Abstract
Inorganic phosphorus (Pi) is essential for plant growth, and phosphate (P) deficiency is a primary limiting factor in Pinus tabulaeformis development in northern China. P acquisition in mycorrhizal plants is highly dependent on the activities of phosphate transporters of their root-associated fungi. In the current study, two phosphate transporter genes, RlPT and LbPT, were isolated from Rhizopogon luteolus and Leucocortinarius bulbiger, respectively, two ectomycorrhizal fungi forming symbiotic interactions with the P. tabulaeformis. Phylogenetic analysis suggested that the sequence of the phosphate transporter of L. bulbiger is most closely related to a phosphate transporter of Hebeloma cylindrosporum, whereas the phosphate transporter of R. luteolus is most closely related to that of Piloderma croceum. The subcellular localization indicated that RlPT and LbPT were expressed in the plasma membrane. The complementation assay in yeast indicated that both RlPT and LbPT partially compensated for the absence of phosphate transporter activity in the MB192 yeast strain, with a K m value of 57.90 μmol/L Pi for RlPT and 35.87 μmol/L Pi for LbPT. qPCR analysis revealed that RlPT and LbPT were significantly up-regulated at lower P availability, which may enhance P uptake and transport under Pi starvation. Our results suggest that RlPT and LbPT presumably play a key role in Pi acquisition by P. tabulaeformis via ectomycorrhizal fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants, such as wheat, require at least 16 macro- and micro-nutrients for their growth and reproduction (Miransari and Mackenzie 2011); among these nutrients, phosphorus (P) is one of the limiting factors that influence forest productivity and crop yields. In addition, P is particularly important for plant water uptake. It has been known for some time that the hydraulic conductivity of intact roots and root cortical cells is lower under P deficiency (Faustino et al. 2013). In soil, concentrations of free inorganic orthophosphate (Pi), which is the only form of P directly accessible to plants, are often very low, generally ranging from 1 to 10 μmol/L. The low availability of Pi in soil is due to the negative charges on orthophosphate that result in rapid sequestration by cations (Vance et al. 2003), which renders Pi highly immobile in soil (Hinsinger 2001). Thus, the uptake of phosphate by plant roots quickly generates a depletion zone. Consequently, supplementation and/or maintenance of the P supply is important to ensuring normal plant growth.
Mycorrhizal symbiosis is widely distributed in terrestrial ecosystems (Smith and Read 2008) and occupies a protected ecological niche by improving host plant nutrient uptake, especially P uptake (de Campos et al. 2013). It is well established that mycorrhizal plants have two pathways of P absorption: a direct uptake pathway through the epidermis and root hairs and a mycorrhizal uptake pathway, in which P absorbed by external fungal hyphae is translocated to structures inside the roots and thus across the symbiotic interface to the plant cortical cells (Bucher 2007; Stonor et al. 2014). In most cases, plants cannot obtain enough P via the direct uptake pathway because of low P availability in the rhizosphere; consequently, P absorption by host plants has been shown to rely heavily on the mycorrhizal uptake pathway (Smith and Smith 2012).
In the mycorrhizal uptake pathway, a high-affinity phosphate transporter (PT) of mycorrhizal fungi plays an important role (Rausch and Bucher 2002), and many partial or full-length cDNAs codes for mycorrhizal PTs have been identified. Harrison and van Buuren (1995) first cloned a PT from the arbuscular mycorrhizal fungus Diversispora epigaea and then cloned the PT genes of Rhizophagus intraradices (Maldonado-Mendoza et al. 2001) and Funneliformis mosseae (Benedetto et al. 2005). In 2011, Sokolski et al. (2011) obtained 25 PT gene fragments from 10 species of arbuscular mycorrhizal fungi by using 4 pairs of special primers (P3, P4, P6, and P7). For ectomycorrhizal (ECM) fungi, the PT genes of Boletus edulis (Wang et al. 2014), Hebeloma cylindrosporum (Tatry et al. 2009), Laccaria bicolor (Martin et al. 2008), Pholiota nameko (Tasaki et al. 2002), and Tuber melanosporum (Martin et al. 2010) have been characterized. In the ongoing project entitled “Exploring the genome diversity of mycorrhizal fungi to understand the evolution and functioning of symbiosis in woody shrubs and trees,” at least 34 PT genes of 11 ECM fungi species, including Amanita muscaria, Laccaria amethystine, Paxillus involutus, Paxillus rubicundulus, Piloderma croceum, Pisolithus microcarpus, Pisolithus tinctorius, Scleroderma citrinum, Sebacina vermifera, Suillus luteus, and Tulasnella calospora, were retrieved from the Joint Genome Institute (JGI, (http://genome.jgi-psf.org/) database (Casieri et al. 2013). These fungal PTs are of great benefit to their host plants by absorbing P from outside of the P depletion zone (Facelli et al. 2014).
Pinus tabulaeformis is a dominant species of coniferous tree in northern China (Chen et al. 2008) and has been widely planted in protected forests and landscape enhancement projects (Bai et al. 2009). However, P deficiency in northern China (Yan et al. 2006) negatively affects the growth, development, and spatial distribution of P. tabulaeformis trees, resulting in substantial losses. Furthermore, P. tabulaeformis is a typical mycorrhizal plant that shows high dependence on the ectomycorrhizae (Bai et al. 2009; Wu et al. 1999; Zhang et al. 2010). Our previous study indicated that inoculation with Rhizopogon luteolus and Leucocortinarius bulbiger significantly improves the growth status and P absorption of P. tabulaeformis seedlings (Bai et al. 2009). Therefore, understanding the mechanism underlying P uptake and use efficiency along with ECM interactions is critical for P. tabulaeformis in northern China. Although many ECM fungal high-affinity PTs have been predicted or identified, most ECM fungi species develop specific associations with their host plants (Roy et al. 2013). Unfortunately, there is no information in the literature on the PT of P. tabulaeformis’ native ECM fungi. In the present study, two high-affinity PT genes were cloned, identified, and functionally characterized from R. luteolus and L. bulbiger.
Materials and methods
Strains, plasmids, and culture conditions
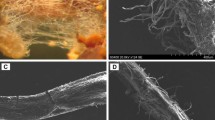
Two fungal strains were originally isolated from the mixed coniferous-broadleaved P. tabulaeformis forest on Daqingshan Mountain, Inner Mongolia, northern China (longitude 109° 41′ E to 112° 17′ E, latitude 40° 34′ N to 41° 14′ N) in August 2004. The strains were well maintained, and more than 40 generations were transferred in modified Melin Norkans medium (MMN, Boon et al. 2000). Based on the fungal sporocarp morphology described by Mao (2009), these two strains were identified as R. luteolus and L. bulbiger. The sporocarp morphology and ECM characteristics (associated with the P. tabulaeformis) are shown in Fig. S1, and the internal transcribed spacer (ITS) sequences of these two fungi species were deposited in the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/) and are accessible using the following accession numbers: KC984860 for R. luteolus and KC984861 for L. bulbiger. The mycelia of R. luteolus and L. bulbiger were incubated in MMN medium at 25 °C for 20 days and collected for RNA and DNA extraction and gene cloning. Escherichia coli DH5α was used for the Luria-Bertani (LB) medium transformation (Sezonov et al. 2007) at 37 °C. Yeast strains, including a wild-type (WT) strain and the corresponding mutant MB192 (MA Tapho3-1 Δpho84:HIS3 ade2 leu2-3,112 his3-532 trp1-289ura3-1,2 canl), were grown in both a yeast extract peptone dextrose medium (1 % yeast extract and 2 % peptone supplemented with 2 % glucose) and a synthetic defined medium (6.7 g/L of a yeast nitrogen base without Pi, contained all of the amino acid supplements, and supplemented with a 200 mg/L geneticin (G418)) at 30 °C. The pUG23 vector with a KanMx-tag was used for the heterogeneous expression of the RlPT and LbPT gene in yeast. The modified pUG23 vector with a cleavable green fluorescent protein gene (GFP) and a KanMx-tag was used for the subcellular localization of the RlPT and LbPT in yeast. The pMD18-T Simple Vector (TaKaRa, Dalian, China) was used for thymine and adenine (TA) cloning and DNA sequencing.
Gene cloning and bioinformatic analysis
Nucleic acid extraction and cDNA synthesis
The total RNAs were prepared using an RNA Easy Spin Plus Kit (Aidlab, Beijing, China) from approximately 0.5 g fresh mycelia of two strains according to the manufacturer’s protocol. Then, the quantity and purity of the RNA were determined by UV measurement (NanoDrop 2000c spectrophotometer, Thermo Scientific, Loughborough, UK) and 1.2 % agarose gel electrophoresis. First-strand cDNAs were synthesized from the total RNA using a Revert Aid First-Strand cDNA synthesis Kit (Thermo, Shanghai, China). The synthesized first-strand cDNAs were used as a PCR template as in the following description. The genomic DNA was prepared using a DP305 Plant Genomic DNA Kit (Tiangen, Beijing, China) according to the manufacturer’s protocol.
Cloning the RlPT and LbPT genes
Based on degenerate primers (see Table S1) designed from the published PT sequence alignments of cDNA contigs, the core fragments of RlPT and LbPT were amplified from the cDNA templates using touchdown PCR conditions. In total, the 50-μL PCR mixture contained 5 μL PCR buffer, 3 μL dNTP mixture (2 mmol/L), 4 μL degenerate primers (5 mmol/L each), 0.5 μL Taq DNA Polymerase (Toyobo, Shanghai, China), 5 μL cDNA template (2.5 ng), and 32.5 μL ddH2O. The thermal cycler (Eppendorf AG 22331, Hamburg, Germany) was programmed for touchdown PCR with denaturation at 94 °C for 4 min, followed by 35 cycles of the following three steps: denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min. The PCR ended with an extra extension of 10 min at 72 °C. Then, the PCR products were purified using a BioTeke Gel Extraction Kit (BioTeke, Beijing, China). The fragment was cloned into the pMD18-T Simple Cloning Vector (TaKaRa, Dalian, China) and transformed into competent E. coli DH5α for DNA sequencing. The rapid amplification of cDNA end (RACE) technique was applied to determine the full-length sequence based on the obtained cDNA core fragments using the 5′ RACE system (version 2.0, Invitrogen, USA) and a SMARTer RACE cDNA Amplification kit (Clontech, CA, USA). After 5′ and 3′ RACE, PCR was performed with high-fidelity KOD FX (Toyobo, Shanghai, China) using the primers RlPT full length and LbPT full length (Table S1) to obtain the full-length genes.
Bioinformatic analysis
The open reading frame (ORF) was predicted using the ORFFinder (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi). The cloned RlPT and LbPT were analyzed to predict the amino acid sequences using the DNAMAN software package (version 7.0.2.176, LynnonBioSoft, Canada). The analyses of the amino acid sequences of RlPT and LbPT were performed using protein Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST) algorithms. The predicted amino acid sequences were used to search for conserved domains with NCBI Conserved Domain Search database (CDD, http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The isoelectric points (pIs) and molecular masses were predicted using the Compute pI/Mw tool from the Expert Protein Analysis System (ExPASy) database (http://www.expasy.ch/tools/pi_tool.html). The potential transmembrane domains in the protein sequences were predicted using the HMMTOP program (http://www.enzim.hu/hmmtop/) (Tusnady and Simon 2001), TMpred (http://www.ch.embnet.org/software/TMPRED_form.html), and the Network Protein Sequence Analysis (NPSA) (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page?/NPSA/npsa_htm.html). The putative amino acid sequences of RlPT and LbPT and the other PTs from different organisms were aligned using the DNAMAN software package and the Clustal X program, version 1.83 (Thompson et al. 1997). A phylogenetic analysis was performed using the maximum likelihood method (Murshudov et al. 1997) in the MEGA5 program (Tamura et al. 2011).
Functional analysis of RlPT and LbPT
Functional complementation of yeast mutants involving RlPT and LbPT
Based on the sequences of these two PTs (RlPT and LbPT) and the homologous sequence of the pUG23 vector (Cormack et al. 1997) with the KnaMx marker, two pairs of specific functional verification primers (see Table S1) were designed to verify the function of RlPT and LbPT. First, the pUG23 vector was cut with a smaI enzyme. Then, the PCR products were purified and mixed with the linear vector and the recombinant yeast expression vector pUG23+PT was constructed using the ClonExpress cloning Kit (Vazyme, Nanjing, China). Finally, the pUG23+PT vectors were transformed into MB192 yeast strains using electro-transformation (Faber et al. 1994). The WT yeast strains and the mutant strain MB192, which was transformed using the empty pUG23 vector, were used as positive and negative controls, respectively.
Transformed cells were transferred into YPD medium (Zinser et al. 1991) that contained all of the amino acid supplements with a 200-mg/L resistance concentration of G418 at 30 °C. The four yeast strains were collected during the exponential growth phase, and cells with an initial OD 600 nm of 0.5 ± 0.01 were incubated in yeast nitrogen base (YNB) medium (Chandra et al. 2001) without Pi and at 30 °C for 4 h P starvation treatment. The yeast cells were rinsed with 0.9 % NaCl solution after centrifugation at 3500 rpm for 5 min, then 2 μL of resuspended (10 times dilution) yeast cells was dropped into the low-P (60 μmol/L) and normal-P (200 μmol/L) YNB medium and cultured at 30 °C; the cell growth status of each treatment was observed after 3 days. Each treatment was replicated five times.
The effects of pH on RlPT and LbPT activities in yeast
To substantiate the pH dependence of Pi uptake in the pUG23+PT, WT, and MB192 yeast strains, different extracellular pH levels, which ranged from 4.0 to 8.0, were used with 80 mmol/L Pi in YNB medium. A 2-(N-morpholino) ethanesulfonic acid (MES) buffer was used to maintain a stable pH value in the medium. Yeast cells with an initial OD 600 nm of 0.5 ± 0.01 were incubated in a different pH YNB medium and cultured at 30 °C for 24 h. Then, the OD 600 nm values of the yeasts were determined (Wu et al. 2011). Each treatment was replicated five times.
Subcellular localization of RlPT and LbPT in yeast
A modified pUG23 vector with a cleavable GFP and a KanMx-tag (Melikant et al. 2004) and two pairs of specific subcellular localization primers (see Table S1) were used in the subcellular localization experiment. The recombinant vector’s construction and transformation, screening the positive clones and P starvation culture, were carried out as in the above description in the functional complementation experiment. The centrifuged (3500 rpm) yeast cells were incubated in a low-P (60 μmol/L) YNB medium after treatment with P starvation. The 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate (Dil) was added to the medium to set the final concentration of Dil at 3 μg/mL, then the medium was incubated in a water bath at 37 °C for 17 min. In order to wash the float, all aliquots were rinsed three times with the 0.9 % NaCl solution. The resuspended yeast cells (add 1 mL 0.9 % NaCl solution) were observed using a confocal laser scanning microscope (Olympus FV10, Tokyo, Japan), and the excitation wavelength was 960 nm. The transformed MB192 with the empty pUG23+GFP vector was also checked as the control.
Determination of acid phosphatase enzyme activities of RlPT and LbPT in yeast
The single clone yeast (pUG23+PT) cells were cultured in a high-P (200 μmol/L) YNB medium at 37 °C for 36 h; then, they were centrifuged (3500 rpm) and incubated in YNB medium (without P) for 2 h of P starvation. The yeast cells that were treated with P starvation were transferred into the same volume at different P concentrations (the final concentrations of P in each medium were 20, 60, 100, 200 μmol/L, respectively) in YNB medium for culturing for 1 h. All of the cultures were centrifuged at 3500 rpm, after which 200 μL of ground buffer (including 90 mmol/L citrate and 10 mmol/L chloride, pH = 4.8) was added to the fungal pellet for low-temperature grinding. The grinding mixtures were centrifuged at 15,000 rpm; then, 100 μL of ground supernatant and 100 μL P-nitrophenol sodium phosphate solution (4 mg/L) were reacted at 37 °C for 2 h; finally, a 1-mL sodium hydroxide solution (0.1 mol/L) was added to the reaction liquid to stop the reaction. The absorbance of the final reaction liquid at 420 nm wavelength was determined, as was the YNB medium without incubated yeast cells, which served as the control. A unit enzyme activity was defined as the amount of acid phosphatase needed by 1 g yeast cell to hydrolyze P-nitrophenol sodium phosphate to 1 μmol/L P-nitrophenol for 1 min at 37 °C and pH = 4.8 (Classics-Barka and Anderson 1962).
The kinetic properties of RlPT and LbPT
The kinetic properties of RlPT and LbPT were analyzed by feeding the transformed yeast cells with 32P, labeled Pi (Liu et al. 2014). The washed and Pi-starved cells were resuspended in a 3 % glucose solution to energize the plasma membrane. The Pi uptake by intact yeast cells was assayed by adding 2 mL of 32P-orthophosphate (0.05 mCi; 1 mCi = 37 MBq) to 50 mL aliquots of cells, suspended in a 25-mmol/L Tris–succinate (pH = 6.5) solution, and then supplemented with 3 % glucose, resulting in final Pi concentrations varying from 0 to 100 μmol/L in a 15-mmol/L NaCl solution. The data were analyzed using SIGMAPLOT (v10.0) to determine the K m values of the RlPT and LbPT in Pi uptake.
Expression of two PT genes in mycelium at different Pi concentrations
Real-time reverse transcription PCR (RT-PCR) analysis, including the RlPT expression in R. luteolus and LbPT expression in L. bulbiger, was conducted in response to the addition of nutrient-enriched (5 and 10 g/L), nutrient-deficient (0.01 and 0.1 g/L), and normal (1 g/L) Pi in Ohta medium (Ohta 1990). Two ECM strains were cultured in a modified Ohta medium supplemented with 0.01, 0.1, 1, 5, and 10 g/L of KH2PO4 for 20 days. The fresh RNA was isolated using an RNA Easy Spin Plus Kit (Aidlab, Beijing, China) from the mycelia of each treatment, which was purified with absolute alcohol and treated with DNase (EN0521, Thermo). Real-time PCR was performed using SYBR Premix Ex Taq (TaKaRa, Dalian, China), and quantitative RT-PCR reactions were conducted using the LightCycler 480 real-time PCR system (Roche) following the manufacturer’s instructions. The reaction mixture (25 μL) contained 2× Maxima SYBR Green qPCR Master Mix (12.5 μL), 1 μmol/L each of the forward and reverse primers (1 μL, the primers are listed in Table S1), 2 μL of template cDNA, and 9.5 μL of nuclease-free water. PCR amplification was conducted under the following conditions: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s. Three independent biological replicates of each sample and three technical replicates of each biological replicate were done in qPCR analysis. The gene expression of these two PTs was normalized against an internal reference gene, γ-actin (Zheng et al. 2014). The relative transcript expression was calculated using the 2-△△Ct method (Livak and Schmittgen 2001).
Results
Cloning of the RlPT and LbPT
Based on degenerate primer pair and PCR amplification, we obtained two conserved region sequences, with 898 and 971 bp lengths. These two conserved region sequences exhibited significant similarity with fungal PT sequences from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov) database (data not shown). Using the 5′ RACE and 3′ RACE techniques, we cloned the full-length cDNA sequences of two PTs and named them RlPT with 2017 bp and LbPT with 1890 bp length (GenBank accession numbers were KM594556 and KM594557, respectively).
The ORF of RlPT consisted of 1653 bp predicted to code 550 amino acids, and the ORF of LbPT with 1680 bp length corresponded to 559 amino acids. Based on the amino acid sequences of two proteins, we found 52.6 % of hydrophobic and 32.1 % of hydrophilic amino acids in the RlPT whereas 50.6 % of hydrophobic and 35.1 % of hydrophilic amino acids in the LbPT. And the ExPASy online hydrophobic predications of these two proteins were also shown in Fig. S2. The pI and the calculated molecular mass were 8.376 and 59.786 kDa for RlPT and 7.190 and 61.26 kDa for LbPT, respectively. The DNA sequences of these two genes analyzed by the HTMM software revealed RlPT contained 11 exons and 10 introns and LbPT contained 16 exons and 15 introns (Fig. S3).
The results from the NCBI Conserved Domain Search further confirmed that RlPT and LbPT belong to the major facilitator superfamily (MFS). Furthermore, we found RlPT had 10 transmembrane-spanning (TMs) domains by using TMHMM software, and LbPT had 9 TMs (Fig. S4). The signal peptide prediction analysis indicated that no signal peptide sequence was observed in RlPT or LbPT.
Phylogenetic analysis of RlPT and LbPT
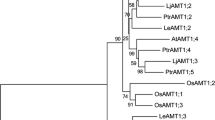
The deduced RlPT and LbPT proteins were compared with other fungal PTs, including 10 arbuscular mycorrhizal PTs and 21 ectomycorrhizal PTs (the species name, gene IDs, and accession number are shown in Table 1). ScPHO84 (Bun-Ya et al. 1991) was also considered because of its well-known function. Two main clades were distinguished (Fig. 1), one branch for the LbPT and HcPT1 and the second branch for the RlPT, another 20 ectomycorrhizal PTs, 10 arbuscular mycorrhizal PTs, and ScPHO84. RlPT was closely related to three PTs of P. croceum, including PcPT1 (KIM88284, 77 % similarity), PcPT2 (KIM78090, 76 % similarity), and PcPT3 (KIM84110, 71 % similarity), and LbPT was closely related to HcPT1 (KIM36204.1, 82 % similarity).
Unrooted phylogenetic tree of RlPT, LbPT, and other fungal PTs based on the maximum likelihood method. RlPT and LbPT are labeled using a red font, and ScPHO84 is labeled using a blue font. The gene IDs and accession numbers of other fungal PTs are shown in Table 1. Values of the major clusters are indicated in the node or branch of the tree, which represents the bootstrap confidence, tested using 1000 replicates of the dataset
Functional complementation verification of RlPT and LbPT in yeast
RlPT and LbPT were transformed into the MB192 mutant using the pUG23 vector to examine their P transport functions. With the gradually dilution of the yeast cells, the growth of four types of yeast cells, including MB192 with empty pUG23 vector, the transformed MB192 cells with RlPT and LbPT, and the WT yeast cells with empty pUG23 vector, decreased in two YPD media with different Pi concentrations (60 and 200 μmol/L, Fig. S5). Compared with the strongly inhibited growth of MB192 yeast cells transformed with the empty pUG23 vector, RlPT and LbPT expression recovered the yeast growth using the P absorption ability of these two genes (Fig. S5a, b).
The effect of pH on the activities of RlPT and LbPT
To determine the pH dependence of RlPT and LbPT, the optical density of the yeast cell lines was measured at a range of 4.0–8.0 pH values with 80 μmol/L of phosphate. The growth of MB192+RlPT and MB192+LbPT strains was similar to that of the WT yeast strain, but the growth of the control MB192 strain with the empty pUG23 vector was significantly inhibited under all of the tested pH levels (Fig. 2). In addition, four types of yeast strain exhibited the best growth under pH = 6, and their growth was repressed when the pH value exceeded 6. These results suggested that both the RlPT and LbPT were H+-transporters. Subsequently, the growth curves of the four yeast mutants (MB192+eV, MB192+RlPT, MB192+LbPT, and WT+eV) were determined at the optimal pH value (pH = 6, Fig. S6).
The effects of different pH levels (4–8) on the growth (OD 600 values) of the MB192 mutant yeast strains transformed by a recombinant vector with RlPT (MB192+RlPT) and LbPT (MB192+LbPT), a WT yeast strain (WT+eV) and the MB192 mutant yeast strain (MB192+eV) transformed with empty pUG23 vector in YNB medium containing 80 μmol/L of phosphate at 30 °C and at 180 rpm for 24 h. Different lowercase letters above the error bars indicate samples that differ significantly from each other: Duncan’s multiple range tests at the 5 % level (n = 5)
Subcellular localization of RlPT and LbPT in yeast
In contrast to the dispersive distribution of GFP proteins in transformed MB192 yeast with the empty vectors pUG23+GFP (Fig. 3a), GFP+RlPT (Fig. 3b), and GFP+LbPT (Fig. 3c), fusion protein expressions were restricted to the plasma membrane in the transformed yeast cells. These findings were consistent with the subcellular localization prediction in the plasma membrane of the online WoLF PSORT (Horton et al. 2007) protein predictor (In the predicted results, RlPT and LbPT have maximum likelihood with the plasma membrane protein ScPHO84, 44.14 % for the RlPT and 31.41 % for the LbPT).
The acid phosphatase enzyme activities of RlPT and LbPT in yeast
In contrast to the lack of color change in the MB192 yeast strain with the empty pUG23 vector in 20 μmol/L Pi supplement, and the minor color changes in 60, 100, and 200 μmol/L Pi, bromocresol purple staining in the MB192+RlPT and MB192+LbPT yeast strains revealed an obvious color shift from pale brown to yellow, which was similar to the color change in the WT yeast strain during the acidification of the liquid medium (Fig. S7). The acid phosphatase enzyme activities of these four types of yeast strains at different Pi concentrations are shown in Fig. 4. For all four yeast strains, the acid phosphatase enzyme activities decreased with increasing phosphate concentrations. Furthermore, the acid phosphatase enzyme activity of the MB192 mutant transformed yeast with the empty vector pUG23 (MB192+eV) was higher than the activity in the other three yeast strains. It is important to note that the P absorption ability of the MB192 strain transformed with RlPT and LbPT would recover to the same levels as the WT yeast strain.
The acid phosphatase enzyme activities of the MB192 mutant yeast transformed with the empty vector pUG23 (MB192+eV), pUG23+RlPT (MB192+RlPT), and pUG23+LbPT (MB192+LbPT) and WT yeast transformed with the empty vector pUG23 (WT+eV). All aliquots were cultivated in tubes containing 20, 60, 100, and 200 μmol/L of phosphatase YNB medium (pH = 4.8) at 30 °C and at 180 rpm for 24 h. The values of the lower vertical coordinate are the Lg (acid phosphatase enzyme activity). In the same phosphatase concentration YNB medium, different lowercase letters above the error bars indicate samples that differ significantly from each other: Duncan’s multiple range tests at the 5 % level (n = 5)
The Michaels constant (K m) of RlPT and LbPT
As the data show in Fig. 5, the MB192 mutant yeast can secrete an acid phosphatase enzyme, the K m value of which is 144.79 μmol/L. However, the K m values of the proteins encoded by the RlPT and LbPT after they were transformed into the MB192 strain were 57.90 and 35.87 μmol/L (Fig. 5), respectively. These results indicate that the RlPT and LbPT encode for two high-affinity phosphate transporters.
The relative expression of RlPT and LbPT at different Pi concentrations
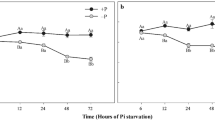
Compared with the expression levels of RlPT and LbPT in Ohta medium which added normal Pi (1 g/L), RT-PCR analysis revealed that both RlPT and LbPT were significantly up-regulated in a nutrient-deficient medium (0.01 and 0.1 g/L KH2PO4) and down-regulated in a nutrient-enriched medium (5 and 10 g/L KH2PO4, Fig. 6). Furthermore, compared with the level of LbPT expression, it was clear that the RlPT expression showed a increasing tendency under the same Pi concentration (e.g., the relative expression of RlPT in 0.01 g/L of KH2PO4 of Ohta medium was 72.36, whereas it was 60.23 for LbPT).
Relative expression of RlPT and LbPT measured by RT-PCR in mycelium cultured in Ohta medium with normal Pi (1 g/L of KH2PO4), nutrient-enriched (5 and 10 g/L), and nutrient-deficient (0.01 and 0.1 g/L) conditions. The gene expression of these two PTs was normalized against an internal reference gene, γ-actin
Discussion
The low concentration of available Pi in the soil (Marschner and Rimmington 1988) is a major challenge faced by all plants (Nussaume et al. 2011). Fortunately, mycorrhizal fungi can help most plants absorb Pi using external mycelia (Facelli et al. 2014), a process governed by fungal high-affinity PTs (Bucher 2007). In the present study, two PTs were isolated, identified, and functionally characterized in R. luteolus and L. bulbiger, which form symbiotic ectomycorrhizae with P. tabulaeformis. For ECM fungi, many genes encoding ECM fungal PTs have been identified (Casieri et al. 2013; Wang et al. 2014). Among all PTs identified thus far in ECM fungi, few PTs, such as HcPT1, HcPT2 (Tatry et al. 2009), and BePT (Wang et al. 2014), have been characterized by yeast complementation (The main properties, including ORF length, predicted molecular weight, predicted isoelectric point, hydrophobicity/hydrophilicity, transmembrane regions, optimal pH, and K m values of these genes are listed in Table S2).
The homology analysis revealed that RlPT and LbPT were highly homologous to other ectomycorrhizal PTs, including 77 % similarity to the PcPT1 protein in RlPT, 81 % identity with HcPT1 (Tatry et al. 2009) in LbPT, and 68 % of RlPT amino acids identity with the BePT (Wang et al. 2014). In addition, the variation of five identified ECM PTs’ ORF length is very small (Table S2). The two full lengths of PT cDNAs, named HcPT1 and HcPT2, are 1647 and 1686 bp long, respectively (Tatry et al. 2009); the ORF length of BePT is 1629 bp (Wang et al. 2014); and the data for RlPT and LbPT are 1653 and 1680 bp, respectively. These results indicated that ECM PTs are highly conserved throughout evolution and share high protein sequence identities.
The PT proteins are present in most living organisms and are usually divided into H+- (Pht1) and Na+-dependent (Pht2) transporters (Casieri et al. 2013; Ravera et al. 2007). Many fugal PTs are recognized as Pht2 transporters (Ai et al. 2009), but for the ECM fungi, most of these transporters belong to the Pht1 subfamily (Pi:H+ transporters), except for TmPT3, which encodes a PT that clusters with Pi:Na+ transporters (Casieri et al. 2013). The predicted products of RlPT and LbPT consisted of 10 and 9 trans-membrane domains, respectively, which were separated into two groups connected with a hydrophilic loop. In addition, the amino acid sequences of RlPT and LbPT include the signature sequence GGDYPLSATIxSE (data not shown); these structural arrangements are typical for Pht1 transporters (Karandashov and Bucher 2005; Liu et al. 2014). The uptake of Pi by the members of the Pht1 family is generally accompanied by an increase in the extracellular pH and an acidification of the cytoplasm (DiTusa et al. 2015; Pedersen et al. 2013). The optimal pH value of the MB192 mutant yeast transformed with the pUG23+RlPT and pUG23+LbPT is 6, and the subcellular localization of RlPT and LbPT (Fig. 3) indicated that these two genes were expressed in the plasma membrane. Based on the two genes’ sequences, optimal pH value of transformed yeast cells, and subcellular localization results, we confirmed that RlPT and LbPT encode the Pi:H+ transporter.
Several approaches have been used to investigate the Pi transport properties of the various Pht1 family members (Glassop et al. 2005; Kobae and Hata 2010). In the current study, RlPT and LbPT were able to complement the loss of the high-affinity Pi transporter activity of the MB192 yeast mutant. The K m values of RlPT and LbPT were 57.9 and 35.7 μmol/L Pi, respectively, values that are in the high-affinity concentration range (Fig. 5). To the best of our knowledge, only three mycorrhizal PTs K m have been measured by this method, including HcPT1 (5 μmol/L, Tatry et al. (2009)), DePT (18 μmol/L, Harrison and van Buuren (1995)), and HcPT2 (55 μmol/L, Tatry et al. (2009)). The K m of RlPT lies outside of the K m range of other mycorrhizal high-affinity PTs, but these data lie within the K m range of most Pht1 high-affinity PTs, e.g., MtPT5 (13 μmol/L, Medicago truncatula, Liu et al. (2008)), PtPT6 (22.6 μmol/L, Populus trichocarpa, Loth-Pereda et al. (2011)), CmPT1 (35.2 μmol/L, Chrysanthemum morifolium, Liu et al. (2014)), GmPT1 (68.9 μmol/L, Glycine max, Fan et al. (2013)), and OsPT6 (97 μmol/L, Oryza sativa, Ai et al. (2009)).
As shown in Fig. 1, RlPT, LbPT, and the other 32 fungal PTs (31 mycorrhizal PTs and ScPHO84) can be distinguished in 2 branches by phylogenetic analysis, one branch for the LbPT and HcPT1 and the second branch for the RlPT, another 20 ECM PTs, 10 AM PTs, and ScPHO84. The second branch also can be divided into 3 small clades, one clade for the 20 ECM PTs (RlPT in this clade), the second clade for 10 AM PTs, and the last clade for the TcPT. The phylogenetic analyses of all mycorrhizal PTs used in present study were basically consistent with the traditional morphological classification of mycorrhizal fungi and L. bulbiger is most closely related to H. cylindrosporum, whereas R. luteolus is most closely related to P. croceum.
In the present study, both RlPT and LbPT were up-regulated in their mycelia under Pi starvation conditions. Also, R. luteolus and L. bulbiger were the two most efficient ECM fungi for P. tabulaeformis in Pi absorption and inoculation effects among five species (L. bulbiger, R. luteolus, Suillus grevillei, Tricholoma fulvum, and Tricholoma terreum) of ECM fungi when P. tabulaeformis seedlings were inoculated with these fungal strains (Bai et al. 2009). These research results presumably explain why P. tabulaeformis exhibits high dependence on ectomycorrhizae at the P depletion area in the natural forest ecosystems of northern China.
Conclusion
In conclusion, RlPT and LbPT encode for two typical high-affinity phosphate transporter in R. luteolus and L. bulbiger. RlPT and LbPT are expected to encode the polypeptide with 550 and 559 amino acid residues, respectively. The pI and the calculated molecular mass are 8.376 and 59.786 kDa for RlPT and 7.190 and 61.26 kDa for LbPT. These two proteins are the hydrophobic protein, RlPT exhibits 10 TMs but LbPT consists of 9 TMs. RlPT is closely related to PcPT1, and LbPT is closely related to HcPT1. RlPT and LbPT might function in a wide range of Pi environments (0.01 to 10 g/L KH2PO4 in Ohta medium) and presumably play an important role in phosphate acquisition by P. tabulaeformis under natural conditions.
References
Ai P, Sun S, Zhao J, Fan X, Xin W, Guo Q, Yu L, Shen Q, Wu P, Miller AJ (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57:798–809. doi:10.1111/j.1365-313X.2008.03726.x
Bai S-L, Li G-L, Liu Y, Dumroese RK, Lv R-H (2009) Ostryopsis davidiana seedlings inoculated with ectomycorrhizal fungi facilitate formation of mycorrhizae on Pinus tabulaeformis seedlings. Mycorrhiza 19:425–434. doi:10.1007/s00572-009-0245-2
Benedetto A, Magurno F, Bonfante P, Lanfranco L (2005) Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 15:620–627. doi:10.1007/s00572-005-0006-9
Boon N, Goris J, De Vos P, Verstraete W, Top EM (2000) Bioaugmentation of activated sludge by an indigenous 3-chloroaniline-degrading Comamonas testosteroni strain, 12gfp. Appl Environ Microbiol 66:2906–2913. doi:10.1128/AEM.66.7.2906-2913.2000
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173:11–26. doi:10.1111/j.1469-8137.2006.01935.x
Bun-Ya M, Nishimura M, Harashima S, Oshima Y (1991) The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol 11:3229–3238. doi:10.1128/MCB.11.6.3229
Casieri L, Lahmidi NA, Doidy J, Veneault-Fourrey C, Migeon A, Bonneau L, Courty P-E, Garcia K, Charbonnier M, Delteil A (2013) Biotrophic transportome in mutualistic plant–fungal interactions. Mycorrhiza 23:597–625. doi:10.1007/s00572-013-0496-9
Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183:5385–5394. doi:10.1128/JB.183.18.5385-5394.2001
Chen K, Abbott RJ, Milne RI, Tian XM, Liu J (2008) Phylogeography of Pinus tabulaeformis Carr. (Pinaceae), a dominant species of coniferous forest in northern China. Mol Ecol 17:4276–4288. doi:10.1111/j.1365-294X.2008.03911.x
Classics-Barka T, Anderson P (1962) Histochemical methods for acid phosphatase using hexazonium pararosanalin as coupler. J Histochem Cytochem 10:741–753
Cormack BP, Bertram G, Egerton M, Gow NA, Falkow S, Brown AJ (1997) Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans. Microbiology 143:303–311. doi:10.1099/00221287-143-2-303
de Campos MC, Pearse SJ, Oliveira RS, Lambers H (2013) Viminaria juncea does not vary its shoot phosphorus concentration and only marginally decreases its mycorrhizal colonization and cluster-root dry weight under a wide range of phosphorus supplies. Ann Bot 111:801–809. doi:10.1093/aob/mct035
DiTusa SF, Fontenot EB, Wallace RW, Silvers MA, Steele TN, Elnagar AH, Dearman KM, Smith AP (2015) A member of the phosphate transporter 1 (Pht1) family from the arsenic‐hyperaccumulating fern Pteris vittata is a high‐affinity arsenate transporter. New Phytologist. doi:10.1111/nph.13472
Faber KN, Haima P, Harder W, Veenhuis M, Geert A (1994) Highly-efficient electrotransformation of the yeast Hansenula polymorpha. Curr Genet 25:305–310. doi:10.1007/BF00351482
Facelli E, Duan T, Smith SE, Christophersen HM, Facelli JM, Smith FA (2014) Opening the black box: outcomes of interactions between arbuscular mycorrhizal (AM) and non‐host genotypes of Medicago depend on fungal identity, interplay between P uptake pathways and external P supply. Plant Cell Environ 37:1382–1392. doi:10.1111/pce.12237
Fan C, Wang X, Hu R, Wang Y, Xiao C, Jiang Y, Zhang X, Zheng C, Fu Y-F (2013) The pattern of phosphate transporter 1 genes evolutionary divergence in Glycine max L. BMC Plant Biol 13:48. doi:10.1186/1471-2229-13-48
Faustino LI, Bulfe NM, Pinazo MA, Monteoliva SE, Graciano C (2013) Dry weight partitioning and hydraulic traits in young Pinus taeda trees fertilized with nitrogen and phosphorus in a subtropical area. Tree Physiol 33:129. doi:10.1093/treephys/tps129
Glassop D, Smith SE, Smith FW (2005) Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 222:688–698. doi:10.1007/s00425-005-0015-0
Harrison MJ, van Buuren ML (1995) A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378:626–629. doi:10.1038/378626a0
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195. doi:10.1023/A:1013351617532
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35(suppl 2):W585–W587. doi:10.1093/nar/gkm259
Karandashov V, Bucher M (2005) Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci 10:22–29. doi:10.1016/j.tplants.2004.12.003
Kobae Y, Hata S (2010) Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol 51:341–353. doi:10.1093/pcp/pcq013
Liu J, Versaw WK, Pumplin N, Gomez SK, Blaylock LA, Harrison MJ (2008) Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. J Biol Chem 283:24673–24681. doi:10.1074/jbc.M802695200
Liu P, Chen S, Song A, Zhao S, Fang W, Guan Z, Liao Y, Jiang J, Chen F (2014) A putative high affinity phosphate transporter, CmPT1, enhances tolerance to Pi deficiency of chrysanthemum. BMC Plant Biol 14:18. doi:10.1186/1471-2229-14-18
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Loth-Pereda V, Orsini E, Courty P-E, Lota F, Kohler A, Diss L, Blaudez D, Chalot M, Nehls U, Bucher M (2011) Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa. Plant Physiol 156:2141–2154. doi:10.1104/pp.111.180646
Maldonado-Mendoza IE, Dewbre GR, Harrison MJ (2001) A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol Plant Mic Interact 14:1140–1148. doi:10.1094/MPMI.2001.14.10.1140
Mao X-L (2009) Macromycetes of China. Since Press, Beijing
Marschner H, Rimmington G (1988) Mineral nutrition of higher plants. Plant Cell Environ 11:147–148. doi:10.1111/j.1365-3040.1988.tb01130.x
Martin F, Aerts A, Ahrén D, Brun A, Danchin E, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452:88–92. doi:10.1038/nature06556
Martin F, Kohler A, Murat C, Balestrini R, Coutinho PM, Jaillon O, Montanini B, Morin E, Noel B, Percudani R (2010) Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464:1033–1038. doi:10.1038/nature08867
Melikant B, Giuliani C, Halbmayer-Watzina S, Limmongkon A, Heberle-Bors E, Wilson C (2004) The Arabidopsis thaliana MEK AtMKK6 activates the MAP kinase AtMPK13. FEBS Lett 576:5–8. doi:10.1016/j.febslet.2004.08.051
Miransari M, Mackenzie A (2011) Development of a soil N test for fertilizer requirements for wheat. J Plant Nutr 34:762–777. doi:10.1080/01904167.2011.540922
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Cryst D53:240–255. doi:10.1107/S0907444996012255
Nussaume L, Kanno S, Javot H, Marin E, Pochon N, Ayadi A, Nakanishi TM, Thibaud M-C (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci 30:83. doi:10.3389/fpls.2011.00083
Ohta A (1990) A new medium for mycelial growth of mycorrhizal fungi. Trans Mycol Soc Jpn 31:323–334
Pedersen BP, Kumar H, Waight AB, Risenmay AJ, Roe-Zurz Z, Chau BH, Schlessinger A, Bonomi M, Harries W, Sali A (2013) Crystal structure of a eukaryotic phosphate transporter. Nature 496:533–536. doi:10.1038/nature12042
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37. doi:10.1007/s00425-002-0921-3
Ravera S, Virkki LV, Murer H, Forster IC (2007) Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol 293:C606–C620. doi:10.1152/ajpcell.00064.2007
Roy M, Rochet J, Manzi S, Jargeat P, Gryta H, Moreau PA, Gardes M (2013) What determines Alnus‐associated ectomycorrhizal community diversity and specificity? A comparison of host and habitat effects at a regional scale. New Phytol 198:1228–1238. doi:10.1111/nph.12212
Sezonov G, Joseleau-Petit D, D’Ari R (2007) Escherichia coli physiology in Luria-Bertani broth. J Bacteriol 189:8746–8749. doi:10.1128/JB.01368-07
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, New York
Smith SE, Smith FA (2012) Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 104:1–13. doi:10.3852/11-229
Sokolski S, Dalpé Y, Piché Y (2011) Phosphate transporter genes as reliable gene markers for the identification and discrimination of arbuscular mycorrhizal fungi in the genus Glomus. Appl Environ Microbiol 77:1888–1891. doi:10.1128/AEM.00919-10
Stonor RN, Smith SE, Manjarrez M, Facelli E, Smith FA (2014) Mycorrhizal responses in wheat: shading decreases growth but does not lower the contribution of the fungal phosphate uptake pathway. Mycorrhiza 1–8 doi:10.1007/s00572-014-0556-9
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Tasaki Y, Kamiya Y, Azwan A, Hara T, Joh T (2002) Gene expression during Pi deficiency in Pholiota nameko: accumulation of mRNAs for two transporters. Biosci Biotechnol Biochem 66:790–800. doi:10.1271/bbb.66.790
Tatry MV, El Kassis E, Lambilliotte R, Corratgé C, Van Aarle I, Amenc LK, Alary R, Zimmermann S, Sentenac H, Plassard C (2009) Two differentially regulated phosphate transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. Plant J 57:1092–1102. doi:10.1111/j.1365-313X.2008.03749.x
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 25:4876–4882. doi:10.1093/nar/25.24.4876
Tusnady GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850. doi:10.1093/bioinformatics/17.9.849
Vance CP, Uhde‐Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. doi:10.1046/j.1469-8137.2003.00695.x
Wang J, Li T, Wu X, Zhao Z (2014) Molecular cloning and functional analysis of a H+-dependent phosphate transporter gene from the ectomycorrhizal fungus Boletus edulis in southwest China. Fungal Biol 118:453–461. doi:10.1016/j.funbio.2014.03.003
Wu B, Watanabe I, Hayatsu M, Nioh I (1999) Effect of ectomycorrhizae on the growth and uptake and transport of 15N-labeled compounds by Pinus tabulaeformis seedlings under water-stressed conditions. Biol Fert Soils 28:136–138. doi:10.1007/s003740050474
Wu Z, Zhao J, Gao R, Hu G, Gai J, Xu G, Xing H (2011) Molecular cloning, characterization and expression analysis of two members of the Pht1 family of phosphate transporters in Glycine max. PLoS One 6:e19752. doi:10.1371/journal.pone.0019752
Yan X, Wu P, Ling H, Xu G, Xu F, Zhang Q (2006) Plant nutriomics in China: an overview. Ann Bot 98:473–482. doi:10.1093/aob/mcl116
Zhang H-H, Tang M, Chen H, Zheng C-L (2010) Effects of inoculation with ectomycorrhizal fungi on microbial biomass and bacterial functional diversity in the rhizosphere of Pinus tabulaeformis seedlings. Eur J Soil Biol 46:55–61. doi:10.1016/j.ejsobi.2009.10.005
Zheng R, Wang J-G, L-h T, Bai S-L, Niu Y-F (2014) Cloning and expression analysis of γ-actin gene from Rhizopogon luteolus. Sci Silvae Sinicae 50:80–85
Zinser E, Sperka-Gottlieb C, Fasch E-V, Kohlwein SD, Paltauf F, Daum G (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173:2026–2034
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31060110 and 31360125). We would like to express our appreciation to Prof. J. Hegemann (Institut fur Mikrobiologie, Heinrich-Heine-Universitat Dusseldorf, Germany) for generously providing the yeast mutants (MB192) and two types of vectors (pUG23 and pUG23+GFP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Rong Zheng and Jugang Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Sporocarps and ectomycorrhizal (ECM) characteristics of the fungi used in the present study. (a) Rhizopogon luteolus; (b) Leucocortinarius bulbiger; (c) mycorrhizal characteristics of Rhizopogon luteolus–Pinus tabulaeformis; (d) mycorrhizal characteristics of Leucocortinarius bulbiger–Pinus tabulaeformis. (GIF 81 kb)
Fig. S2
The ExPASy online hydrophobic prediction of RlPT (a) and LbPT (b). (GIF 123 kb)
Fig. S3
The DNA structures of RlPT and LbPT. The lavender boxes represent the exons, the white boxes represent the introns, and the characters in the box indicate the number of bases. (GIF 28 kb)
Fig. S4
The transmembrane-spanning domains predication of RlPT (a) and LbPT (b) by using TMHMM software. (GIF 182 kb)
Fig. S5
Functional expression of RlPT and LbPT in the MB192 strains and YPD medium. A staining test was performed to determine PT activity in the MB192 mutant yeast transformed with the expression vector pUG23 harboring no insert (MB192+eV) and pUG23+PT (MB192+RlPT/LbPT), and a WT yeast strain transformed with empty expression vector pUG23 (WT+eV) was cultured as a positive control. All aliquots (a and b are the experiment for the RlPT, c and d are the experiment for the LbPT) were cultivated in dishes containing 60 μmol/L (a and c) and 200 μmol/L (b and d) phosphate in a YPD medium at 30 °C for 3 days. The green arrows indicate that all aliquot concentrations decreased along the arrow directions (10-fold dilution each time). (GIF 361 kb)
Fig. S6
The growth (OD 600 nm) curves of MB192+eV, MB192+RlPT, MB192+LbPT, and WT+eV at optimal pH (pH = 6). All of the yeast strains were cultured in YNB medium that contained 80 μmol/L of phosphate at 30 °C and at 180 rpm, and the OD (600) values were determined every 5 h. (GIF 23 kb)
Fig. S7
The functional expression of RlPT and LbPT in yeast. A staining test was applied to determine the acid phosphatase enzyme activities in the MB192 mutant yeast transformed with the empty vector pUG23 (MB192+eV), pUG23+RlPT (MB192+RlPT), and pUG23+LbPT (MB192+LbPT), and a WT yeast strain transformed with empty pUG23 vector (WT+eV) was cultured as a positive control. All aliquots were cultivated in tubes containing 20, 60, 100, and 200 μmol/L of phosphatase YNB medium (pH = 4.8) at 30 °C and at 180 rpm for 24 h. Bromocresol purple was added to the culture medium as a color indicator, and a color shift reflected medium acidification, which correlates with the growth of the yeast cells. (GIF 104 kb)
Table S1
Primers used in this study. In the primers for functional verification and subcellular localization, the cDNA complementary sequences are the underlined bases and the homologous sequences of vectors are the bases without underlining. (XLS 17 kb)
Table S2
The main properties, including ORF length, predicted molecular weight, predicted isoelectric point, hydrophobicity/hydrophilicity, transmembrane regions, optimal pH, and K m values of functionally characterized PTs from ECM fungi. “–” means no data at present time. (XLS 33 kb)
Rights and permissions
About this article
Cite this article
Zheng, R., Wang, J., Liu, M. et al. Molecular cloning and functional analysis of two phosphate transporter genes from Rhizopogon luteolus and Leucocortinarius bulbiger, two ectomycorrhizal fungi of Pinus tabulaeformis . Mycorrhiza 26, 633–644 (2016). https://doi.org/10.1007/s00572-016-0702-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0702-7