Abstract
Mango (Mangifera indica Linn.) has been widely studied as a folk medicines and it has become a common ingredient in cosmeceutical products. However, there was no report on the efficacy of Thai mango leaf extracts against inflammation. Thus, the aim of this study was to ascertain the anti-inflammatory and antioxidant activities of methanol (MeOH) and aqueous (Aq) extracts from the leaves of three mango varieties: Keaw Morakot, Nam Doc Mai, and Mahajanaka. To assay in vitro anti-inflammatory activity, the amount of nitric oxide (NO) released from macrophage cells was indirectly measured. Since free radicals play an important role in the inflammatory process, the antioxidant activity of M. indica leaf extracts was measured by ABTS and DPPH radical scavenging, ascorbic acid, and total phenolic assays. Mangiferin was also analyzed by HPLC. The most effective extract was selected and tested for anti-inflammatory activity in an animal model, an acute ear edema test in albino rats. The MeOH extract of Mahajanaka (M-MeOH) reduced nitric oxide production by LPS-stimulated macrophage cell lines RAW 264.7. M-MeOH showed the strongest antioxidant activity and it was, therefore, selected for an in vivo anti-inflammatory assay. An acute ear edema test in albino rats showed that M-MeOH exhibited significant anti-inflammatory activity compared to the control; ethyl phenylpropiolate-induced ear edema (P < 0.05). Moreover, mangiferin, a bioactive compound in M. indica leaves was detected in MeOH and Aq extracts. We concluded that the MeOH extract of M. indica leaves exhibits an anti-inflammatory property, both in vitro and in vivo, due to the presence of efficacious antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a necessary part of the body’s immune response. It is the body trying to heal itself after an injury and protect itself against foreign invaders (Mohan 2002). When inflammation happens, various pathological changes occur. Active inflammatory mediators are produced by microbial products or host proteins, such as complements, kinins, and coagulation factors. There are many mediators of inflammation such as histamine, nitric oxide (NO), prostaglandins (PGs), leukotrienes (LTB4), bradykinin, lipoxins, serotonin, growth factors, and cytokines (Dray, 1995) and the inhibition of these pro-inflammatory mediators is an important goal in the treatment of various inflammatory diseases.
Nitric oxide (NO) is synthesized by nitric oxide synthase (NOS). There are three main NOS isoforms: the first one is neuronal isoforms (nNOS), another one is endothelial isoforms (eNOS), and the last isoform is inducible isoforms (iNOS). The inducible forms of NOS are the pro-inflammatory enzymes, which are responsible for raising the NO levels and for the vasodilation and hypotension observed during inflammatory process. Since the lipopolysaccharides (LPS) stimulated NO secretion, LPS-stimulated RAW 264.7 cells (murine macrophage cell line) have been extensively used to determine inflammatory responses (MacMicking et al. 1997). Recently, numerous plant species have been reported for their anti-inflammatory and antioxidant properties. Antioxidation has been proposed as the mechanism underlying these properties.
Many plants have been consumed worldwide for health benefits, and mango (Mangifera indica Linn.) has become one of the most popular choices used to treat diseases. The plant is classified in the family Anacardiaceae and is cultivated in many tropical and subtropical regions. M. indica offers many important health benefits, for it has anti-cancer (El-Hawary and Rabeh 2014), hypocholesterolemic (Anila and Vijayalakshmi 2003), anti-bacterial (Hannan et al. 2013), and anti-inflammatory properties (Garrido et al. 2004a, b). Among others not only the fruit, but the leaves also have a lot of benefits. While the pharmaceutical information about mango leaves from other countries has been well documented, information about Thai mango leaves is limited. The ability of the fruit extract of mango to exhibit anti-inflammatory activities gained our interest so we tested the properties of the leaf extracts of three varieties of mango grown in Thailand, Keaw Morakot, Nam Doc Mai, and Mahajanaka, which are the commercially important fruits and easy to grow in Thailand. We focused on their antioxidant and anti-inflammatory properties.
Materials and methods
Plant materials and extraction
Three varieties of M. indica leaves were collected from Ban Hong District, Lamphun Province, Thailand. The plant species and varieties were identified by a botanist of the Queen Sirikit Botanic Garden, Mae Rim, Chiang Mai, Thailand (the plant identification number: Mahajanaka (M) is WP 5059, Nam Doc Mai (N) is WP 5060, and Keaw Morakot (K) is WP5061). The leaves were extracted by two solvents (methanol: MeOH and distilled water: Aq). Ground dry leaves of mango were macerated in both solvents for 24 h at room temperature. Each extract was filtered with Whatman No. 1 and the solvents were removed by vacuum rotary evaporator. Then, the crude extracts were lyophilized in a freeze dryer. They were then weighed to calculate the actual percentage yield (K-MeOH 0.52%, K-Aq 0.25%, N-MeOH 0.91%, N-Aq 0.45%, M-MeOH 0.73%, M-Aq 1.29%).
Animals
Male Wistar rats (Rattus norvegicus) age 18–20 days obtained from the National Laboratory Animal Center, Mahidol University, Thailand, were kept in cages and had free access to tap water and a standard pellet diet (SmartHeart® Hamster Food: Complete and Balanced Formula). The laboratory room temperature was maintained at 24–26 °C in a 12-h light/dark cycle. All procedures encompassing the animals were approved under permission number of Re. 004/13.
Determination of antioxidant activity
DPPH radical scavenging activity
The DPPH radical scavenging assay is to measure a stable free radical with violet-color of DPPH (1,1-diphenyl-2-picrylhydrazyl). A colorless of DPPH is observed after mixing the DPPH solution with an antioxidant. Following this, various concentrations of the extracts were added in methanolic DPPH solution and the absorbance was recorded at 517 nm (Brand-William et al. 1997). Gallic acid was used as a standard radical scavenger. The IC50 (a haft maximal inhibitory concentration) of each extract was then calculated and compared to that of gallic acid to obtain gallic acid equivalent antioxidant activity (GAEA).
ABTS radical scavenging activity
ABTS or 2,2′-azinobis (3-ethylbenzo thiazoline-6-sulfonic acid) free radical cation (ABTS+) decolorization assay (Re et al. 1999) was used to investigate antioxidant efficacy. A solution of ABTS+ was prepared by mixing ABTS with hydrogen peroxide (H2O2) for 16–18 h in the dark at a low temperature. The degree of green ABTS+ solution was measured at 734 nm. The extracts at various concentrations were added to the ABTS+ radical cation solution before measuring the optical density. The IC50 of each extract was then calculated and compared to that of trolox, a vitamin E analog, to establish the difference.
Ascorbic acid assay
Following the method of Schlessier et al. (2002), the content of ascorbic acid (vitamin C) analog was determined by extracting it from the samples using trichloroacetic acid. Dinitrophenylhydrazine reagent was mixed and then the sample was heated to 60 °C. The mixture was then cooled in an ice tank and sulfuric acid (H2SO4) was added. After 20 min of incubating in the dark, the amount of vitamin C was recorded at 520 nm before calculating its concentration against (standard) ascorbic acid. The amount of ascorbic acid was expressed as milligram ascorbic acid equivalent per gram of extract (mg AAE/g extract).
Total phenolic assay
To investigate the amount of phenolics in the extracts, the method of Singleton and Rossi (1965) was applied by mixing Folin-ciocalteu reagent in sodium carbonate decahydrate (Na2CO3.10H2O) with tested extracts for 2 h. The reaction was then spectrophotometrically read at 750 nm. We calculated total phenolic by comparing absorbance with a standard gallic acid monohydrate. The amount of phenolic was expressed as milligram gallic acid equivalent per gram of extract (mg GAE/g extract).
In vitro anti-inflammatory activity
Cell culture
Murine macrophage cells (RAW 264.7) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) containing streptomycin and penicillin (100 μg/ml and 100 U/ml, respectively) at 37 °C in a 5% CO2 incubator.
Cell viability
A modified MTT assay was used to assess the viability of RAW 264.7 cells (Mosmann 1983). A portion of MLE at different concentrations (1.25, 2.5, 5, 10, 20, and 40 μg/ml) was added to 96-well plate containing 5 × 105 cells each. After mixing 20 μl of MTT solution in phosphate-buffered saline, the wells were further incubated for 4 h at 37 °C. To solubilize any deposited formazan, a volume of 200 μl of dimethyl sulfoxide (DMSO) was added to each well and the optical density (OD) was recorded at 550 nm by a microplate reader.
Determination of nitric oxide production
We indirectly measured the amount of nitric oxide (NO) released from macrophage cells by pre-incubating RAW 264.7 cells (5 × 105 cells/well) with lipopolysaccharide (LPS) (2 μg/ml) for 24 h. Following this, a volume of 100 μl of cell culture medium was collected and mixed with 100 μl of Griess reagent. After incubating the mixture at 25 °C for 10 min, the level of NO was measured at 550 nm using a microplate reader. All experiments were performed in triplicate (Griess reagent: 1% sulfanilamide and 0.1% naphthyl ethylenediamine dihydrochloride in 2.5% phosphoric acid) (Yoon et al. 2009; Lee et al. 2013; Joo et al. 2014).
In vivo anti-inflammatory activity
The most effective extract from in vitro study was selected and tested for anti-inflammatory activity in an animal model. The EPP-induced ear edema method was used to test for acute inflammation in male Wistar rats (age 18–20 days) using the adapted method of Keardrit et al. (2010). Firstly, the rats weighing 35 to 40 g were divided into four groups (6 rats each). Both ears of each rat in the first three groups were topically applied with the tested extracts or phenylbutazone (PHBZ) at a dose of 1 mg/ear and served as two treatment groups and one positive control group. The negative control group was applied with the vehicle. After pre-treatment, induction of ear edema was carried out by topically applying ethyl phenylpropiolate (EPP) dissolved in acetone (50 mg/ml) to the inner and outer surfaces of both ears (20 μl/ear). Ear thickness was measured using a digital vernier caliper at 15, 30, 60, and 120 min after the edema induction.
Mangiferin analysis
Mangiferin was analyzed by HPLC following Gholkar and Laddha (2015) with some modifications. An Agilent system (quaternary pump, VWD detector at 257 nm, degasser) coupled with the Zorbax eclipse XDB-C18 column (4.6 × 150 mm) was used. The mobile phase consisted of acetonitrile: water containing 0.1% v/v of o-phosphoric acid (15:85). The flow rate was 1.0 ml/min; the injection volume was 20 μl. The retention time of a standard mangiferin was used to identify the peak of mangiferin being presented in the tested extracts. The amounts of mangiferin were determined by comparing the peak area of the tested samples with those peak areas from the standard (0.01–0.10 mg/mL).
Data analysis
All data are expressed as mean ± standard error of mean (S.E.M). One-way ANOVA followed by Tukey’s post hoc test for multiple comparisons in SPSS version 17 was used, while a statistical significance was considered when P < 0.05. In vitro assays were performed in triplicate. The IC50 (concentration that produced 50% inhibition) values were calculated using linear regression.
Results
DPPH and ABTS radical scavenging activity
The IC50 of DPPH and ABTS scavenging activities in the leaf extracts of the three varieties of mango grown in Thailand are shown in Table 1. M-MeOH had the lowest IC50 of DPPH and ABTS (0.129 ± 0.015 and 0.275 ± 0.008 mg/ml, respectively). The lowest IC50 correlates with the highest antioxidant capacity. The IC50 values of DPPH of M-MeOH was significantly lower than the K-Aq and M-Aq (P < 0.05), while, an IC50 value of ABTS of M-MeOH was significantly lower than other extracts, except for K-Aq. From our result, it was found that the IC50 of trolox, a standard antioxidant, was 0.211 mg/ml and it had the representative regression coefficient (R2) at 0.9951, while the linear regression equation was y = 2.7725x + 44.148. Furthermore, an IC50 value of gallic acid was 0.05 mg/ml (y = 0.1111x + 0.0445, R2 = 0.9995).
Ascorbic acid and total phenolic contents
The results for ascorbic acid contents showed higher values in the methanolic extracts and a significant increase when compared with the distilled water extracts. M-MeOH had the highest value (71.524 ± 2.341 mg AAE/g extract). The total phenol content was also higher in the methanolic extracts. The highest value occurred in the M-MeOH (0.295 ± 0.022 mg GAE/g extract) (Table 2).
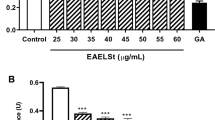
Effect of MLE on macrophage RAW 264.7 cell viability
Figure 1 illustrates the viability of RAW 264.7 cells in the presence of MLE. With this result, concentrations of the samples which produced 95% of cell viability were 1.25 and 2.5 μg/ml. On the contrary, less number of cell viability was significantly observed in the presence of the extracts at concentrations of 5–40 μg/ml when compared to 1.25 and 2.5 μg/ml (P < 0.05). Thus, the extracts at concentrations up to 2.5 μg/ml were selected for subsequent NO inhibition experiment.
Inhibitory efficacy against nitric oxide production
The inhibitory efficacy of MLE was evaluated against NO production in LPS-stimulated RAW 264.7 cells. Accumulation of nitrite in the cells increased due to the stimulation by LPS. Our results indicate that the percentage of inhibition of NO production induced by LPS increased in a concentration-dependent manner when treated with MLE, with 0.075, 0.15, 0.3125, 0.625, 1.25, and 2.5 μg/ml of MLE. Interestingly, the M-MeOH effectively inhibited NO production, at as high as just over 80%, when compared with other five extracts (Fig. 2).
EPP-induced ear edema in rats: acute inflammation
Inhibition of acute inflammation by MLE was clearly seen in Table 3. Edema thickness of the phenylbutazone group and the extract at both doses used (1 and 2 mg/ear) was significantly reduced when compared to a negative control group. The percentage inhibition of ear edema in rats receiving a low dose of the extract was lower than that of rats treated with phenylbutazone (1 mg/ear) but the extract at a high dose (2 mg/ear) showed higher percentage inhibition (87.88%) at 120 min after topical application when compared with phenylbutazone (79.80%).
HPLC
The HPLC chromatograms of a standard mangiferin and tested extracts are highlighted in Fig. 3. The regression equation of a standard mangiferin was Y = 56,680.2882X + 193.99831, with a correlation coefficient (r2) of 0.99561. Mangiferin was found in extracts whereas high mangiferin contents were found in the MeOH extracts, M-MeOH had the highest mangiferin contents (31.423% W/W) (Fig. 4). Therefore, mango leaves extracted with MeOH are a good source of mangiferin.
Discussion
Macrophages have a major effect on inflammatory responses via the generation of various pro-inflammatory molecules, especially NO. If NO over-produced, it causes various inflammatory diseases. Since LPS isolated from the cell walls of gram-negative bacteria is extensively used to activate macrophage in a variety of anti-inflammatory experiments (Nicholas et al. 2007), this study used the LPS to stimulate nitric oxide production from RAW 264.7 cells. Therefore, we used LPS-treated macrophage cells to screen various anti-inflammatory plants. MLE effectively reduced NO, which shows that it might be useful for suppressing the inflammatory process. The anti-inflammatory property of these plants might involve in their underlying antioxidant mechanisms. Chain-breaking and removal properties have been proposed to be key mechanisms against inflammation (Rice-Evans and Diplock 1993). The former is the primary antioxidant, which donates an electron to the free radical presented in the system. The latter acts as secondary antioxidant which involves in removing initial ROS/reactive nitrogen species and quenching the chain-initiating catalyst. Moreover, antioxidants also prevent any damage being occurred in human body through different ways, including electron donation, co-antioxidants, metal ion chelation, and gene expression regulation (Krinsky 1992). Phenolic compounds, which are found in all plants, are an essential part of the human diet. Thus, they act as antioxidant properties, which inhibit the production of NO and peroxynitrite (Conforti and Menichini, 2011).
The M-MeOH had a higher level of total phenolics when compared with other extracts in this experiment. It was highly potent for antioxidation and confirmed the result of DPPH and ABTS radical scavenging activity by having the lowest IC50. Both of these radicals are commonly used to evaluate antioxidant activity in vitro. In addition, ascorbic acid or vitamin C content was measured to confirm those properties. In general, vitamin C is essentially responsible for maintaining the normal functions of cells in various organ systems. Therefore, it is known as a powerful antioxidant. The M-MeOH extract in this experiment showed the highest ascorbic acid content. Therefore, we selected this extract to determine its anti-inflammatory property in vivo. EPP-induced ear edema in murine is a good model and usually used for rapid in vivo screening of anti-inflammatory activity. This assay is simple and provides a reliable result with several anti-inflammatory drugs, including phenylbutazone. EPP produces local swelling by causing vasodilatation, vascular permeability and fluid accumulation, and several inflammatory mediators such as histamine, kinins, serotonin (5-HT), and prostaglandins (PGs) are responsible for signs of inflammation. Disruption or prohibition of the synthesis and release of the key mediators of acute inflammation is the mechanism underlying the anti-edematous effect of phenylbutazone. The results obtained from this study suggested a similar mechanism for MLE at a dose of 2 mg/ear (Brattsand et al. 1982; Sireeratawong et al. 2012).
From our preliminary studies, the MLE showed antioxidant and anti-inflammatory properties, due to the mangiferin in MLE. Mangiferin is a xanthone, and this substance is known as the most potent antioxidants. Mangiferin has been reported to possess anti-inflammation property by inhibiting the transcription factor NF-κB and different pro-inflammatory cytokines. These efficacies provide protective effects against a wide range of pathophysiological conditions and organ dysfunctions (Benard and Chi 2015). Inhibitions of IL-1 (interleukin 1) receptor-associated kinase 1 (IRAK1), NF-κB, and MAPK (mitogen-activated protein kinase) phosphorylation are the results of mangiferin in ameliorating peptidoglycan- or LPS-stimulated peritoneal macrophage. Moreover, the study of Garrido et al. (2004a, b) shows that mangiferin-rich extract from M. indica (VIMANG®) acted as an anti-inflammatory agent by reducing ear edema in mice. In addition, mangiferin may reduce inflammation via various mechanisms such as decreases in overpowering cytokines and pro-inflammatory mediators, inhibition of inflammatory cellular activations, regulations of inflammatory gene expressions, and enhancements of the cellular resistance against inflammatory injuries (Sánchez et al. 2000; Garrido et al. 2004a, b; Carvalho et al. 2009).
Conclusions
From the results of this study, we can conclude that the Thai mango leaf extracts, Keaw Morakot, Nam Doc Mai, and Mahajanaka, exhibited anti-inflammatory properties both in vitro and in vivo, and antioxidation proved to be the mechanism underlying those properties. The most effective one was methanolic extract of Mahajanaka.
References
Anila L, Vijayalakshmi NR (2003) Antioxidant action of flavonoids from Mangifera indica and Emblica officinalis in hypercholesterolemic rats. Food Chem 83:569–574
Benard O, Chi Y (2015) Medicinal properties of mangiferin, structural features, derivative synthesis, pharmacokinetics and biological activities. Mini Rev Med Chem 15(7):582–594
Brand-William W, Bondet V, Berset C (1997) Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm-Wiss Technol 30:609–615
Brattsand R, Thalen A, Roempke K, Kallstrom L, Gruvstad E (1982) Influence of 16 alpha, 17 alpha-acetal substitution and steriod nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J Steroid Biochem 16(6):779–786
Carvalho RR, Pellizzon CH, Justulin LJ, Felisbino SL, Vilegas W, Bruni F, Lopes-Ferreira M, Hiruma-Lima CA (2009) Effect of mangiferin on the development of periodontal disease: involvement of lipoxin A4, anti-chemotaxic action in leukocyte rolling. Chem Biol Inter 179:344–350
Conforti F, Menichini F (2011) Phenolic compounds from plants as nitric oxide production inhibitors. Curr Med Chem 18:1137–1145
Dray A (1995) Inflammatory mediators of pain. Br J Anaesth 75:125–131
El-Hawary SS, Rabeh MA (2014) Mangifera indica peels: a common waste product with impressive immunostimulant, anticancer and antimicrobial potency. JNSR 4:102–115
Garrido G, González D, Lemus Y, García D, Lodeiro L, Quintero G, Delporte C, Núñez-Sellés AJ, Delgado R (2004b) In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol Res 50:143–149
Garrido G, Delgado R, Lemus Y, Rodríguez J, García D, Núñez-Sellés AJ (2004a) Protection against septic shock and suppression of tumor necrosis factor alpha and nitric oxide production on macrophages and microglia by a standard aqueous extract of Mangifera indica L. (VIMANG). Role of mangiferin isolated from the extract. Pharm Res 50:165–172
Gholkar M, Laddha K (2015) Seasonal variation in the content of mangiferin in leaves of Mangifera indica L. Int J Pharm Pharm Sci 7:578–580
Hannan A, Asghar S, Naeem T, Ikram Ullah M, Ahmed I, Aneela S, Hussain S (2013) Antibacterial effect of mango (Mangifera indica Linn.) leaf extract against antibiotic sensitive and multi-drug resistant Salmonella typhi. Pak J Pharm Sci 26:715–719
Joo T, Sowndhararajan K, Hong S, Lee J, Park SY, Kim S, Jhoo JW (2014) Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi. J Biol Sci 21(5):427–435
Keardrit K, Rujjanawate C, Amornlerdpison D (2010) Analgesic, antipyretic and anti-inflammatory effects of Tacca chantrieri Andre. J Med Plants Res 4:1991–1995
Krinsky NI (1992) Mechanism of action of biological antioxidants. Exp Biol Med 200:248–254
Lee SY, Kim HJ, Han JS (2013) Anti-inflammatory effect of oyster shell extract in LPS-stimulated Raw 264.7 cells. Prev Nutr Food Sci 18(1):23–29
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350
Mohan H (2002) Inflammation and healing. In: Textbook of pathology. Jaypee Publication, New Delhi
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI (2007) Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol 179:7121–7127
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans CA (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rice-Evans C, Diplock AT (1993) Current status of antioxidant therapy. Free Radic Biol Med 15:77–96
Sánchez GM, Re L, Giuliani A, Núñez-Sellésd AJ, Davisona GP, León-Fernández OS (2000) Protective effects of Mangifera indica L. extract mangiferin and selected anti-oxidants against TPA induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharm Res 42:565–573
Schlessier K, Harwat M, Bohm V, Bitsch R (2002) Assessment of antioxidant activity by using different in vitro test methods. Free Radic Res 36:177–187
Singleton VL, Rossi JJA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Sireeratawong S, Itharat A, Lerdvuthisopon N, Piyabhan P, Khonsung P, Boonraeng S, Jaijoy K (2012) Anti-inflammatory, analgesic and antipyretic activities of the ethanol extract of Piper interruptum Opiz. and Piper chaba Linn. ISRN Pharmacol 2012:480265
Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W (2009) Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol 125:286–290
Acknowledgements
We would like to thank the Science Achievement Scholarship of Thailand, the Department of Biology in the Faculty of Science at Chiang Mai University and the graduate school, Chiang Mai University and Center of Excellence in Bioresources for Agriculture, Industry and Medicine, Faculty of Science, Chiang Mai University.
Funding
This study was funded by the Science Achievement Scholarship of Thailand, the graduate school, Chiang Mai University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures encompassing the animals were conducted with strict adherence to guidelines and procedures reviewed and approved by the Institutional Animal Care and Use Committee of the Biology Department, Faculty of Science, Chiang Mai University, permission number Re. 004/013.
Rights and permissions
About this article
Cite this article
Khumpook, T., Saenphet, S., Tragoolpua, Y. et al. Anti-inflammatory and antioxidant activity of Thai mango (Mangifera indica Linn.) leaf extracts. Comp Clin Pathol 28, 157–164 (2019). https://doi.org/10.1007/s00580-018-2809-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2809-z