that had appeared to decrease unsaturated fat oxidation, which thus prolonged the levels of hepatic and plasma TG and caused the unnecessary accretion of TGs in the lipid stores (Swapna et al. 2019). The eminent level of PL in this study was perhaps due to the lofty levels of FFA and TC. However, high HDL-C levels make possible the transportation of excess cholesterol to the liver for emission in the form of bile (Meriga et al. 2017). Oral administration of ZB considerably suppressed the levels of TC, TGs, FFAs, and PLs and the LDL-C levels of the rats with NAFLD. Meriga et al. (2017) documented that supplementation of piperine which is a phytoconstituent isolated from Piper nigrum caused a hypolipidemic state by plummeting cholesterol absorption and secretion from the intestine, which leads to the decreased availability of FFAs to the liver. The results of our study are in line with these findings.
Deregulated lipid metabolism is a trademark of NAFLD and derivatives of fatty acids and non-TG lipids cause lipotoxic hepatic damage (Haberl et al. 2020). The obese rats demonstrated abnormalities in hepatic tissue lipid biomarkers that impersonate the commencement of a cluster of metabolic risks encompassing dyslipidemia. In the present study, we found a significant elevation in the levels of TC, TGs, FFA, and PL in group II rats. On the other hand, ZB significantly lowered the HFD-induced rise in FFA level, representing that the reduction in FFA noted in group IV might have endorsed in ameliorating the IR and recovering the insulin signaling by the ZB or vice versa, as lessening of IR can result in lowering of FFA influx to liver (Sasidharan et al. 2014). Previous data evidently incriminate hepatic IR as the major feature accountable for the accretion of FFA as TGs in hepatocytes in NAFLD (Hamad et al. 2011). Thus, treatment strategies for NAFLD, right now, are chiefly determined on weight loss and utilize insulin-sensitizing agents (Attar and Van Thiel 2013). In this study, we noted noticeable reduction of hepatic TG level as observed with ZB administration that can be well authenticated with the reduction in FFA level and in addition to enhancement in insulin sensitivity and decreased body weight.
Lipid homeostasis is continued by adipocytes by lipolysis and lipogenesis and prominent lipogenesis stoutly connected with obesity, NAFLD, IR, and T2DM (Uddandrao et al. 2020). Liver is well known to be a key provider to boost cholesterol manufacture in obesity. HMGR, an endoplasmic reticulum bond and peroxisomal enzyme, catalyzes the diminution of HMG-CoA to CoA and mevalonate, the rate-limiting reaction in the de novo synthesis of TC. HMGR in obese control rats substantiate a well-known fact that obesity is correlated to a prominent TC balance and an enthused synthesis of cholesterol, predominantly in the liver (Brahma Naidu et al. 2016). FAS, a vital lipogenic enzyme, regulates the conversion of acetyl-CoA to long-chain fatty acids, and eminent activity leads to lofty lipogenesis (Ferre and Foufelle 2010). ACC is the major enzyme in de novo lipogenesis and a physiologic halt of CPT, which manufactures malonyl CoA in the liver (Brahma Naidu et al. 2016). Therefore, these enzymes play an important role in the lipid metabolism in the liver, and alterations may lead to dyslipidemia then NAFLD. In the current study, we found that elevation in the activities of HMGR, FAS, and ACC and concomitant reduction in CPT-1 activity in the liver of group II rats indicate the elevated lipogenesis and might be the reason behind altered lipid profiles in both blood and tissue. Interestingly, ZB supplementation to the obese rats (group IV) successfully restored these altered activities of enzymes in to normal range. Overall, this study suggests that ZB might inhibit cholesterol biosynthesis by blocking substrate access to the active site of the HMGR, and it also regulates FAS which in turn markedly attenuates hyperlipidemia through decreased fatty acid synthesis. On the other hand, ZB suppressed the activity of ACC and enhanced the CPT-1 activity via attenuation of increased glucose and lipid levels (Kalaivani et al. 2019). Hence, this study suggests that ZB can suppress the lipogenesis and thereby protect the liver.
NAFLD is robustly connected with the occurrence of oxidative stress, and conflict in lipid metabolism shows the way to hepatic lipid build up, which affects diverse reactive oxygen species generators (Chen et al. 2020). At high concentrations, reactive oxygen species cause oxidative changes to cellular macromolecules such as lipids, proteins, DNA, etc. and leads to the accretion of damaged macromolecules, inducing damage of the liver (Serviddio et al. 2013). Oxidative stress caused by the expenditure of a HFD is obvious in most investigational models and individuals with clinical conditions (Jansy et al. 2021; Mohammadi et al. 2015). Similarly, the levels of the lipid peroxidation product TBARS were increased in group II rats. TBARS are products of lipid peroxidation frequently considered to find out the equilibrium between antioxidation and oxidation. The present study demonstrated an increase in TBARS levels and suggests an augmented production of reactive species with possibility to cause damage to cell membranes. Physiologically, an increase in H2O2 production can increase the levels of TBARS and obesity associated with a dyslipidemic profile and promotes lipid peroxidation through development of free radicals and ROS (Chielle and Casarin 2017). From this study, we found that ZB can suppress the levels of TBARS by preventing lipid peroxidation by exerting its antioxidant potential.
Oxidative damage in cells is consistently linked with instability in multiple enzymatic and non-enzymatic antioxidant defense systems that occur in the cells. Enzymatic antioxidants such as CAT, GPx, and SOD synergistically put off lipid peroxidation and hunt reactive oxygen species (Rameshreddy et al. 2018). The raise in oxidative stress parameters might be coupled with the decreased SOD, GPx, and CAT activities and associated diminishing in GSH content noted in the present study. On the other hand, in our study, ZB supplementation prevented the oxidative stress and restored the tissue antioxidants in obese rats. GSH is a principal endogenous antioxidant that balances for the damage caused by free radicals, and it is concerned in the defense of normal cell function and organization through provisions of the extinguishing of free radicals, redox homeostasis, and contribution in detoxification reactions (Sathibabu Uddandrao et al. 2019). SOD is the defending antioxidant enzyme that eliminates superoxide anion free radicals by transforming extreme ROS to low reactive H2O2 radicals and maintains the altitude of O2•− in the body. CAT is an iron-containing antioxidant enzyme that decomposes H2O2 radicals, eliminating its toxic effect. GPx is a selenium-containing antioxidant enzyme and blocks the free radical chain reaction and translates the intermediate and end products of lipid peroxidation reaction into particular alcohols and also decomposes H2O2 to H2O molecules (Pavithra et al. 2020). During obesity, free radicals formed at the damage site amend the levels of CAT, GPx, and SOD. Therefore, a decrease in the activity of antioxidant enzymes results in the gathering of free radicals, including superoxide anion radicals and H2O2, which damages the organs (Sathibabu Uddandrao et al. 2019; Uddandrao et al. 2022). This phenomenon robustly proposes that superoxide radicals and other free radicals are generated subsequent supplementation of HFD in experimental rats. At the same time, treatment of ZB significantly restored the levels of CAT, GPx, and SOD by increasing antioxidant enzyme activity, prevented the damage of liver by scavenging the free radicals, and exerted its antioxidant potential.
In conclusion, supplementation of ZB to HFD-induced NAFLD rats can restore the IR, altered glucose homeostasis, and lipid profiles along with elevation of the antioxidant system in the liver. Overall, the results of the present study suggested that ZB might able to attenuate the HFD-induced NAFLD by suppressing lipogenesis and oxidative stress in the liver. However, further detailed study is necessary to validate this therapeutic activity in advanced level before developing as a drug for NAFLD.
References
Attar BM, Van Thiel DH (2013) Current concepts and management approaches in nonalcoholic fatty liver disease. Sci World J 2013:481893
Bagherniya M, Nobili V, Blesso CN, Sahebkar A (2018) Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: a clinical review. Pharmacol Res 130:213–240
Byrne CD, Targher G (2020) NAFLD as a driver of chronic kidney disease. J Hepatol 72(4):785–801
Chen Z, Tian R, She Z, Cai J, Li H (2020) Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med 152:116–141
Chielle EO, Casarin JN (2017) Evaluation of salivary oxidative parameters in overweight and obese young adults. Arch Endocrinol Metab 61(2):152–159
Fargion S, Porzio M, Fracanzani AL (2014) Nonalcoholic fatty liver disease and vascular disease: State-of-the-art. World J Gastroenterol 20:13306–13324
Ferre P, Foufelle F (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab 12:83–92
Haberl EM, Pohl R, Rein-Fischboeck L et al (2020) Hepatic lipid profile in mice fed a choline-deficient, low-methionine diet resembles human non-alcoholic fatty liver disease. Lipids Health Dis 19:250
Hamad EM, Taha SH, Abou Dawood AGI, Sitohy MZ, Abdel-Hamid M (2011) Protective effect of whey proteins against nonalcoholic fatty liver in rats. Lipids Health Dis 10:57
Hu Y, Yin F, Liu Z, Xie H, Xu Y, Zhou D, Zhu B (2020) Acerola polysaccharides ameliorate high-fat diet-induced non-alcoholic fatty liver disease through reduction of lipogenesis and improvement of mitochondrial functions in mice. Food Funct 11(1):1037–1048
Ioannou GN (2016) The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab 27:84–95
Jahn D, Kircher S, Hermanns HM, Geier A (2019) Animal models of NAFLD from a hepatologist’s point of view. Biochim Biophys Acta Mol Basis Dis 1865(5):943–953
Jansy A, Uddandrao VVS, Sangeethadevi G, Saravanan G, Chandrasekaran P, Sengottuvelu S, Tamilmani P, Sethumathi PP, Vadivukkarasi S (2021) Biochanin A attenuates obesity cardiomyopathy in rats by inhibiting oxidative stress and inflammation through the Nrf-2 pathway. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2021.1874017
Kalaivani A, Uddandrao VVS, Parim B, Ganapathy S, Sushma NPR, Kancharla C, Rameshreddy P, Swapna K, Sasikumar V (2019) Reversal of high fat diet-induced obesity through modulating lipid metabolic enzymes and inflammatory markers expressions in rats. Arch Physiol Biochem 125(3):228–234
Kasper P, Martin A, Lang S et al (2020) NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01709-7
Kucera O, Cervinkova Z (2014) Experimental models of non-alcoholic fatty liver disease in rats. World J Gastroenterol 20(26):8364–8376
Meriga B, Parim B, Chunduri VR, Naik RR, Nemani H, Suresh P, Ganapathy S, Sathibabu Uddandrao VV (2017) Antiobesity potential of piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr Metab (lond) 14:72
Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J et al (2013) Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab 15(5):1–3
Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z, Djalali M, Tehrani-Doost M, Hosseini M, Eghtesadi S (2015) Effects of probiotics on biomarkers of oxidative stress and inflammatory factors in petrochemical workers: a randomized, double-blind, placebo-controlled trial. Int J Prev Med 6:82
Musso G, Gambino R, Cassader M (2013) Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res 52:175–191
Naidu PB, Uddandrao VV, Naik RR, Suresh P, Meriga B, Begum MS, Pandiyan R, Saravanan G (2016) Ameliorative potential of gingerol: Promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol 419:139–147
Pavithra K, Sathibabu Uddandrao VV, Chandrasekaran P, Brahmanaidu P, Sengottuvelu S, Vadivukkarasi S, Saravanan G (2020) Phenolic fraction extracted from Kedrostis foetidissima leaves ameliorated isoproterenol-induced cardiotoxicity in rats through restoration of cardiac antioxidant status. J Food Biochem 44(11):13450
Peng K, Mo Z, Tian G (2017) Serum lipid abnormalities and nonalcoholic fatty liver disease in adult males. Am J Med Sci 353(3):236–241
Rameshreddy P, Uddandrao VVS, Brahmanaidu P, Vadivukkarasi S, Ravindarnaik R, Suresh P, Swapna K, Kalaivani A, Parvathi P, Tamilmani P, Saravanan G (2018) Obesity-alleviating potential of asiatic acid and its effects on ACC1, UCP2, and CPT1 mRNA expression in high fat diet-induced obese Sprague-Dawley rats. Mol Cell Biochem 442(1–2):143–154
Sangeethadevi G, VV SU, Jansy Isabella RAR, Saravanan G, Ponmurugan P, Chandrasekaran P, Sengottuvelu S, Vadivukkarasi S (2021) Attenuation of lipid metabolic abnormalities, proinflammatory cytokines, and matrix metalloproteinase expression by biochanin-A in isoproterenol-induced myocardial infarction in rats. Drug Chem Toxicol https://doi.org/10.1080/01480545.2021.1894707
Sasidharan SR, Joseph JA, Anandakumar S, Venkatesan V, Madhavan CN, Agarwal A (2014) Ameliorative potential of Tamarindus indica on high fat diet induced nonalcoholic fatty liver disease in rats. Sci World J 2014:507197
Sathibabu Uddandrao VV, Brahmanaidu P, Ravindarnaik R, Suresh P, Vadivukkarasi S, Saravanan G (2019) Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic rats. Eur J Nutr 58(6):2425–2437
Serviddio G, Bellanti F, Vendemiale G (2013) Free radical biology for medicine: Learning from nonalcoholic fatty liver disease. Free Radic Biol Med 65:952–968
Shaunak M, Byrne CD, Davis N, Afolabi P, Faust SN, Davies JH (2021) Non-alcoholic fatty liver disease and childhood obesity. Arch Dis Child 106(1):3–8
Souza MR, Diniz Mde F, Medeiros-Filho JE et al (2012) Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol 49:89–96
Swapna K, Sathibabu Uddandrao VV, Parim B, Ravindarnaik R, Suresh P, Ponmurugan P, Santhanaraj B, Vadivukkarasi S, Harishankar N, Prathap Reddy K, Nivedha PR, Saravanan G (2019) Effects of asiatic acid, an active constituent in Centella asiatica (L.): Restorative perspectives of streptozotocin-nicotinamide induced changes on lipid profile and lipid metabolic enzymes in diabetic rats. Comp Clin Pathol 28:1321–1329
Tchang BG, Saunders KH, Igel LI (2021) Best practices in the management of overweight and obesity. Med Clin North Am 105(1):149–174
Than NN, Newsome PN (2015) A concise review of nonalcoholic fatty liver disease. Atherosclerosis 239:192–202
Togar B, Turkez H, Tatar A, Hacimuftuoglu A, Geyikoglu F (2015) Cytotoxicity and genotoxicity of zingiberene on different neuron cell lines in vitro. Cytotechnology 67(6):939–946
Uddandrao VVS, Parim B, Singaravel S, Ponnusamy P, Ponnusamy C, Sasikumar V, Saravanan G (2022) Polyherbal formulation ameliorates diabetic cardiomyopathy through attenuation of cardiac inflammation and oxidative stress Via NF-κB/Nrf-2/HO-1 pathway in diabetic rats. J Cardiovasc Pharmacol 79(1):75–86
Uddandrao VVS, Rameshreddy P, Brahmanaidu P, Ponnusamy P, Balakrishnan S, Ramavat RN, Swapna K, Pothani S, Nemani H, Meriga B, Vadivukkarasi S, Nivedha PR, Ganapathy S (2020) Antiobesity efficacy of asiatic acid: Down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Arch Physiol Biochem 126(5):453–462
Wang H, Mehal W, Nagy LE et al (2021) Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol 18:73–91
Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM (2013) Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev 34(1):84–129
Yeh H, Chuang C, Chen H, Wan C, Chen T, Lin L (2014) Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT-Food Sci Technol 55:329–334
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The protocol of the study was approved by the Institutional Animal Ethical Committee, Nandha College of Pharmacy (Approval No: 688/PO/Re/S/02/CPCSEA), and the experiments were carried out as per the guidelines described by CPCSEA, Government of India.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sivaraj, R., Jaikumar, S. & Sengottuvelu, S. Zingiberene attenuates high fat diet–induced non-alcoholic fatty liver disease through suppression of lipogenesis and oxidative stress in rats. Comp Clin Pathol 31, 201–209 (2022). https://doi.org/10.1007/s00580-022-03321-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03321-9