Abstract
Diseases in captive wildlife constitute a major challenge to the function of zoological gardens only second to management. These diseases vary in etiology depending on susceptibility, the environment, and husbandry method used. Exposure to infectious diseases is known to be responsible for the declining figures in wild animal population especially wild felids. Sick or dead animals subjected to laboratory diagnosis, diagnostic imaging, and necropsy from three (3) zoological gardens in Nigeria between 2014 and 2018 were included in this study, while dead animals not subjected to any form of clinical, laboratory, and necropsy diagnosis were excluded. Between 2014 and 2018, carcass submission at the Jos, Kano, and Unilorin zoo comprised wild ungulates, non-human primates, small mammals, reptiles, felids, and aviary from the selected zoological gardens combined. In total, sixty-six (66) cases were studied, and this included a wide variety of animal species. All cases resulted in mortalities distributed across these zoological gardens. Non-infectious conditions accounted for 23% of total cases reported including fractures, nutritional deficiencies, toxicity, and dystocia, among others. Infectious diseases including bacterial, parasitic, and viral were the dominant groups of diseases of captive wild animals in these zoos. Bacterial infections were the most common infectious causes accounting for 41% of the cases. Isolates include Mannheimia haemolytica biotype A serotype 2 (A2), Salmonella spp, and Escherichia coli. While non-infectious causes were responsible for some mortalities either singly or as co-morbidities with bacterial agents, mixed causes accounted for 15% of all the cases. This study enumerates the common diseases, species affected, in wild captive animals in three zoological gardens in Nigeria, making the ation available to clinicians, biologists, pathologists, public health workers, and policy makers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild animals are held in captivity in zoological gardens majorly for recreational, educational, and entertainment purposes (Agoramoorthy and Hsu 2005). Also, these zoological gardens serve conservation efforts of endangered wild animal species (Kohn 1994). Amongst the exhibits of zoological gardens are reptiles, birds, primates, ungulates, felines, and other mammals. In 1999, a survey enumerated that the Jos zoological garden housed 62 mammals of 25 species, 60 birds of 18 species, and 39 reptiles of 9 species (Galleria Media Limited 2018). These included chimpanzees, baboons, lions, hyenas, monkeys, crocodiles, tortoise, geese, stork, horses, camel, snake, rabbits, owl, pigeons, and varied species of eagles, which totaled about 130 animals including those in seclusion (Galleria Media Limited 2018). Still within the north-central of the country, the University of Ilorin zoological garden houses primates, carnivores, hoofed mammals, birds, and reptiles. They are 25 carnivores (only 3 lions), 18 ungulates, 18 primates, and 9 reptiles, equids (Mules, donkeys, and a horse), and camels and several birds (Kolapo and Jegede 2017). In the south-west, other zoos including the university of Ibadan zoological gardens exhibit species that include reptiles, birds, primates, herbivores, and carnivores (Emikpe et al. 2016).
There is a dearth of information regarding diseases of captive wildlife that plague various collections and various terrains in Nigeria (Emipke et al. 2016). A detailed investigation of the causes, pathology, and pathogenesis of the mortalities in captive wild animals on exhibition in Nigerian zoological gardens is an integral component of a good conservation effort aimed at not only bringing wildlife closer to the society but also militating against the extinction of wildlife. Wild animals that are sick are said to be sluggish in their activity, and may appear dull with depression (Jani 2006). Body part movement are said to be restricted including the movements of ear, trunk, tail, and legs (Jani 2006). These animals become partially or completely anorexic (lack of interest in feeding). Abdominal pains are expressed as grunting or groaning sound with restlessness, lying down, and getting up. Urination, rumination, defecation, and lacrimation are also said to be reduced or increased in some disease conditions (Jani 2006), like increase defecation in gastrointestinal diseases. Diseases in captive wildlife constitute a major challenge to the running of zoological gardens second to management. These diseases vary in etiology depending on susceptibility, the environment, and husbandry method used. Exposure to infectious diseases is known to be responsible for the declining figures in wild animal population especially wild felids (Thalwitzer et al. 2010). Also, as reported earlier, most spontaneous diseases of wild animals remain unpublished or is in departmental archives as pathology reports (Aguirre and Pearl 2005), making such information inaccessible by veterinarians and policy makers for database archiving purposes and for national wildlife disease control programs.
Infectious and non-infectious diseases continue to plague wild animals kept in captivity. Infectious diseases vary considerably based on the infectious agent, be it bacteria, parasites, viruses, and protozoal, which have been described separately according to the infected species (Jani 2006). The major non-infectious diseases of captive animals are described as a common for all species (Jani 2006), and may include tooth and tusk problems, choke, tympani, indigestion, constipation, intestinal obstruction, colic and enteritis, heat stroke, stress, pneumonia, fractures, overgrown soles, and poisoning to mention but a few (Jani 2006). Infectious parasitic diseases of captive wild animals seem to be the most reported; for instance, reports on gastrointestinal parasites dominate in southwest zoos (Kolapo and Jegede 2017; Emikpe et al. 2016; Otegbade and Morenikeji 2014; Adetunji 2014; Adetunji and Adesope 2014), because of the ease of conducting fecal sampling.
Since the founding of the Jos zoo in 1956 (Galleria Media Limited 2018), the Kano zoo in 1972 (Adams and Salome 2014), and the University of Ilorin zoo in 1985 (Kolapo and Jegede 2017), occasional and limited disease reporting have been made, which justifies the need for a holistic disease investigation. Therefore, this study is aimed at documenting the fatal diseases of the different species of captive wild animals housed within the 3 selected zoos in Nigeria (Jos, Kano, and University of Ilorin zoological gardens) based on occasional reports about disease incidence and presence of disease agents in these captive animals. Therefore, knowledge about pathology of the common diseases, species affected, including those in rare felids, is important for clinicians, biologists, pathologists, and public health workers.
Materials and methods
Animals
Inclusion criteria for this study were animals necropsied, dead animals subjected to laboratory diagnosis, and dead animals subjected to diagnostic imaging, while the exclusion criteria include dead animals not subjected to any form of clinical, laboratory, and post-mortem diagnosis, and dead but not necropsied animals due to autolysis of carcasses. Between 2014 and 2018, carcass submission at the Jos, Kano, and Unilorin zoo comprised reptiles, birds, wild ungulates, non-human primates, small mammals, and felids. In total, sixty-six (66) cases were studied, and this included a wide variety of animal species (see Table 1).
Physical and clinical examination
Thorough physical and clinical examinations were carried out on wild captive animals antemortem to determine obvious causes of ill health in the three zoological gardens. Physical manipulation following physical, or chemical restrain, sample collection, and diagnostic imaging were carried out. All non-infectious illness were determined during these examinations.
Laboratory investigation ante mortem and postmortem
Bacteria
For bacteria isolation, postmortem samples of liver, spleen, and heart were cultured aerobically and anerobically at 37 °C on blood agar and McConkey agar using standard procedure as previously described (Kumbish et al. 2006). Salmonella spp. and Escherichia coli were identified using standard laboratory methods (Parmer and Davies 2007). Mannheimia spp from lung samples that were collected from the necropsied captive animals were isolated and identified by standard procedures as earlier described by Ekong et al. (2014). Isolates identified as Mannheimia haemolytica were biotyped and capsule serotyped. Detection of mycobacteria from suspected tuberculous lesions were examined microscopically by Ziehl-Nielsen (Z-N) staining technique for the detection of Acid-Fast Bacillus (AFB) and cultured using the Lőwenstein-Jensen (L-J) medium cultured at 37 °C for 8 weeks on paired L-J media enriched with pyruvate (L-J-P medium) and enriched with glycerol (L-J-G medium) as previously described (Cadmus et al. 2004).
Parasites
Fecal/intestinal samples or whole worms and external parasite routinely sent for parasitology analysis, turned out for different parasites which aided the diagnoses or were the main diagnosis in some of the cases. At postmortem examination, carcasses in which gastrointestinal worms were found and identified were sent for parasitic examination. As a routine, intestinal content was scooped in all cases and sent for parasitology, where they were physically examined for consistency and presence of nematode larva or tapeworm proglottids. Fecal analysis was done using simple concentration techniques: flotation and sedimentation tests (Tessaro 1989; Kolapo and Jegede 2017). Recovered ova identification was based on morphologic and micrometric measurements as provided by Bowman and Lynn (1999) and Kolapo and Jegede (2017).
Viruses
Tissue samples especially from ungulates were routinely sent for virological detection or isolation. For virus isolation, suspensions (1:10) in sterile phosphate-buffered saline (PBS) of tissue samples (liver, heart, spleen, kidney, lung, trachea, duodenum, and caecum) were made. The suspension was clarified by centrifugation at 1500 rpm for 15 min, and supernatant was treated with a mixture of penicillin (10,000 IU/ml of supernatant) and streptomycin (10,000 μg/ml of supernatant) for 45 min at 37 °C. One vial of freeze-dried vaccine of each tested virus was reconstituted in 1 ml PBS (positive control). All the field and vaccine (0.2 ml) sample were inoculated onto the chorio allantoic membrane (CAM) of minimal-disease- free 10-day-old developing chicken embryos as described earlier (Akanbi and Taiwo 2014). Following inoculation, the embryos were incubated at 37 °C and checked daily for mortality. Five days post inoculation (PI), CAM were harvested and examined. Subsequent passages were required for adaptation of the virus in CAM.
Necropsy techniques
All dead captive wild animals were either necropsied immediately or preserved in − 20 °C until necropsied. At necropsy, detailed observations were made and samples were taken for ancillary laboratory investigation.
Results
Animal species distribution
In total, sixty-six (66) zoo animals were examined and studied, and this included a wide variety of animal species separated into 7 major groups—reptiles, birds, ungulates, non-human primates, small mammals, canids, and felids (Fig. 1; Tables 1 and 2).
Physical and clinical examination findings
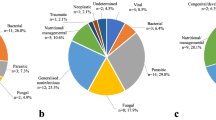
Following physical or chemical restraint on wild captive animals antemortem, thorough physical and clinical examinations revealed several obvious non-infectious causes of ill health in the three zoological gardens. Non-infectious conditions accounted for 23% of total cases reported including fractures, nutritional deficiencies, toxicity, and dystocia. Calorie, protein, and vitamin deficiencies contributed to the fair to poor bodily conditions observed in the lions, civet, monkeys, baboons, and crocodiles, and in some bird species (black kite, Indian peafowl, grey crown crane). Diagnostic imaging was useful to determine cases of fractures and their classifications.
Laboratory investigation
Bacterial
Tissue samples routinely sent for bacteriology turned out for different bacteria isolates which aided the diagnoses or were the main diagnosis in some of the cases. Culture of tissue from the samples yielded mild to heavy growth of different bacteria on a case-by-case basis. Bacterial infections were the most abundant findings as they were reported in 41% of the cases. Isolates include M. haemolytica biotype A serotype 2 (A2), Salmonella spp, and E. coli. Detailed bacteriological findings are given in the individual cases under case reports in Table 3.
Parasites
Detailed parasitological findings are given in the individual cases under case reports in Table 2, while details of various parasites in each species assessed are found in Table 3.
Viral
Detailed virological findings are given in the individual cases under case reports in Table 3. In particular, a sample yielded PPR virus isolate. Viral disease incidence was low, probably due to lack of viral isolation or use of detection tools in most cases.
Non-infectious
This accounted for 23% of total cases reported which include fractures, nutritional inadequacies, toxicity, and dystocia, among others. See Table 2 for details. Some caused mortality on their own, while others were complicated by infectious agents, especially bacterial (Fig. 2).
Mixed
Mixed causes accounted for 15% of cases. These were classified as such when more than one agent or factor contributed to the death of the animal.
One (1) case was classified as inconclusive due to lack of empirical data to arrive at a cause of death.
Necropsy findings
Of the sixty-six (66) carcasses examined, twelve (12) were from Kano zoo and only six (6) were submitted from the Jos zoological garden. The two duikers were in good nutritional status, while one of the lions was emaciated and the other was severely cachectic. The two (monkeys) were in fair bodily condition. Three (3) of the baboons were cachectic, while one was moderately emaciated, and the 5th was in moderately good bodily condition. The three monkeys were all moderately emaciated. The lions and the Civet cat were moderately emaciated. The Crocodiles were moderately emaciated. The other ruminants (3 Sitatunga) were in good bodily condition. The ostrich and the goose were in good bodily condition, while all other bird species (white stork, White peafowl, Black Kite, Indian peafowl, Grey Crowned crane) were moderately emaciated.
Discussion
Due to the fact that the zoological garden serves a safe meeting point for humans and wild animals, there is need to know diseases prevalent in them for control and prevention of zoonotic diseases. This group constitutes a major public health problem (Kruse et al. 2004) and represents more than 75% of human diseases from sources related to wildlife and domestic animals (Taylor et al. 2001).
Many animal deaths evidenced husbandry errors (e.g. wrong diet, disregard for social stratification, and species differences, among others). These include emaciation, cachexia, muscular atrophy, and loss of body fat depots which were determined during physical and postmortem examination. Husbandry inadequacies have been reported as major cause of mortalities in captive animals (Kolapo and Jegede 2017; Emikpe et al. 2016; Anga and Akpavie 2002) in Nigeria. Husbandry/feed problems have also led to similar issues as incriminated by Jegede et al. (2018) in African greater cane rats fed with aflatoxin imbued feed. This is why zoo personnel (e.g. keepers, veterinarians, curators, and biologists) have to be continually reminded that all husbandry practices should be based on principles which minimize stress (Fowler 1996). Malnutrition is an important stressor; others include toxins, parasites, infectious agents, and confinement (Fowler 1996). Although sick captive animals are known to become partially or completely anorexic (Jani 2006), if this was the case in these animals, the zoo attendants should have observed it and bring it to the attention of the zoo veterinarians.
For non-infectious conditions, especially cases of fracture in the African green monkey at Jos zoo and in the crown crane at the Unilorin zoo in which in both cases there were no evidence of external force, the only probable cause is a nutritional deficiency. Nutrition inadequacies were observed as a major issue at all zoos besides health management issues; this therefore is the most rational reason for fractures as nutritional deficiencies (e.g. Vitamin D) have been incriminated as a reason for fractures in captive animals (Lin et al. 2005); therefore, the role of nutrition in captive animal fracture incidence is a researchable area.
Infectious diseases are the dominant groups of diseases of captive wild animals, and infectious diseases vary considerably based on the infectious agent, be it bacteria, parasites, viruses. and protozoal (Jani 2006). The species groups of captive animals studied which included reptiles, birds, ungulate ruminants, non-human primates, and carnivorous felids (Fig. 1; Table 1) all showed various stages of infection and incapacity. Importantly, amongst the ungulate ruminants are co-infections with bacteria (M. haemolytica, E. coli, and Salmonella spp), parasite (Eimeria spp), and a virus (Peste des petits ruminants). Earlier study showed that all the herbivores at the Unilorin zoo except the horse had no intestinal parasite during the dry season as compared to the 72.2% gastrointestinal (GIT) parasite prevalence during the raining season (Kolapo and Jegede 2017), whereas these herbivores were battling severe conditions that had compromised their lungs as shown in Table 2.
Amongst the carnivorous felids especially in two lions and a civet cat (Table 2) were principally severe nematode (Toxocara cati, Trichuris spp) and cestode (Ancylostoma spp and Taenia spp) helminthic infections. The remaining two (2) lions died because of non-infectious causes, which included intestinal obstruction, intussusception and torsion, and oesophageal obstruction with tracheal obtrusion. Choke (oesophageal obstruction and tracheal obtrusion) and intestinal obstruction have been known to be major non-infectious conditions of captive animals (Nemat et al. 2013; Akanbi et al. 2020). Novel, fatal non-infectious findings, which hitherto were not reported in literatures, were recorded among the lions especially at the Unilorin zoo, which is the case of intussusception and choke.
Of the three (3) zoonotic like parasites—Trichuris spp, Toxocara canis, and Ancylostoma spp which can affect the health of handlers and visitors to the zoo (Kolapo and Jegede 2017), both Trichuris and Toxocara infections were seen in two lions examined at the Jos zoo. Zoonotic helminthic infections in lions (toxocariasis and trichuriasis) at the Jos zoo were severe and had a fatal outcome. Heavy intestinal parasitism such as reported here is rare and was unfortunate that such infections could go unnoticed until the fatal outcome at the Jos zoo. Toxocara cati, Trichuris spp, Ancylostoma spp, and Taenia spp causes various syndromes in the host. Cats get infected with hookworms either by skin penetration, by infective larvae or by ingestion of the larvae in the environment or in the paratenic host (rodents). In heavy infestations, hookworms cause anemia and death due to their voracious blood sucking habits. Toxocara spp (roundworm, Order Ascaridida) are small intestinal nematodes of dogs and cats (Zajac et al. 2012). Cats acquire T. cati infection by ingestion of the larvated eggs or paratenic hosts (rodents). Toxocara is an important pathogen in puppies and kittens. T. cati causes chronic ill-thrift in infected animals (Zajac et al. 2012). Trichuris felis is a nematode of the caecum and large intestine of wild cats. Heavy infections cause weight loss, unthriftiness, and profuse bloody diarrhea which may result in death. Trichuris felis and other spp are known to be parasitic worms of the caecum and large intestine of wild cats; in this case, numerous adult worms were found all through the GIT (stomach, duodenum, jejunum, ileum, and large intestine). The same applies to Ancylostoma spp and Taenia spp which were not only found in the small intestine, but in the stomach, and large intestine up to the rectum. The presence of Toxocara cati (JZ), Trichuris spp (JZ), and Ancylostoma spp (UZ) in these zoos where children visit raise public health concerns. Toxocara cati and Trichuris spp are responsible for visceral and ocular larva migrant, while Ancylostoma spp causes eosinophilic enteritis and cutaneous larva migrant in humans (Zajac et al. 2012), and handlers and visitors may also be infected.
One of the primates submitted from the Jos zoo, i.e., the African green monkey died of helminthiasis (Filaria spp) with pulmonary migration and intraerythrocytic parasite (Table 3). This carcass also had a femoral fracture. While the other monkey (Mona) died because of a protozoal (toxoplasmosis) infection. At the Unilorin zoo, the primates (Anubis baboons) suffered from an outbreak of salmonellosis (Salmonella spp) and one baboon died of mycobacteriosis and two of the three patas monkeys died of congestive heart failure and salmonellosis, while the third died of salmonellosis (Table 2). One Anubis baboon suffered from a cerebral haemorrhage (stroke) which was secondary to physical injury. Stroke in baboon and hepatocoel/hepatocyst in patas monkey from the Unilorin zoo was also additional novel findings of this study as this was not reported before now. Among the non-human primates, findings were consistent with documented reported cases (Kalter 1989; Emikpe et al. 2007; Kim et al. 2020). Helminthiasis and pneumonia were the cause of death in the two crocodile reptiles from Unilorin zoological gardens (Table 2). The result of this present studies confirmed earlier findings of parasites in the reptiles (Kolapo and Jegede 2017), as the crocodiles had intestinal nematode worms identified as Ascaris spp.
External parasitism (Pediculosis) was responsible for the death of a white Stork, while atrial rupture and septicaemia were the cause of death in a goose (Table 2). An infant bushbuck also had severe pediculosis which agrees with reports by Durden et al. (2015) that these organisms are vectors for hemoparasites like Anaplasma spp (Egri 2018). Septicaemia secondary to humeral compound fracture caused the death in a crown crane, while acute heart failure secondary to myocardial infarction and hardware disease was responsible for death of an adult ostrich. In the white peafowl, Indian peafowl and a Kite, co-infection with E. coli bacteria and an intestinal protozoal parasite (Eimeria spp), were the cause of death respectively. According to a recent study, the peafowls were the only avian species in which gastrointestinal parasites were isolated of all avian species at the Unilorin zoo (Kolapo and Jegede 2017), but this study showed that the white Stork was heavily infested with lice during the study period which resulted in its death. Also, the Kite had coccidiosis as shown by this report.
The findings that among the ungulates, bacterial and viral septicaemias were the main cause of death (Table 2) at the university of Ilorin zoo is similar to a 23-year study of zoo disease patterns in 262 carcasses comprising ruminants, primates, carnivores, reptiles, equids, rodents, and aviary revealed (Emikpe et al. 2016) that infectious diseases caused by bacteria, parasitic, fungi, and viral agents were the most common disease condition in captive animals in Nigeria. Also, parasitic organisms were most common in different species of wildlife exhibited at the Unilorin zoo (Kolapo and Jegede 2017). At the Jos zoo, parasitic survey had identified an array of parasites in exhibited animals (Dawet et al. 2013) and a much earlier report from the Jos zoo, reported Salmonella spp isolate from Chimpanzee (Ocholi et al. 1987). Much earlier documentation thus exist (Isoun et al. 1972; Idowu et al. 1975; Ikede et al. 1976; Akinyemi and Ikede 1982).
The cause of death of the two (2) duikers at the Jos zoo was rather challenging, tissues were tested by PCR, but gave an unusual band with PPR primer. This needs further sequence analysis or other virological testing to determine which another virus or agent may be responsible. The cases turned out to be multiple co-infections with Maheimmia, Escherichia, and Eimeria.
Among the felids, helminthiasis, gastrointestinal obstruction, intussusceptions, and nutritional inadequacies resulted in the death of this group of animals. However, the prevalence of macroparasite infections in large carnivores has been largely overlooked. The interaction between parasite prevalence and intensity, physiological condition, and susceptibility to disease is well demonstrated in some species, but poorly understood in large carnivores (Berentsen et al. 2012). Furthermore, some wild canids have evolved to cope with a certain level of chronic parasitic infection that has little or no adverse health effects (Kennedy-Stoskopf 2003; Berentsen et al. 2012). It is quite unfortunate that these zoos have lost five (5) lions to avoidable infections and conditions from the during the study periods, whereas helminthic infections in two (2) lions (toxocariasis and trichuriasis) respectively were the cause of the fatal outcome at the Jos zoo, intussusception and choke resulted in the death of two (2) lions at the Unilorin zoo, and a systemic fungal infection in Kano zoo. The findings at the Jos zoo, especially where nematode parasites Toxocara spp and Trichuris spp were responsible for the fatal outcome of infection in two lions, were at variant with earlier study by Dawet et al. (2013) where these GIT parasites were prevalent in non-human primates. The findings in the Jos zoo monkeys (African green monkey and Mona’s monkey) that died were that of bacterial septicaemia. In the non-human primates examined, protozoal, helminthic, and bacterial septicaemias including a granulomatous pneumonia were the major cause of mortalities at the Unilorin zoo. Parasitic infections are common in both zoos studied and in other Nigerian zoos (Kolapo and Jegede 2017; Emikpe et al. 2016; Adetunji and Adesope 2014; Otegbade and Morenikeji 2014; Adetunji 2014; Dawet et al. 2013).
Helminthic parasitic infection was responsible for the death recorded among the crocodiles (Table 2), while bacterial septicaemias, ectoparasitism, physical injury, and heart failure were the cause of mortalities in most of the avian species (Table 2). Colibacillosis was common in the avian species. Generally, captive birds are most susceptible to viral and bacterial septicaemias which have been reported in literatures and this was the case in the study conducted by Emikpe et al. (2016).
Only one virus (PPRV) isolate was recovered from this study, and this is not uncommon as already reported (Emikpe et al. 2016). This is mainly due to the lack of readily available diagnostic facilities for viral detection and isolation.
The detection of the presence of parasites termed zoonotic-like, i.e., Trichuris spp, Toxocara canis, and Ancylostoma spp, raise the zoonotic threat level at the university of Ilorin zoo, and calls for adequate control measures against transmission to handlers and visitors to the zoo (Kolapo and Jegede 2017). This also goes to the Jos zoological garden, where some of these zoonotic-like parasites (whipworm and hookworm) were found in the non-human primates (Dawet et al. 2013). Although, a study of gastrointestinal parasites in primates and their keepers from two zoological gardens in Ibadan showed that there was no evidence of cross transmission of GIT helminths between the non-human primates and zoo keepers (Adetunji 2014).
Stress associated with captivity and closeness to humans has been hypothesized to contribute to how resistant animals are to infections in captivity (Egbetade et al. 2014). This has led to reason some diseases overwhelming the animals within a short period. Although some diseases like mycobacteriosis are rarely diagnosed before death in zoo animals (Lécu and Ball 2011), this therefore gives rise to the need for regular (annual/ni-annual) screening and checks to be done on captive animals especially in Third World countries. Better training should also be given to zoo staff on quick recognition and reporting of captive animal behavioral and corporeal changes.
References
Adams A, Salome AA (2014) Impacts of zoological garden in schools (a case study of zoological garden, Kano State Nigeria). Open J Ecol
Adetunji VE (2014) Prevalence of gastro-intestinal parasites in primates and their keepers from two zoological gardens in Ibadan, Nigeria, Sokoto. J Vet Sci 12(2):25–30
Adetunji V, Adesope A (2014) Some causes of mortalities in captive wild animals in Ibadan, Nigeria: a retrospective study. Niger Vet J 35(2):989–994
Agoramoorthy G, Hsu MJ (2005) Use of nonhuman primates in entertainment in Southeast Asia. J Appl Anim Welfare Sci 8:141–149
Aguirre AA, Pearl MC (2005) New technology and sorta situ: conservation medicine linking captive and wildlife populations. In: Joint Conference, American Association of Zoo Veterinarians; health and conservation of captive and free-ranging wildlife; 2004; San Diego, California, USA
Akanbi OB, and Taiwo, VO (2014) Mortality and pathology associated with highly pathogenic avian influenza H5N1 outbreaks in commercial poultry production systems in Nigeria. Int Sch Res Notices
Akanbi OB, Adam M, Jegede HO, Ajadi A, Atata JA, Raufu I, Aiyedun JO, Shoyinka SV (2020) Fatal multiple intestinal intussusceptions and torsion secondary to a non-degradable foreign body gastrointestinal obstruction in a captive Lion (Panthera leo). Braz J Vet Pathol 13(3):609–614
Akinyemi JO, Ikede BO (1982) Verminous pneumonia in a gabon viper. Zool Gart 52:78–80
Anga TJ, Akpavie SO (2002) Pathology of zoo animals at the University of Ibadan Zoological Garden. Niger Vet J 23(1):40–46
Berentsen AR, Becker MS, Stockdale-Walden H, Matandiko W, McRobb R, Dunbar MR (2012) Survey of gastrointestinal parasite infection in African lion (Panthera leo), African wild dog (Lycaon pictus) and spotted hyaena (Crocuta crocuta) in the Luangwa Valley. Afr Zool 47(2):363–368
Bowman DD, Lynn RC (1999) Georgis’ parasitology for veterinarians. Saunders, Philadelphia
Cadmus SIB, Atsanda NN, Oni SO, Akang EEU (2004) Bovine tuberculosis in one cattle herd in Ibadan in Nigeria. Vet Med 49(11):406–412
Dawet A, Yakubu DP, Butu HM (2013) Survey of gastrointestinal parasites of non-human primates in Jos Zoological Garden. Am J Primatol 2:1–3
Durden LA, Mihalca AD, Sándor AD, Kanyari PW (2015) A new species of sucking louse (Phthiraptera: Anoplura: Linognathidae) from Günther’s Dikdik (Madoqua guentheri) in Kenya. J Parasitol 101(2):140–144
Egbetade A, Akinkuotu O, Jayeola O, Niniola A, Emmanuel N, Olugbogi E, Onadeko S (2014) Gastrointestinal helminths of resident wildlife at the Federal University of Agriculture Zoological Park. J Vet Sci 12(3):26–31
Egri B (2018) Louse infestation of ruminants. In Bovine science—a key to sustainable development (pp. 79–88). IntechOpen, London
Ekong PS, Akanbi BO, Odugbo MO (2014) A case report of respiratory mannheimiosis in sheep and goat complicated by Bordetella parapertussis. Niger Vet J 35(2):968–974
Emikpe BO, Adeniran GA, Alaka OO, Ohore OG, Antia RE, Ajayi OL, Omobowale OT (2007) Valvular endocarditis in a captive monkey in Ibadan, Nigeria: a case report. Niger Vet J 28(3):49–52
Emikpe BO, Morenikeji, OA, Jarikre TA (2016) Zoo animals’ disease pattern in a university zoological garden, Ibadan, Nigeria. Asian Pac J Trop Dis 6(2):85–89
Fowler ME (1996) An overview of wildlife husbandry and diseases in captivity. Rev Sci Tech off Int Epiz 15(1):15–22
Galleria Media Limited (2018) Jos Zoo https://www.nigeriagalleria.com/Nigeria/States_Nigeria/Plateau/Jos-Zoo.html Accessed 30 March 2018
Idowu AI, Golding RR, Ikede BO, Hill DH, Cunningham JH (1975) Oral fibroma in captive python. J Wildl Dis 11:201–204
Ikede BO, Falade S, Golding RR (1976) Anthrax in captive carnivores in Ibadan. J Wildl Dis 12:130–132
Isoun TT, Losos GJ, IKEDE, B.O. (1972) Diseases of zoo animals in Nigeria. J Wildl Dis 8:335–339
Jani RG (2006) Common diseases in wild animals, their treatment &Wound management. A Seminar on review of livestock policy, 2006 India. Reading Material, SLTC. Pune pp 59–65
Jegede HO, Akeem AO, Daodu OB, Adegboye AA (2018) Aflatoxicosis in African greater cane rats (Thryonomys swinderianus). Vet World 11(7):1001
Kalter SS (1989) Infectious diseases of nonhuman primates in a zoo setting. Zoo Biol 8(S1):61–76
Kennedy-Stoskopf S (2003) Canidae. In: Zoo and Wild Animal Medicine, (eds) M.E. Fowler & R.E. Miller, 5th edn, 482–491. W.B. Saunders, St. Louis, MO
Kim J, Habing GG, Salyards GW, and Coble DJ (2020) Antimicrobial stewardship in captive monkeys. In Neglected diseases in monkeys (pp. 141–170). Springer, Cham
Kohn B (1994) Zoo animal welfare. Rev Science and Technology 13:233–245
Kolapo TU, Jegede HO (2017) A survey of gastrointestinal parasites of captive animals at the University of Ilorin zoological garden. Vom J Vet Sci 12:17–27
Kruse H, Kirkemo AM, Handeland K (2004) Wildlife as source of zoonotic infections. Emerg Infect Dis 10(12):2067
Kumbish PR, Jambalang AR, Damina MS, Hussaini BA, Oyetunde IL, Akanbi BO, Jwander LD, Danbirni S, Elisha IL, Solomon P, Woma TY, Bako B, Nanbol D, Chukwukere S, Ardo A, Bunshia (2006) Outbreak of highly pathogenic avian influenza in local chickens in Nigeria, Vom. J Vet Sci pg 32–36, special edition November 2006
Lécu A, Ball R (2011) Mycobacterial infections in zoo animals: relevance, diagnosis and management. Int Zoo Yearb 45(1):183–202
Lin RC, Engeli E, Prowten AW, Erb HN, Ducharme NG, Goodrich LR (2005) Antebrachial fractures in four captive polar bears (Ursus maritimus). Vet Surg 34(4):358–365
Nemat A, Ali Z, Ahmad S, Sikander SK, Hussain Z (2013) Study of disease records of zoo animals in Lahore zoo, Pakistan. J Anim Plant Sci 25(3):483–492
Ocholi RA, Enurah LU, Odeyemi PS (1987) Fatal case of Salmonellosis (Salmonella pullorum) in a Chimpanzee (Pan troglodytes) in the Jos Zoo. J Wildl Dis 23(4):669–670
Otegbade AC, Morenikeji OA (2014) Gastrointestinal parasites of birds in zoological gardens in south-west Nigeria. Trop Biomed 31(1): 54–62
Parmer D, Davies R (2007) Fowl typhoid in small backyard laying flock. Vet Rec 160:348
Taylor LH, Latham SM, Woolhouse MEJ (2001) Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 356(1411):983–989
Tessaro SV (1989) Review of the disease, parasites and miscellaneous pathological conditions of North America bison. Can Vet J 30:416–422
Thalwitzer S, Wachter B, Robert N, Wibbelt G, Muller T, Lonzer J et al (2010) Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian Farmland. Clin Vaccine Immunol 17(2):232–238
Zajac AM, Conboy GA, Greiner EC, Smith SA and Snowden KF (2012) Helminth parasite, In: Veterinary clinical parasitology 8th Edition 2012. Vet Parasitol Wiley Blackwell
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akanbi, O.B., Jegede, H.O., Adam, M. et al. Disease and mortalities in selected zoological gardens in Nigeria. Comp Clin Pathol 30, 743–753 (2021). https://doi.org/10.1007/s00580-021-03273-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-021-03273-6