Abstract
Truffles are the fruiting structures of ascomycetes in the genus Tuber. Because of their economic importance, truffles have been cultivated for many years using artificially inoculated host plants. Nevertheless, the life cycle and reproductive mode of Tuber spp. are still poorly understood. In filamentous ascomycetes, sexual reproduction is genetically controlled by the mating-type (MAT) locus. Among Tuber spp., the MAT locus has been recently characterized in the black truffles Tuber melanosporum and Tuber indicum. Here, by using sequence information derived from these species and from a Tuber borchii expressed sequence tag (EST) showing similarity to the mat1 gene of Alternaria brassicicola, we embarked on a chromosome-walking procedure to sequence the complete MAT region of T. borchii. This fungus produces highly commercialized whitish truffles and represents a model species for addressing basic questions concerning the life cycle of Tuber spp. We show that T. borchii is heterothallic, as its MAT locus is organized into two idiomorphs, each harbored by different mycelial strains. The alignment of the MAT locus from black truffles and T. borchii reveals that extensive sequence rearrangements and inversions occurred between these species. Moreover, by coupling mating-type analyses to karyological observation, we show that mycelia isolated from ascocarps and mycorrhizae are formed by homokaryotic hyphae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuber borchii Vittad. is an ectomycorrhizal ascomycete belonging to the order Pezizales that produces edible hypogeous fruit bodies. These are known as whitish truffles for the color of their peridium, and they are characterized by a pungent garlic-like odor. According to Montecchi and Sarasini (2000), this species belongs to the “puberulum” group, which comprises many European, Asiatic, and North American species (Jeandroz et al. 2008; Wang et al. 2007; Bonito et al. 2010, 2013). T. borchii is one of the most widespread Tuber species. It has a broad distribution in Europe, being found from southern Finland to Sicily and from Ireland to Hungary and Poland (Hall et al. 2007). It is well adapted to various environments (e.g., from coastal pine forests to hilly areas of the hinterland), it can grow associated with many different plant species (e.g., Pinus spp., Corylus avellana, Ostrya carpiniofolia, Quercus spp., Cystus spp.), and its truffle production is generally abundant. Although T. borchii truffles are widely commercialized in Italy and other European countries, their market value is low compared to other cultivable truffle species (e.g., Tuber melanosporum Vittad. and Tuber aestivum Vittad.). For all these reasons, there are only a few T. borchii plantations in Europe (Zambonelli et al. 2002).
Aside from the cultivation and commercial perspectives, T. borchii is of interest as, in contrast to other Tuber species, its mycelium is easy to isolate and cultivate in vitro (Sisti et al. 1998). Efficient inoculation procedures have also been developed to induce the synthesis of T. borchii mycorrhizae on a number of host species both in greenhouses and in vitro (Sisti et al. 1998; Zeppa et al. 2000; Giomaro et al. 2005; Iotti et al. 2012a). Due to these features, T. borchii has become a model species among Tuber spp. to perform biochemical and genetic studies and gain a better understanding of the cross-talk that governs the mutual recognition and symbiotic relationship between Tuber spp. and their hosts (Zeppa et al. 2000; Giomaro et al. 2005; Ceccaroli et al. 1999, 2003; Zeppa et al. 2002; Lacourt et al. 2002; Ambra et al. 2004; Polidori et al. 2007).
Despite the interest in the biology, ecology, and cultivation of this Tuber species, only a few studies have been dedicated to understanding its life cycle and reproductive modes. In this regard, we note that recent years have witnessed a tremendous leap toward these insights in other truffle species. Early population genetics studies using co-dominant SSR markers revealed an apparent complete absence of heterozygosis in T. melanosporum ascocarps, which was explained by a closed mating system or even an exclusive selfing in this species (Bertault et al. 1998). These conclusions were based on the assumption that the Tuber spp. life cycle, including the symbiotic (ectomycorrhiza) and reproductive (ascocarp) stages, was sustained by a secondary (dikaryotic) mycelium, as the hyphae of mycelia isolated in vitro and of mycorrhizae frequently display paired nuclei (Fasolo-Bonfante and Brunel 1972; Lanfranco et al. 1995). However, these findings have more recently been superseded. It was in fact shown that in Tuber magnatum and T. melanosporum, not only mycorrhizae were haploid, but ascocarps were also formed by haploid hyphae (Rubini et al. 2005; Paolocci et al. 2006; Riccioni et al. 2008). Specifically, it was shown that these species can outcross and that the haploid hyphae forming the non-ascogenous tissues of the gleba originate from only one of the two partners involved in the fertilization process, the maternal one. Furthermore, in mature truffles, it has been shown that the paternal contribution is present only in the ascospores, and because only few of these structures are damaged during standard DNA isolation methods, paternal DNA can be detected only when either DNA is isolated from purified pools of ascospores or a high number of PCR cycles is used. From these considerations, it has been argued that the development of secondary (dikaryotic) mycelium is presumably limited to the first stage of fruiting body development and that the life cycle of T. magnatum and T. melanosporum is prevalently haploid (Rubini et al. 2005, 2011b, 2012; Paolocci et al. 2006; Riccioni et al. 2008).
Although these studies have provided more insight on the reproductive modes of Tuber spp., the fertilization process, a key step for entering in the sexual phase, remains elusive. The typical gametangia of ascomycetes (i.e., ascogonia and antheridia), in fact, have never been identified with certainty in any truffle species (Rubini et al. 2007). Indeed, a structure resembling an ascogonium has been observed only once in T. melanosporum (Callot 1999). Thus, whether fertilization in Tuber is mediated by the differentiation of gametangia or simply results from a somatogamic process has yet to be fully elucidated (Rubini et al. 2007, 2014; Le Tacon et al. 2015). Regardless of how it performs, according to the abovementioned life cycle model, the fertilization step should occur after the formation of the ectomycorrhizae and should be temporarily linked to the formation of fruit bodies (Rubini et al. 2012).
Whether a filamentous ascomycete (Pezizomycotina) reproduces by haploid-selfing or outcrossing is genetically controlled by the mating-type (MAT) locus (Turgeon and Yoder 2000). This locus contains the two master mating-type genes MAT1-1-1 and MAT1-2-1, which encode transcription factors with an α1 domain and a high mobility group (MATA_HMG) domain, respectively (Debuchy et al. 2010). Heterothallic ascomycetes are characterized by the presence of two different versions of the MAT locus called idiomorphs, with each haploid strain containing only one MAT gene, either MAT1-1-1 or MAT1-2-1. Cross-fertilization is an obligate step in these species. Conversely, in homothallic species, both MAT genes are present in each haploid strain. Thus, homothallic fungi do not have distinctive mating types and each haploid strain can self-fertilize or cross with any other (Billiard et al. 2012).
Although the MAT genes of several Pezizomycotina have been characterized (Debuchy et al. 2010), cloning of these regions in Tuber spp. has been hampered for a long time by their poor sequence conservation among fungi of different lineages (Rubini et al. 2007). Despite the fact that an expressed sequence tag (EST) sequence of T. borchii showing similarity to mat1 of Alternaria brassicicola was previously reported (Zeppa et al. 2002), only the recent sequencing of T. melanosporum genome has made the identification of the MAT locus of a Tuber species possible (Martin et al. 2010a). More specifically, as the MAT locus in the sequenced genome contains the MAT1-2-1 gene only, and the second MAT gene, MAT1-1-1, has been identified in a different strain, it has been concluded that T. melanosporum is heterothallic (Rubini et al. 2011b). The characterization of the MAT genes of T. melanosporum has also been instrumental to clone their orthologs in T. indicum, the Tuber species being phylogenetically closest to T. melanosporum, and to show that this species is also heterothallic (Belfiori et al. 2013). MAT sequences have also been cloned from other Tuber species, and commercial use of this information is covered by a patent application (Martin et al. 2012).
In this study, the MAT locus of T. borchii has been sequenced with the aims of (i) ascertaining the reproductive mode of this species; (ii) comparing the structure and sequences of MAT genes and idiomorphs of T. borchii with those of other Tuber species; and (iii) providing markers (e.g., PCR primers) for typing T. borchii strains according to their mating type. Additionally, to gain insight into the life cycle of this species, the growth rate, morphology, and distribution of nuclei in free-living mycelia isolated from ascocarps and mycorrhizae were investigated.
Overall, the definition of the T. borchii life cycle and the identification of its reproductive mode are of interest to address basic questions concerning the dynamics and biology of the fertilization step in Tuber spp. These studies are also of relevance for improving the cultivation of these fungi.
Materials and methods
Sample sources
A set of 26 T. borchii ascocarps collected between 2007 and 2013 in Central Italy at different producing sites and under different host species was used in this study (Table 1). The truffles from each site were collected with the help of truffle pickers. All the ascocarps were identified by a morphological examination according to Pegler et al. (1993) and Montecchi and Sarasini (2000) and by sequencing the PCR-amplified internal transcribed spacer (ITS) region of the nuclear ribosomal genes (rDNA) according to Rubini et al. (1998).
The T. borchii ectomycorrhizae (ECMs) were collected under Pinus nigra trees in a natural truffle-producing site in central Italy. The ECMs were identified by morphotyping (Zambonelli et al. 1993; Giomaro et al. 2000) and PCR analysis with the species-specific ITS primer pair TB1-TB2 according to Amicucci et al. (1998).
Isolation and characterization of T. borchii mycelia
T. borchii mycelia were isolated from ascocarps and ECMs. Fresh ascocarps were carefully washed under running tap water and surface-sterilized by immersion in 95 % ethanol for 1 min. From each ascocarp, a small portion of the internal gleba was then removed with a scalpel under sterile environment and placed in an agarized medium in Petri dishes. Single ECMs, collected under a stereomicroscope, were maintained in water and placed for 3 min in a 0.2 % (v/v) NaClO solution. The ECMs were then washed three times in sterile water, dried in sterile filter paper, and placed in an agarized medium in Petri dishes. The modified Melin-Norkrans (MMN) medium (Marx 1969) supplemented with 6 g/l agar and 50 mg/l ampicillin (pH 6.6) was used. The Petri dishes were incubated at 22–24 °C in the dark and inspected daily under a stereomicroscope. The isolated mycelia were grown in agarized MMN medium without antibiotic. All the isolated mycelia (Table 1) were subcultured to fresh MMN plates every 3–4 months and maintained at 4 °C. Dual cultures of mycelia with the same or opposite mating types were performed according to Iotti et al. (2012b). The pairs with opposite mating types tested were tb15/tb16, tb7/tb6, tb15/tb6, and tb16/tb7. Control experiments were performed with the pairs tb15/tb7 and tb16/tb6 or by double inoculation of the same strain.
Morphological analyses were performed with stereo and light microscopes. The hyphae were stained with Tripan Blue (Sigma, Milan, Italy) according to Brundrett et al. (1995), and nuclei within the hyphae were stained with 4′,6-diamidino-2-phenylindole (DAPI) according to Rubini et al. (2011b). To evaluate the growth rate, Petri dishes (10-cm diameter) filled with 25 ml agarized MMN were inoculated with mycelium plugs (6-mm diameter) taken from the periphery of colonies grown for 20 days on the same medium. The plates were incubated at 24 °C in the dark, and the growth curve was determined by measuring the colony diameter in two perpendicular axes every 3–4 days and by averaging the measure of three replicates according to Mischiati and Fontana (1993).
DNA and RNA isolation, PCR amplification, and sequencing
DNA isolation was performed according to Paolocci et al. (1999) and PCR amplification of the ITS region was according to Rubini et al. (1998), using the ITS1-ITS4 primer pair (White et al. 1990). The isolation and sequencing of the mating-type region was performed with the primers reported in Supplemental Table S1 and Fig. S1. PCR amplifications were performed in a 50 μl mixture containing 10× PCR buffer (Euroclone, Milan, Italy), 2.5 mM MgCl2, 0.2 mM each dNTP, 10 μM each primer, 1 U Taq polymerase (Euroclone, Milan, Italy), and 20 ng genomic DNA. PCR reactions were performed in a 9700 PCR system (Life Technologies, Carlsbad, CA, USA) with the following thermal profile: 2 min at 94 °C, followed by 40 cycles of denaturation at 94 °C for 20 s, annealing at 50–65 °C for 20 s depending on the primers used, extension at 72 °C for 1 min, and a final extension at 72 °C for 7 min. Long-range PCR amplification of the MAT1-1 region (approximately 10 kb, see “Results”) was performed with LA-Taq DNA polymerase (Takara Bio Inc., Otsu, Japan) using the following two-step thermal profile: 30 s at 94 °C, 30–40 cycles of denaturation at 94 °C for 30 s and annealing/extension at 68 °C for 15 min, and a final extension of 7 min at 72 °C.
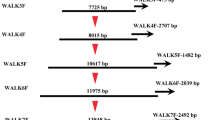
A partial sequence of T. borchii MAT1-1 was obtained from GenBank, and a short fragment of the MAT1-2-1 gene was isolated by PCR. These fragments were used as templates to sequence the complete MAT genes and to extend the sequences of both idiomorphs toward their 5′ and 3′ ends by genome walking. To this purpose, both the Universal GenomeWalker™ Kit (Clontech, Palo Alto, CA, USA) and the inverse PCR (IPCR) procedure (Ochman et al. 1988) were used. GenomeWalker libraries of adapter-ligated fragments were prepared according to the supplier’s instructions from DNA isolated from tb15 and tb16 mycelia (Table 1). Multiple steps of genome walking were performed using either AP1 or AP2 adapter primers provided with the kit, coupled with specific primers. The IPCR was performed to extend the 5′ region of MAT1-2 and the 3′ region of MAT1-1 obtained with the GenomeWalker kit. For this purpose, 1 μg of genomic DNA from tb15 and tb16 were digested with the enzyme HindIII and were circularized by self-ligation. IPCR and nested IPCR on the tb15 library were performed with the b30/b31 and b29/b32 primer pairs, respectively. IPCR and nested IPCR on the tb16 library were performed with the b15/b17 and b16/b18 primer pairs, respectively. All primers used are reported in the Supplemental Table S1 and Fig. S1.
RNA isolation and rapid amplification of cDNA ends (RACE) analysis were performed according to Rubini et al. (2011b). RNA was isolated from a 20-day-old culture of tb15 strain. For MAT1-2-1 cDNA amplification, the gene-specific reverse primers b23 and the 3′ RACE adaptor primer were used.
The PCR amplicons were purified using the JetQuick PCR purification kit (Genomed, GmbH, Löhne, Germany) or cloned in E. coli using standard procedures (Sambrook et al. 1989). Sequencing was performed with the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Life Technologies, Carlsbad, CA, USA) according to the supplier’s instructions and run on an ABI 3130 Genetic Analyzer (Life Technologies, Carlsbad, CA, USA). Sequence visualization and assembly were performed using FinchTV (Geospiza, www.geospiza.com/finchtv) and Bioedit software (Hall 1999). Primers were designed with the help of PerlPrimer software v. 1.1.16 (Marshall 2004). All sequences were deposited in GenBank under the following accession nos.: KT165325-KT165350 (ITS from T. borchii ascocarps), KT165351-KT165362 (ITS from mycelial isolates), KM210558, and KM210559 (assembled MAT1-2 and MAT1-1 contigs).
Identification of T. borchii MAT1-1-1 and MAT1-2-1 genes
The T. melanosporum mating-type genes were used to search for T. borchii orthologous sequences deposited in public databases. By using the T. melanosporum MAT1-1-1 sequence as query in BLASTn searches, the T. borchii EST AF487329 with 85 % sequence identity was retrieved. Conversely, neither BLASTn nor TBLASTn searches identified any T. borchii sequence with a significant similarity to the T. melanosporum MAT1-2-1 nucleotide or amino acid sequences, respectively.
To test for the presence of the putative MAT1-1-1 gene in the T. borchii ascocarps and mycelia under investigation, the AF487329 sequence was aligned with the corresponding MAT1-1-1 gene of T. melanosporum (Supplemental Fig. S2a) and the T. borchii MAT1-1-1-specific primers b1 and b3 were designed. These primers produced the expected amplicon of approximately 530 bp from seven ascocarps and seven mycelia isolated in vitro (Table 1). All the remaining samples did not produce any amplicons, despite the fact that their DNA yielded the expected PCR product when amplified with ITS primers (Table 1 and data not shown). Because only some strains produced the expected MAT1-1-1-specific product, heterothallism in T. borchii was inferred and these samples were regarded as the putative MAT1-1 strains. Conversely, the samples that did not produce any PCR amplicon were considered as putative MAT1-2 strains and were used as targets to search for the MAT1-2-1 gene by PCR with the primers P1 and P2, previously designed on the T. melanosporum MAT1-2-1 gene (Rubini et al. 2011b). Because these primers did not produce detectable amplicons in any T. borchii sample, a new primer pair (b38/b39, Supplemental Table S1, Fig. S1) was designed on the HMG domain conserved between the T. melanosporum and T. indicum MAT1-2-1 genes. When tested on T. borchii DNA using low-stringency PCR conditions consisting of an annealing temperature of 50 °C and high MgCl2 concentration (4 mM), this primer pair produced an amplicon of approximately 1 kbp in those T. borchii ascocarps and mycelia that failed to produce the MAT1-1-1-specific amplicon (Table 1). The PCR amplicon from ascocarp Tar415 was cloned, sequenced, and aligned with the T. melanosporum MAT1-2-1 gene (Supplemental Fig. S2b). BLASTX search against the T. melanosporum Gene Models database (http://mycor.nancy.inra.fr/IMGC/TuberGenome/blast.php) also confirmed the similarity (68 % amino acidic identity) of Tar415 fragment with the T. melanosporum MAT1-2-1 predicted protein (GSTUMT00001090001). The MAT1-2-1-specific primers b23 and b33 were then designed on the T. borchii HMG-box region to produce a DNA fragment of approximately 580 bp (Supplemental Table S1, Fig. S1). To test for the mating type of the T. borchii samples, a multiplex PCR procedure was set by combining the primer pairs b1-b3 and b23-b33
Isolation of T. borchii MAT idiomorphs
The two mycelial strains tb15 and tb16, harboring the MAT1-2-1 and MAT1-1-1 genes, respectively, were used as templates to isolate, by means of genome walking and IPCR, the two T. borchii MAT idiomorphs.
In tb16 the partial MAT1-1-1 sequence (AF487329) was extended toward the 5′ and 3′ ends (Supplemental Fig. S1). In tb15, the genome walking approach, starting from the MAT1-2 gene fragment obtained as reported above, allowed us to extend the MAT1-2 idiomorph by approximately 5 kbp toward its 5′ end only. The 3′ end of this idiomorph was then isolated using sequence information retrieved from tb16. Within the tb16 contig itself, a sequence corresponding to the T. melanosporum gene model GSTUMT00001092001 was found and named TbOrf2 (Supplemental Fig. S1). Notably, this gene was present at the 3′ flanking regions of both T. melanosporum idiomorphs (Rubini et al. 2011b).
Under the hypothesis that this sequence was also flanking the 3′ end of the T. borchii MAT1-2 region, the primers b34 and b35 were designed to target the tb16 TbOrf2 gene (Supplemental Table S1, Fig. S1) and were employed in PCR. These primers produced an amplicon of approximately 320 bp not only from tb16, as expected, but also from tb15. The sequence analysis confirmed that these amplicons showed a high sequence similarity to each other (approximately 90 % of sequence identity) and to the T. melanosporum gene model GSTUMT00001092001. Subsequently, to specifically amplify the 3′ end of the MAT1-2 idiomorph from tb15, the b36 primer was designed on TbOrf2 and used in combination with the MAT1-2-1 gene-specific primer b33 (Supplemental Table S1, Fig. S1). This primer pair, however, did not produce any PCR amplicon in tb15. Conversely, a 6870-bp-long amplicon was obtained when the primer b37, designed in inverse orientation with respect to b36, was used in combination with the primer b33.
Results
Characterization of T. borchii ascocarps and in vitro isolated mycelia
The ITS rDNA sequences from the 26 ascocarps identified as T. borchii by morphological observation (Table 1) were analyzed to confirm the species identity and assess genetic polymorphism. All the samples displayed an amplicon of approximately 600 bp, and both BLAST analysis and alignment with T. borchii ITS sequences retrieved from GenBank confirmed that all specimens belonged to T. borchii. The comparison of the ITS sequences did not show any remarkable polymorphism: all samples shared the same haplotype, with the only exception of Tar042, which differed from the other samples by a single nucleotide substitution. The two haplotypes identified in this study clustered within the T. borchii clade I described by Bonuso et al. (2010) (Supplemental Fig. S3).
Four mycelial strains from ECMs and eight from ascocarps were isolated (Table 1). The identity of all the isolates was confirmed by sequencing the ITS region. The mycelia growing in Petri dishes in MMN agar formed circular colonies with submerged and aerial hyphae (Fig. 1a). Microscopical observations showed that the aerial hyphae often carried a small condensation drop at the tip and frequently collapsed on the agar surface, forming coiled structures (Fig. 1b). Staining with DAPI revealed that the cells were multinucleated, with the number of nuclei ranging from 2 to 7. Nuclei were frequently paired, and several pairs of nuclei within single cells were observed (Fig. 1c, d).
Morphology of T. borchii mycelium grown in vitro. a Mycelium growing on solid MMN media, scale bar 13 mm; b details of hyphae on the agar surface and forming coiled structures (Trypan Blue staining), scale bar 30 μm; c apical cell; and d internal cell of a multinucleated hypha showing the presence of paired nuclei, scale bars 10 μm
The mycelial strains exhibited different growth rates (Supplemental Fig. S4). The strains tb136, tb7, and tb209 grew faster than the others, reaching the border of the plate in 27, 34, and 55 days, respectively. Also, the growth of tb147 and tb15 was fast initially, but when their colonies reached the diameter of about 5.5 and 7 cm after 34 days of cultivation, their growth increased slowly or not at all. Tb6 was the slowest; it stopped growing after 22 days, forming small colonies with a diameter of approximately 2.5 cm. All the other strains stopped growing after 55 days with colonies of 4, 5, and 6 cm in diameter. Dual cultures of mycelia with the same or opposite mating types did not show any relevant morphological changes: the two mycelial colonies grew normally, regardless of the mating type of the strains employed in the assay. When in contact, they continued to grow without showing any polarized growth or the formation of inhibition zones (data not shown).
Identification of T. borchii MAT genes and idiomorphs
The MAT genes of T. borchii were identified by different strategies. A EST sequence (AF487329) corresponding to a putative T. borchii MAT1-1-1 gene was identified by performing BLAST searches based on the T. melanosporum MAT sequences. Conversely a PCR fragment belonging to a MAT1-2-1 gene was isolated from T. borchii using primers derived from the T. melanosporum and T. indicum MAT1-2-1 sequences. Using MAT-specific primers designed on these fragments in a multiplex PCR, we tested the mating type of the ascocarps and mycelia reported in Table 1. When a low number of PCR cycles (25) was performed, a single PCR fragment was obtained from the ascocarps; however, when the PCR cycles were increased to 50, both the MAT1-1 and MAT1-2 fragments were produced in some samples (Fig. 2b). Conversely, a single PCR fragment, either MAT1-2 or MAT1-1, was obtained from each mycelial strain irrespective of the number of PCR cycles performed (Fig. 2a, b, Table 1).
Multiplex PCR amplification of DNA isolated from T. borchii mycelia and ascocarps. The b23, b33, b1, and b3 MAT-specific primers were used with either 25 (a) or 50 (b) PCR cycles, respectively. Lane 1, DNA ladder mix (Fermentas); lane 2, negative control (no DNA template); lanes 3–6, DNA from mycelium strains tb6, tb15, tb7, and tb16; lanes 7–14, DNA from ascocarps Tar325, Tar178, Tar146, Tar202, Tar210, Tar209, Tar165, and Tar240
Starting from the above described partial MAT sequences, the two T. borchii idiomorphs were sequenced by adopting a strategy based on genome walking and IPCR and by using as template the two mycelial strains tb15 and tb16, harboring the MAT1-2-1 and MAT1-1-1 genes, respectively. As a result, 11.4- and 14.6-kbp-long contigs were assembled (Supplemental Fig. S1, Fig. 3).
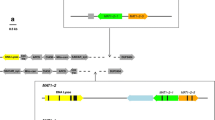
Structure of T. borchii MAT locus compared with those of T. indicum and T. melanosporum. a, f MAT1-1 and MAT1-2 regions of T. indicum sample Ti_U986, respectively (Belfiori et al 2013). b, e MAT1-1 and MAT1-2 regions of T. melanosporum, respectively (Rubini et al 2011b). c, d MAT1-1 and MAT1-2 regions of T. borchii, respectively. The white boxes indicate the idiomorphic regions. The sequences (region A) shared between the T. borchii idiomorphs are indicated with light gray boxes. The MAT1-1-1, MAT1-2-1, Orf2, Orf3, Orf4, and GSTUMT00001088001 genes are indicated with arrowed boxes. The IR1 inverted repeat bordering the idiomorphs, the sequences (region B) conserved upstream the two MAT genes, and the degenerate ORFs sharing similarity to Orf3 are indicated by hatched boxes. The hatching pattern and dotted lines indicate the inverse orientation of similar sequences
Structure of T. borchii MAT idiomorphs and genes
The sequences within the MAT locus of T. borchii were compared to each other and with those of black truffle species (Fig. 3). The assembled contigs of the two T. borchii strains showed a nearly identical region of approximately 1000 bp, likely representing the common region flanking the 5′ end of the idiomorphs. In this region, an ORF (TbOrf3) was identified, although it was only partially sequenced (Fig. 3). This ORF shared high similarity (approximately 89 %) with TiOrf3, an ankyrin-containing gene previously identified within the MAT1-2 idiomorph of T. indicum (Belfiori et al. 2013). In tb16, a sequence named TbOrf2 was identified downstream the MAT1-1-1 gene. A TbOrf2-like sequence was also present in the genomic region upstream of the MAT1-2-1 gene in tb15 (Fig. 3). This sequence was indeed in an inverted orientation with respect to TbOrf2 of strain tb16, and its putative coding sequence contained several stop codons. Because several attempts to extend downstream the partial TbOrf2-like sequence present in tb15 failed, the 3′ side of the idiomorphic region remains uncertain. Furthermore, in tb16, the putative 3′ region flanking the MAT locus showed a different gene composition with respect to T. melanosporum. More specifically, in tb16, another ORF was identified downstream of TbOrf2, which was named TbOrf4 (Fig. 3). This ORF showed similarity with the origin recognition complex subunit 1 (ORC1) gene (e.g., XP_003710500; XP_003836193) of different fungal species, including the gene model GSTUMT00001242001 of T. melanosporum. This gene, however, is not linked to the MAT locus in T. melanosporum because in this species, it is located in a different genomic region.
A large portion of the non-coding regions of T. borchii idiomorphs also shared similar sequences, but in inverted orientation (Fig. 3, region A). Among these non-coding regions, some sequences also shared similarity with sequences present in the idiomorphs of the black truffle species. More specifically, next to the 5′ end of both MAT genes of all the truffle species considered, an 800-bp-long sequence was present (Fig. 3, region B).
Within the putative idiomorphic region of each strain, a single MAT gene, named TborcMAT1-1-1 in tb15 and TborcMAT1-2-1 in tb16, was identified. These two genes are in opposite orientation to each other and in inverted orientation with respect to those present in T. melanosporum and T. indicum (Fig. 3; Rubini et al. 2011b; Belfiori et al. 2013). However, the MAT genes of T. borchii showed a structure similar to those of T. melanosporum and T. indicum. The alignment of MAT1-2-1 cDNA and MAT1-1-1 EST sequences with those of the corresponding genomic DNA showed that the number and positions of introns were conserved: 2 and 3 in TborcMAT1-1-1 and TborcMAT1-2-1, respectively. Only the first intron of both T. borchii MAT genes was longer than that of the corresponding one in the two black Tuber species (Supplemental Figs. S5a and S5b). The coding sequences of both MAT genes were also highly conserved among these Tuber spp. Compared with those of T. melanosporum and T. indicum, the TborcMAT1-1-1 and TborcMAT1-2-1 deduced proteins showed 86 and 80 % (85 % in the MATA_HMG region) sequence similarity, respectively. Most of the polymorphism was located at the C-terminal region in the MAT1-1-1 protein with the presence of only six amino acid changes within the α-domain (94–96 % of similarity, Fig. 4a) while amino acid changes were scattered all over the sequence in the MAT1-2-1 protein (Fig. 4b).
The two MAT proteins of Tuber also showed approximately 15 % of conserved residues distributed both within and outside the MATA_HMG and α1 domains (Fig. 5) and presence of evolutionarily conserved amino acids between the two functional domains when compared with other Pezizomycotina (Supplemental Fig. S6).
Discussion
In this study, we report the identification of the mating-type locus of T. borchii, a species belonging to the large group of whitish truffles. As this locus is organized into two idiomorphs harbored by different mycelial strains, with each idiomorph containing either MAT1-1-1 or MAT1-2-1, it can be concluded that this species is heterothallic. Thus, T. borchii adds to the black truffles T. melanosporum and T. indicum among the Tuber spp. of economic relevance whose sexual reproductive mode involves obligate outcrossing. Additionally, by coupling the molecular analysis to the morphological and the karyological observations of mycelia isolated from ascocarps and mycorrhizas, we show that the life cycle of this species is prevalently haploid.
Our findings are important for improving the cultivation methods of T. borchii. The availability of MAT1-1-1 and MAT1-2-1 specific markers, for example, will enable us to evaluate the distribution of T. borchii strains with different mating type in the field. Similar researches recently performed for black truffle T. melanosporum revealed the importance of mating-type distribution for truffle production (Rubini et al. 2011a; Murat et al. 2013).
The identification of the MAT locus of T. borchii offers the possibility to approach unsolved questions in the reproductive biology of Tuber species using a model species alternative and/or complementary to T. melanosporum. Additionally, it will facilitate to unveil in the near future the mating type of other similar Tuber spp. and shed more light on species boundaries within the whitish truffle species complex.
T. borchii is heterothallic
T. melanosporum was the first Tuber species where the MAT locus was characterized (Rubini et al. 2011b; Martin et al. 2010a) and represented the bedrock for successive identification of the homologous region in T. indicum (Belfiori et al. 2013). With the aim to identify the MAT locus of T. borchii, we started the present work by searching in public databases for T. borchii MAT sequences using as queries the T. melanosporum MAT1-1-1 and MAT1-2-1 genes. Whereas no putative orthologs of MAT1-2-1 have been found, this in silico screening has allowed us to confirm the T. borchii EST AF487329 as a partial transcript of a MAT gene. This 718 bp EST was originally cloned by means of differential display between mRNA populations from ripe and unripe T. borchii fruit bodies in a study aimed at identifying genes involved in ascocarp development (Zeppa et al. 2002). Interestingly, these authors reported this EST to show 26 % of identity with respect to the mating-type protein mat1 of A. brassicicola, and to be neither expressed in free-living mycelia nor in ripe fruit bodies, but in unripe ascocarps only.
Thus, we designed specific primers on this putative MAT1-1-1 gene to screen a set of T. borchii ascocarps. We observed that, using a low number of PCR cycles, the MAT1-1-1-specific band was produced only by a subset of the ascocarps analyzed. This result has been interpreted as a first indication that T. borchii is heterothallic and is consistent with our previous finding that the non-ascogenous tissue of the gleba in Tuber spp. is mainly formed by uniparental hyphae (Paolocci et al. 2006; Riccioni et al. 2008). To identify the mating type of T. borchii, we therefore pursued the same approach previously followed in T. melanosporum and T. indicum (Rubini et al. 2011b; Belfiori et al. 2013): the ascocarps that did not produce any PCR amplicon with primers specific for a given MAT gene were screened for the presence of the alternative one. Following this approach and by using sequence information from black truffles, we were eventually able to amplify a fragment of the putative MAT1-2-1 gene.
The definitive evidence of heterothallism in T. borchii was obtained when we amplified DNA isolated from mycelia: regardless of the number of cycles performed, multiplex PCR with primers for the two putative MAT genes produced a single band, either MAT1-2-1 or MAT1-1-1, in all 12 mycelial strains tested.
The MAT idiomorphs of T. borchii display extensive rearrangements with respect to those of black truffles
The T. borchii MAT region was isolated from two mycelial strains of opposite mating type named tb15 and tb16 by genome walking and inverse PCR. As results of these strategies, a 14.6- and 11.4-kbp-long contigs were assembled for tb15 and tb16, respectively. The sequencing of the MAT region from these two strains has confirmed an organization similar to that of T. melanosporum and T. indicum, with two idiomorphs (MAT1-1 and MAT1-2) each characterized by the presence of a single MAT gene, TborcMAT1-1-1 and TborcMAT1-2-1, respectively. The T. borchii MAT genes show the same structure as those from T. melanosporum and T. indicum, and the corresponding deduced proteins share 80 to 86 % similarity with those of black truffles.
Because in tb15 the walking procedure did not allow us to clone the flanking region downstream of the MAT locus, the 3′ end of T. borchii idiomorphs remains to be defined. Notwithstanding, we observed some rearrangements and scarce gene synteny around the MAT locus of T. borchii compared with T. melanosporum and T. indicum. For example, TbOrf4, which is linked to TbOrf2 and MAT1-1-1 in tb16, is located elsewhere in the genome of T. melanosporum. The Orf3 gene, located at the 5′ region of both T. borchii idiomorphs, is present within the MAT1-2 idiomorph in T. indicum (Belfiori et al. 2013), whereas only degenerate Orf3 sequences are present in the MAT1-1 idiomorph of T. indicum and in both idiomorphs of T. melanosporum. The Orf2 gene, identified in both mating types of T. melanosporum downstream of the MAT locus (Rubini et al. 2011b) is located at the same position in T. borchii. However, in tb15, this sequence is present in inverse orientation with respect to tb16 and its putative coding sequence contains several stop codons. Thus, the Orf2-like gene detected in tb15 is not functional.
The MAT genes of Tuber provide insight in the evolution of the mating type in Pezizomycotina
The MAT1-1-1 and MAT1-2-1 genes are generally considered non-homologous; however, their evolutionary origin has been recently reconsidered (Martin et al. 2010b; Jackson et al. 2013). More specifically, the tertiary structure prediction and the presence of conserved amino acid residues suggested that the α1 and MATA_HMG domains may be related, and an evolutionary origin of α1 from an ancestral MATA_HMG domain has been proposed. The alignment of MAT1-1-1 and MAT1-2-1 proteins of Tuber spp. not only confirms the presence of these evolutionarily conserved residues between the MATA_HMG and α1 domains but also shows the presence of many conserved amino acid residues outside the two functional domains. Notably, the two MAT genes of T. borchii are in inverse orientation, and conserved sequences in inverse orientations between the two T. borchii idiomorphs are also present in non-coding regions. The presence of conserved sequences in inverted orientation within the MAT locus is a feature that characterizes the black truffles as well as other ascomycetes (Rubini et al. 2011b; Belfiori et al. 2013; Conde-Ferráez et al. 2007; Sun et al. 2012).
These observations reinforce the hypothesis that inversions and the resulting suppression of recombination may have driven the diversification of sex-specific alleles at the MAT locus in ascomycetes and other organisms (Idnurm 2011). Since Tuber spp. belongs to a basal clade within Pezizomycotina, it is likely that their MAT organization represents the ancestral form in this group of fungi.
Furthermore, in T. borchii, both MAT genes are inverted with respect to their homologs from the two black Tuber species. A possible evolutionary scenario may involve the presence of the short inverted repeats (IR) identified at or next to the borders of the idiomorphs of black truffles (Fig. 3, IR1 hatched boxes; Rubini et al. 2011b; Belfiori et al. 2013) but lacking from those of T. borchii. We speculate that the IR may have driven the inversion of the entire MAT locus of black truffles with respect to T. borchii, causing also the inclusion of some genes, such as TiOrf3, within the idiomorphic region of the former species. Mechanisms underlying the inversion of MAT loci and involving IR sequences have been described in yeasts and filamentous ascomycetes (Chitrampalam et al. 2013; Hanson et al. 2014) and might have concurred to rearrange and diversify the MAT regions of different Tuber lineages as well.
Finally, it is interesting to note that in addition to the MAT genes, a short region of approximately 800 bp linked at their 5′ ends is also shared among the Tuber species here considered (Fig. 3, region B). The presence of this conserved sequence in the same position just upstream of each MAT gene suggests that it may be an important regulatory region. In turn, it can be speculated that the two MAT genes from different Tuber species are controlled by a putatively conserved regulatory mechanism. Specific analyses are needed, however, to evaluate this hypothesis.
T. borchii mycelia isolated from ascocarps and mycorrhizae are plurinucleate and homokaryotic and do not show morphological changes in dual cultures
The mycelial strains here isolated show a similar morphology when grown in Petri dishes, and although some differences in growth rate were detected, these are correlated neither with the isolation source (mycorrhiza or ascocarp) nor with the mating type. Neither was any relevant morphological change observed when tests of mating interaction were performed. Similar results were obtained in dual cultures of T. melanosporum strains (Iotti et al. 2012b). Frequently, in mycelial cultures 20–30 days old, coiled hyphae that may resemble sexual structures of ascomycetes (i.e., ascogonia) have been observed. However, these structures were formed in both dual- and single-mating cultures, and they most likely and simply derive from the collapse of the aerial hyphae on the agar surface. The occurrence of similar structures has been previously described in mycelial cultures of T. maculatum (Iotti et al. 2002). The lack of any detectable morphological change upon the interaction between free-living mycelia of opposite mating type suggests that physical contact is not sufficient, at least under in vitro conditions, to promote in sexually compatible strains the switch from the vegetative to the reproductive phase and that other environmental and/or biological factors are required. This is an expected result for a symbiotic species.
The karyological analysis of T. borchii mycelial strains isolated from both ascocarps and ECMs showed the presence of multinucleate cells containing a variable number of nuclei, which are frequently paired. The presence of paired nuclei in mycelia isolated in vitro, as well as in the hyphae emanating from the ECMs of different Tuber species (i.e., T. melanosporum), has been previously reported and was initially interpreted as a dikaryotic vegetative stage for these fungi (Lanfranco et al. 1995). However, analyses carried out using SSR markers have shown that gleba and ECMs in both T. melanosporum and T. magnatum are formed by haploid hyphae (Paolocci et al. 2006; Riccioni et al. 2008) and that individual T. melanosporum mycorrhizae and mycelia isolated in vitro have a single MAT gene (Rubini et al. 2011b; Iotti et al. 2012b). Likewise, the present study shows that the gleba of T. borchii ascocarps as well as mycelia isolated from both ascocarps and single ECM tips harbor either the MAT1-2-1 or MAT1-1-1 genes. Indeed, we never obtained isolates showing both mating types; thus, it is unlikely that the ECMs or the gleba are formed by heterokaryotic hyphae. Additionally, and in keeping with data from other Tuber spp. (Paolocci et al. 2006; Rubini et al. 2011a), the present observations allow us to argue that any T. borchii mycorrhizal root tip results from plant root colonization by a single mycelial strain. Thus, a plausible explanation for the presence of paired nuclei in T. borchii hyphae as well as in those of other Tuber spp. is the occurrence of synchronous mitosis or waves of parasynchronous mitosis that, starting from the apex, propagates to the neighboring cells. Such a phenomenon has been frequently observed in filamentous ascomycetes with multinucleated hyphae (Gladefelter 2006).
Overall, these observations corroborate our previous contentions that the Tuber spp. life cycle is prevalently haploid and, consequently, that fertilization and ascocarp formation are temporally linked in these symbiotic fungi (Riccioni et al. 2008).
Mating types and taxonomic implications
MAT genes are functional and rapidly evolving traits (Wik et al. 2008), which have proven useful to study the taxonomy of closely related fungal species (Turgeon 1998). In this regard, sequences of MAT genes and comparison of the idiomorphs’ structure allowed us to discover the existence of cryptic species in T. indicum (Belfiori et al. 2013). T. borchii belongs to a group comprising many closely related species (Montecchi and Sarasini 2000; Jeandroz et al. 2008; Bonito et al. 2010, 2013), and the polymorphisms of the ITS and EF-1α sequences have recently suggested the presence of cryptic species within this taxon (Bonuso et al. 2010). Thus, MAT genes and idiomorphic sequences may represent ideal genomic regions for gaining further insights into the taxonomy and species boundaries within the whitish truffle species complex.
References
Ambra R, Grimaldi B, Zamboni S, Filetici P, Macino G, Ballario P (2004) Photomorphogenesis in the hypogeous fungus Tuber borchii: isolation and characterization of Tbwc-1 the homologue of the blue-light photoreceptor of Neurospora crassa. Fungal Genet Biol 41:688–697
Amicucci A, Zambonelli A, Giomaro G, Potenza L, Stocchi V (1998) Identification of ectomycorrhizal fungi of the genus Tuber by species-specific ITS primers. Mol Ecol 7:273–277
Belfiori B, Riccioni C, Paolocci F, Rubini A (2013) Mating type locus of Chinese black truffles reveals heterothallism and the presence of cryptic species within the Tuber indicum species complex. PLoS One 8:e82353. doi:10.1371/journal.pone.0082353
Bertault G, Raymond M, Berthomieu A, Callot G, Fernandez D (1998) Trifling variation in truffles. Nature 394:734
Billiard S, López-Villavicencio M, Hood E, Giraud T (2012) Sex, outcrossing and mating types: unsolved questions in fungi and beyond. J Evol Biol 25:1020–1038
Bonito GM, Gryganskyi AP, Trappe JM, Vilgalys R (2010) A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Mol Ecol 19:4994–5008
Bonito G, Smith ME, Nowak M et al (2013) Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PLoS One 8:e52765
Bonuso E, Zambonelli A, Bergemann SE, Iotti M, Garbelotto M (2010) Multilocus phylogenetic and coalescent analyses identify two cryptic species in the Italian bianchetto truffle, Tuber borchii Vittad. Conserv Genet 11:1453–1466
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1995) Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research, Canberra
Callot G (1999) La truffe, la terre, la vie. INRA, Paris
Ceccaroli P, Saltarelli R, Buffalini M, Piccoli G, Stocchi V (1999) Three different forms of hexokinase are identified during Tuber borchii mycelium growth. Mol Cell Biochem 194:71–77
Ceccaroli P, Saltarelli R, Cesari P, Pierleoni R, Sacconi C, Vallorani L, Rubini P, Stocchi V, Martin F (2003) Carbohydrate and amino acid metabolism in Tuber borchii mycelium during glucose utilization: a 13C NMR study. Fungal Genet Biol 39:168–175
Chitrampalam P, Inderbitzin P, Maruthachalam K, Wu BM, Subbarao KV (2013) The Sclerotinia sclerotiorum mating type locus (MAT) contains a 3.6-kb region that is inverted in every meiotic generation. PLoS One 8:e56895
Conde-Ferráez L, Waalwijk C, Canto-Canché BB, Kema GH, Crous PW, James AC, Abeln ECA (2007) Isolation and characterization of the mating type locus of Mycosphaerella fijiensis, the causal agent of black leaf streak disease of banana. Mol Plant Pathol 8:111–120
Debuchy R, Berteaux-Lecellier V, Silar P (2010) Mating systems and sexual morphogenesis in ascomycetes. In: Borkovich KA, Ebbole DJ (eds) Cellular and molecular biology of filamentous fungi. ASM, Washington, pp 501–526
Fasolo-Bonfante P, Brunel A (1972) Caryological features in a mycorrhizal fungus: “Tuber Melanosporum” Vitt. Allionia 18:5–11
Giomaro G, Zambonelli A, Sisti D, Cecchini M, Evangelista V, Stocchi V (2000) Anatomical and morphological characterization of mycorrhizas of five strains of Tuber borchii Vittad. Mycorrhiza 10:107–114
Giomaro GM, Sisti D, Zambonelli A (2005) Cultivation of edible ectomycorrhizal fungi by in vitro mycorrhizal synthesis. In: Declerck S, Strullu DG, Fortin A (eds) In vitro culture of mycorrhizas. Springer, Berlin Heidelberg, pp 253–267
Gladefelter AS (2006) Nuclear anarchy: asynchronous mitosis in multinucleated fungal hyphae. Curr Opin Microbiol 9:547–52
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Res 41:95–98
Hall I, Brown G, Zambonelli A (2007) Taming the truffle. The history, lore, and science of the ultimate mushroom. Timber, Portland
Hanson SJ, Byrne KP, Wolfe KH (2014) Mating-type switching by chromosomal inversion in methylotrophic yeasts suggests an origin for the three-locus Saccharomyces cerevisiae system. Proc Natl Acad Sci 111(45):E4851–E4858
Idnurm A (2011) Sex and speciation: the paradox that non-recombining DNA promotes recombination. Fungal Biol Rev 25:121–127
Iotti M, Amicucci A, Stocchi V, Zambonelli A (2002) Morphological and molecular characterization of mycelia of some Tuber species in pure culture. New Phytol 155:499–505
Iotti M, Piattoni F, Zambonelli A (2012a) Techniques for host plant inoculation with truffles and other edible ectomycorrhizal mushrooms. In: Zambonelli A, Bonito GM (eds) Edible ectomycorrhizal mushrooms. Springer, Berlin Heidelberg, pp 145–161
Iotti M, Rubini A, Tisserant E, Kholer A, Paolocci F, Zambonelli A (2012b) Self/nonself recognition in Tuber melanosporum is not mediated by a heterokaryon incompatibility system. Fungal Biol 116:261–275
Jackson D, Lawson T, Villafane R, Gary L (2013) Modeling the structure of yeast MATa1: an HMG-box motif with a C-terminal helical extension. Open J Biophys 3:1–12
Jeandroz S, Murat C, Wang Y, Bonfante P, Le Tacon F (2008) Molecular phylogeny and historical biogeography of the genus Tuber, the ‘true truffles’. J Biogeogr 35:815–829
Lacourt I, Duplessis S, Abbà S, Bonfante P, Martin F (2002) Isolation and characterization of differentially expressed genes in the mycelium and fruit body of Tuber borchii. Appl Environ Microb 68:4574–4582
Lanfranco L, Arlorio M, Matteucci A, Bonfante P (1995) Truffles: their life cycle and molecular characterization. In: Stocchi V, Bonfante P, Nuti P (eds) Biotechnology of ectomycorrhizae. Molecular approach. Plenum, New York, pp 139–149
Le Tacon F, Rubini A, Murat C et al (2015) Certainties and uncertainties about the life cycle of the Périgord black truffle (Tuber melanosporum Vittad.) Ann For Sci doi:10.1007/s13595-015-0461-1
Marshall OJ (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20:2471–2472
Martin F, Kohler A, Murat C et al (2010a) Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464:1033–1038
Martin T, Lu SW, van Tilbeurgh H, Ripoll DR, Dixelius C, Turgeon BG, Debuchy R (2010b) Tracing the origin of the fungal α1 domain places its ancestor in the HMG-box superfamily: implication for fungal mating-type evolution. PLoS One 5:e15199
Martin F, Murat C, Paolocci F, Rubini A, Riccioni C, Belfiori B, Arcioni S (2012) Molecular method for the identification of mating type genes of truffles species European Patent Application EP2426215
Marx DH (1969) The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153–163
Mischiati P, Fontana F (1993) In vitro culture of Tuber magnatum mycelium isolated from mycorrhizas. Mycol Res 97:40–44
Montecchi A, Sarasini M (2000) Funghi ipogei d’Europa. Associazione Micologica Bresadola, Trento
Murat C, Rubini A, Riccioni C et al (2013) Fine-scale spatial genetic structure of the black truffle (Tuber melanosporum) investigated with neutral microsatellites and functional mating type genes. New Phytol 199:176–187
Ochman H, Gerber AS, Hartl DL (1988) Genetic applications of an inverse polymerase chain reaction. Genetics 120:621–623
Paolocci F, Rubini A, Granetti B, Arcioni S (1999) Rapid molecular approach for a reliable identification of Tuber spp. ectomycorrhizas. FEMS Microbiol Ecol 28:23–30
Paolocci F, Rubini A, Riccioni C, Arcioni S (2006) Reevaluation of the life cycle of Tuber magnatum. Appl Environ Microbiol 72:2390–2393
Pegler DN, Spooner BM, Young TWK (1993) British truffles: a revision of British hypogeous fungi. Royal Botanic Garden, Kew
Polidori E, Ceccaroli P, Saltarelli R, Guescini M, Menotta M, Agostini D, Palma F, Stocchi V (2007) Hexose uptake in the plant symbiotic ascomycete Tuber borchii Vittadini: biochemical features and expression pattern of the transporter TBHXT1. Fungal Genet Biol 44:187–198
Riccioni C, Belfiori B, Rubini A, Passeri V, Arcioni S, Paolocci F (2008) Tuber melanosporum outcrosses: analysis of the genetic diversity within and among its natural populations under this new scenario. New Phytol 180:466–478
Rubini A, Paolocci F, Granetti B, Arcioni S (1998) Single step molecular characterization of morphologically similar black truffle species. FEMS Microbiol Lett 164:7–12
Rubini A, Paolocci F, Riccioni C, Vendramin GG, Arcioni S (2005) Genetic and phylogeographic structures of the symbiotic fungus Tuber magnatum. Appl Environ Microbiol 71:6584–6589
Rubini A, Riccioni C, Arcioni S, Paolocci F (2007) Troubles with truffles: unveiling more of their biology. New Phytol 174:256–259
Rubini A, Belfiori B, Riccioni C, Arcioni S, Martin F, Paolocci F (2011a) Tuber melanosporum: mating type distribution in a natural plantation and dynamics of strains of different mating types on the roots of nursery-inoculated host plants. New Phytol 89:723–735
Rubini A, Belfiori B, Riccioni C et al (2011b) Isolation and characterization of MAT genes in the symbiotic ascomycete Tuber melanosporum. New Phytol 189:710–722
Rubini A, Belfiori B, Riccioni C, Paolocci F (2012) In: Zambonelli A, Bonito GM (eds) Genomics of Tuber melanosporum: new knowledge concerning reproductive biology, symbiosis, and aroma production. Springer Heidelberg, Berlin, pp 57–72
Rubini A, Riccioni C, Belfiori B, Paolocci F (2014) Impact of the competition between mating types on the cultivation of Tuber melanosporum: Romeo and Juliet and the matter of space and time. Mycorrhiza 24:19–27
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning—a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Sisti D, Zambonelli A, Giomaro G, Rossi I, Ceccaroli P, Citterio B, Benedetti PA, Stocchi V (1998) In vitro mycorrhizal synthesis of micropropagated Tilia platyphyllos Scop. plantlets with Tuber borchii Vittad. mycelium in pure culture. Acta Horticult 457:379–387
Sun Y, Corcoran P, Menkis A, Whittle CA, Andersson SG, Johannesson H (2012) Large-scale introgression shapes the evolution of the mating-type chromosomes of the filamentous ascomycete Neurospora tetrasperma. PLoS Genet 8:e1002820
Turgeon BG (1998) Application of mating type gene technology to problems in fungal biology. Annu Rev Phytopathol 36:115–137
Turgeon BG, Yoder OC (2000) Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet Biol 31:1–5
Wang YJ, Tan ZM, Murat C, Jeandroz S, Le Tacon F (2007) Molecular taxonomy of Chinese truffles belonging to the Tuber rufum and Tuber puberulum groups. Fungal Divers 24:301–328
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Gelfand MA, Sninski DH, White TJ (eds) PCR protocols. A guide to methods and applications. Academic Press, San Diego, pp 315–322
Wik L, Karlsson M, Johannesson H (2008) The evolutionary trajectory of the mating-type (mat) genes in Neurospora relates to reproductive behavior of taxa. BMC Evol Biol 8:109
Zambonelli A, Salomoni S, Pisi A (1993) Caratterizzazione anatomo-morfologica delle micorrize di Tuber spp. in Quercus pubescens Willd. Micol Ital 22:73–90
Zambonelli A, Iotti M, Giomaro G, Hall I, Stocchi V, Wang Y, Danell E (2002) T. borchii cultivation: an interesting perspective. In: Hall IR, Wang Y, Zambonelli A, Danell E (eds) Edible mycorrhizal mushrooms and their cultivation. Proceedings of the 2th International Conference on Edible Mycorrhizal Mushrooms. Institute for Crop & Food Research, Christchurch, pp 0–7
Zeppa S, Vallorani L, Potenza L, Bernardini F, Pieretti B, Guescini M, Giomaro G, Stocchi V (2000) Estimation of fungal biomass and transcript levels in Tilia platyphyllos–Tuber borchii ectomycorrhizae. FEMS Microbiol Lett 188:119–124
Zeppa S, Guidi C, Zambonelli A, Potenza L, Vallorani L, Pierleoni R, Sacconi C, Stocchi V (2002) Identification of putative genes involved in the development of Tuber borchii fruit body by mRNA differential display in agarose gel. Curr Gen 42:161–168
Acknowledgments
The authors are grateful to Mr. L. Tanzi and Mr. L. Gallo for providing some truffle samples used in this study and to Dr. F. Martin and Dr. C. Murat (INRA France) for their support and encouragement throughout the progress of the research work. This study was supported by Regione Umbria (project: “Indagini ecologiche, genetiche e molecolari per potenziare la produzione di tartufi pregiati in Umbria”) and the Italian Ministry of Education, Universities and Research (PRIN 2008 project: “Il ciclo biologico del tartufo: interazioni genotipo-ambiente”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors are named on a patent application entitled “Molecular method for the identification of mating-type genes of truffles species,” serial number WO/2012/032098; PCT/EP2011/065501, filed on 07-09-2011 by CNR, Plant Genetics Institute and INRA.
Rights and permissions
About this article
Cite this article
Belfiori, B., Riccioni, C., Paolocci, F. et al. Characterization of the reproductive mode and life cycle of the whitish truffle T. borchii . Mycorrhiza 26, 515–527 (2016). https://doi.org/10.1007/s00572-016-0689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0689-0