Abstract
Mycorrhizal fungi are key microorganisms for enhancing phytoremediation of soils contaminated with heavy metals. In this study, the effects of the arbuscular mycorrhizal fungus (AMF) Funneliformis mosseae (=Glomus mosseae) on physiological and molecular mechanisms involved in the nickel (Ni) tolerance of tall fescue (Festuca arundinacea = Schedonorus arundinaceus) were investigated. Nickel addition had a pronounced negative effect on tall fescue growth and photosynthetic pigment contents, as well as on AMF colonization. Phosphorus content increased markedly in mycorrhizal plants (M) compared to non-inoculated (NM) ones. However, no significant difference was observed in root carbohydrate content between AMF-inoculated and non-inoculated plants. For both M and NM plants, Ni concentrations in shoots and roots increased according to the addition of the metal into soil, but inoculation with F. mosseae led to significantly lower Ni translocation from roots to the aboveground parts compared to non-inoculated plants. ABC transporter and metallothionein transcripts accumulated to considerably higher levels in tall fescue plants colonized by F. mosseae than in the corresponding non-mycorrhizal plants. These results highlight the importance of mycorrhizal colonization in alleviating Ni-induced stress by reducing Ni transport from roots to shoots of tall fescue plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that transport, chelation, and sequestration processes in living organisms function in regulating concentrations of essential metal ions in different cellular compartments and minimizing the damage caused by heavy metal ions entering the cytosol (O’Halloran and Culotta 2000; Clemens 2001). Important components of heavy metal homeostasis and detoxification systems are membrane-localized heavy metal transporters (Williams et al. 2000) and chelation processes (Cobbett 2000). Membrane transport systems are likely to play a central role in the regulation of metal concentrations within different cells and organelles. The ATP binding cassette (ABC) protein superfamily is the largest membrane protein family, well known in plants. Members of this superfamily catalyze the Mg ATP-energized transport of a broad range of substrates across biological membranes. ABC transporters mediate diverse cellular transport processes such as excretion of potentially toxic compounds and conferring of heavy metal tolerance (Martinoia et al. 2002). Once metal ions enter the cell, they are bound by chelators. Metallothioneins (MTs) are the best characterized heavy metal-binding ligands in plant cells. They belong to the superfamily of thiol-containing metal-binding proteins which modulate internal levels of metal concentrations between deficient and toxic concentrations by binding to the toxic metals through closely spaced cystein-thiol groups (Cobbett 2000).
Arbuscular mycorrhizal fungi (AMF) are an important component of the rhizosphere and have been well documented to enhance phytoremediation of heavy metal-contaminated soils (Gonzalez-Chavez 2000; Davies et al. 2001). Environmental stress conditions, such as accumulation of heavy metals, typically impose severe difficulties for plant survival and growth. The AMF symbiosis can alleviate abiotic stresses through improvement of plant growth as a result of enhanced nutrient and water uptake (Davies et al. 2002; Carpio et al. 2005) and by stimulating or modifying specific physiological mechanisms related to the adaptation to stressful environments (Porcel et al. 2003; Auge et al. 2004).
AMF can influence certain plant physiological mechanisms, such as metal avoidance or tolerance to increase plant phytoremediation capability (Perotto and Martino 2001). There is evidence showing that plant-encoded metal transporters and chelators are expressed in mycorrhizal plants when grown under conditions of heavy metal contamination (Rivera-Becerril et al. 2005; Gohre and Paszkowski 2006; Hildebrandt et al. 2007). On the other hand, it has been shown that nickel (Ni) can form chelated compounds, which may replace other heavy metals from physiologically important centers in plant metabolism (Cammack et al. 1988; Kramer et al. 1996). The aim of the present study was to investigate the physiological and molecular mechanisms involved in Ni tolerance of mycorrhizal tall fescue plants inoculated with Funneliformis mosseae. Ni translocation and the expression levels of MT and ABC genes in response to four concentrations of Ni were investigated.
Material and methods
Seeds of Festuca arundinacea (recently renamed Schedonorus arundinaceus) were harvested from plants originally collected from Kordestan Province (Iran) and maintained in the research field of Isfahan University of Technology (Isfahan, Iran). Fescue seeds were pre-germinated on moist filter paper for about 1 week until the radicles appeared. Potting soil was taken from the bed of Zayandeh-rood River near Chelvan (45 km to Shahrekord, Chahar Mahal-Bakhtiari Province). The soil had a loam-sand texture and the following general chemical properties: pH 8.08, ECe 0.37 dS m−1, CEC 9.7 cmol (+) kg−1, total N 0.071 %, organic C 0.28 %, and available P, K, and Ni 5.2, 162, and 13 mg kg−1, respectively. Before the experiment, the soil was air-dried, sieved through a 2-mm sieve, and then sterilized by autoclaving for 2 h at 121 °C. Nickel (as NiCl2) was added to the soil at concentrations of 0, 30, 90, and 180 mg kg−1 (Ni 0, Ni 30, Ni 90, and Ni 180). F. arundinacea plantlets were cultivated in plastic pots (20 cm * 15 cm, diameter * height) filled with soil contaminated with the four levels of Ni. Inoculum of F. mosseae, purchased from the Zist Fanavar Touran Company (Semnan, Iran), consisted of sandy soil substrate (containing spores, mycelium, and mycorrhizal root fragments) from alfalfa cultures and contained 55 AMF spores per gram. For inoculation treatments, 20 g of F. mosseae inoculum was introduced at planting under the tall fescue seedlings in the pots, or an equivalent of sterilized inoculum for non-inoculated treatments was added. Three plants were grown in each treatment (M or NM in four levels of Ni) in a greenhouse (temperature 27 ± 3 °C, relative humidity 45 ± 8 %, 12 ± 0.5 h daylight). Plants were watered as needed (about four times a week).
After 3 months, the plants were harvested and analyzed for a series of parameters, detailed below. The average fresh weight was recorded for all treatments, and the water content was calculated after the cultures were oven-dried at 75 °C. Leaves and roots were used for the analysis of gene expression (frozen in liquid nitrogen and stored at −80 °C until use).
Determination of mycorrhizal root colonization
One gram of roots (with 1 cm length) was used to estimate F. mosseae colonization. Fresh root samples were treated with 10 % KOH and the AMF stained with trypan blue (0.1 %) in lactophenol (Phillips and Hayman 1970). The percentage of root colonization was determined by the grid-intersect method (Giovannetti and Mosse 1980).
Determination of chlorophyll and carotenoid contents
Pigments were extracted using the method of Arnon (1949) and Lichtenthaler (1987). Total leaf chlorophyll and carotenoids were extracted in 80 % (v/v) acetone from 100 mg of fresh leaf sample in the dark at room temperature. Absorbance was measured at 470, 663, and 645 nm in a UV/vis spectrophotometer (Ultrospec™ 3100 pro).
Carbohydrate determination
Soluble carbohydrate concentrations in roots were determined by the anthrone method (Poorter and Villar 1997) with some modifications. Briefly, root dry material was extracted in 80 % ethanol at 30 °C for 30 min followed by centrifugation at 4500 rpm for 10 min. The extraction procedure was repeated for the resulting pellet. To remove lipids, the supernatant was extracted with a solution of chloroform and deionized water. Chloroform was removed by centrifugation for 10 min at 4500 rpm. The resulting ethanol-water fraction was used for determination of carbohydrate content. For soluble sugars, 200 μL of each sample was added to 5 mL anthrone reagent solution and heated at 100 °C for 7.5 min. The absorbance of the resulting solution was measured in a spectrophotometer at 625 nm. A calibration curve was prepared with glucose standards ranging from 0.02 to 0.11 mg.
Mineral concentrations
Dried root samples were ground and digested in concentrated HNO3 at 140–160 °C. After cooling, the extracts were diluted with 1 N HCl up to 25 mL. The P in the digested sample was estimated by the ammonium-vanadate-molybdate method (Allen 1989) and absorbance measured at 700 nm, using a UV spectrophotometer. Approximately 1 g DW was used for the determination of Ni concentration in leaves and roots, separately. Oven-dried samples were ashed in a furnace at 480 °C for 5 h and then dissolved in HCl. The digested material was filtered on 45-mm filters and made up to volume using distilled water. Metal concentration was assessed by atomic absorption spectrophotometry (GBC 932 AB PLUS type).
RNA extraction and real-time polymerase chain reaction
Total cellular RNA was extracted from frozen shoots and roots using QIAzol Lysis reagent (QIAGEN, cat. no. 79306). Quantiscript reverse transcriptase (QIAGEN QuantiTect Reverse Transcription Kit, cat. no. 205311) was used for the synthesis of complementary DNA (cDNA) with oligo-dT and random primers, according to the manufacturer’s protocol. Real-time PCR (RT-PCR) assays were performed using the QIAGEN apparatus (Qiagene, Rotor-Gene, CA, USA). RT-PCR of actin (tall fescue actin housekeeping gene, accession number AY194227), ABC transporter (putative tall fescue ABC transporter gene, accession number CA820687.1), and metallothionein (metallothionein type II gene from rice, accession number CA820683) cDNAs were performed using specific primers (Table 1) and SYBR Premix Ex Taq (TaKaRa, cat. no. RR081Q). The thermocycler program for all reactions included an initial denaturation step at 95 °C for 3 min, followed by 40 amplification cycles consisting of denaturation at 94 °C for 30 s, annealing at 60 °C for 10 s, and extension at 72 °C for 25 s. Real-time PCR experiments were carried out on three biological replications with four technical repetitions for each. Differences in Ct values between an unknown sample and the calibrator were expressed as fold changes relative to the calibrator sample. The relative levels of gene expression were calculated by using the 2−ΔΔCt method.
Statistical analysis
The experiment was a 4 * 2 factorial in a completely randomized design consisting of four levels of Ni (0, 30, 90, and 180 mg kg−1) and two AMF levels (M and NM). All measured data were analyzed by analysis of variance (ANOVA) to test the significance of Ni or AMF status and their interaction effects. Means were shown as mean ± SE. The least significant difference (LSD) (α = 0.05) test was also utilized for mean comparison (SAS, Inc., 1999).
Results
Shoot and root biomass
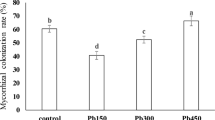
No root colonization was found in non-inoculated F. arundinacea plants, while successful AMF colonization was observed in F. arundinacea-inoculated plants even when they grew in Ni-contaminated soil. Nickel decreased the percentage of mycorrhizal colonization to 46 % in 30 ppm Ni, to 41 % in 90 ppm Ni, and to 32 % in 180 ppm Ni treatments (colonization with 0 ppm Ni was 57 %). Shoot and root growth of tall fescue plants was significantly reduced in Ni treatments at concentrations greater than 30 ppm (Fig. 1). Shoot and root biomass was higher (p < 0.05) in control (non-mycorrhizal plants grown without Ni) and mycorrhizal plants growing in the absence of Ni than in NM and M plants under Ni stress. The application of 180 ppm Ni in NM plants reduced shoot biomass by 20 %, while root biomass was 27 % lower compared to that of the control (Ni 0). However, decreases in root biomass caused by Ni were considerably less (p < 0.05) in mycorrhizal (21 %) than in non-mycorrhizal (27 %) plants. Furthermore, mycorrhization increased the water content of roots by 9 % (NM 50.15 % compared to M 59.14 %). Leaf chlorosis and necrosis at the highest concentration of Ni (180 ppm) were more prominent in NM plants compared to those of their AMF-inoculated counterparts.
Chlorophyll and carotenoid contents
Total chlorophyll content in shoots of both M and NM tall fescue plants was significantly (p < 0.05) decreased in all Ni treatments compared to the 0 ppm Ni treatment (Fig. 2). Mycorrhizal inoculation significantly increased (p < 0.05) chlorophyll content to 58 and 87 % in the treatments of 90 and 180 ppm Ni, respectively, and there was no significant difference between 0 and 30 ppm Ni treatments in chlorophyll content in the shoots (Fig. 2a).
Carotenoid content in the shoots of both M and NM tall fescue plants was also significantly (p < 0.05) decreased after growth in Ni-contaminated soil. The carotenoid content was clearly enhanced in the treatments of 30 and 90 ppm when plants were inoculated by F. mosseae compared to the non-mycorrhizal plants, but not in the case of 180 ppm Ni where the AMF was not effective in increasing carotenoid content (Fig. 2b).
Carbohydrate content
Carbohydrate content in the roots of NM tall fescue plants was significantly (p < 0.05) increased in all Ni treatments compared to the 0 ppm Ni treatment (Fig. 3). In contrast, M plants showed no significant difference in the content of carbohydrate among all Ni treatments compared to 0 Ni. Also, no significant difference was observed in root carbohydrate content between F. mosseae-inoculated and non-inoculated plants.
Mineral concentrations
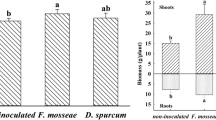
Root P concentration of the tall fescue plants was significantly higher in all treatments as compared to that of the control (non-mycorrhizal plants grown without Ni). However, no significant difference (p < 0.05) was observed regarding the P concentration in shoots of both M and NM plants in all Ni treatments compared to the control. Plants inoculated by F. mosseae showed higher amounts (up to 30 %) of P in their roots than in non- inoculated ones. In shoots, mycorrhizal colonization significantly increased P concentrations (except for Ni 180 ppm) of F. arundinacea (Fig. 4a, b).
Ni concentration significantly increased in roots and shoots when the plants were grown in Ni-contaminated soil (Table 2). Nickel content in roots ranged from 63 to 420 mg kg−1 in the different treatments. Colonization by F. mosseae significantly reduced Ni concentrations in roots and shoots at all Ni levels. Significant and positive correlations (p ≤ 0.05) were obtained between the Ni concentration of roots and shoots of tall fescue plants and Ni content in the soil (for both M and NM plants) (Fig. 5). There was also a significant difference in Ni translocation factor between M and NM plants. Inoculation with F. mosseae led to significantly (p < 0.05) lower root translocation of Ni to the aboveground parts than in non- inoculated plants.
ABC and MET genes expression
Results for the expression of ABC and MET genes in roots and shoots of tall fescue from representative experiments are shown in Fig. 6. The RT-PCR data showed that the ABC gene was highly expressed in roots of M tall fescue plants compared to NM ones (Fig. 6a). In NM plants exposed to 30 and 90 ppm Ni, the ABC gene was significantly upregulated to 2.4 and 1.7 times, respectively, compared to 0 Ni treatment but not in 180 ppm Ni treatment. The expression of ABC gene in roots of M plants was significantly increased at all concentrations of Ni compared with 0 ppm Ni. In contrast, shoots of M plants showed no significant difference regarding the expression of ABC gene in all Ni treatments as compared to Ni 0, while the expression of this gene was significantly upregulated in shoots of NM plants at the Ni concentration of 30 ppm. In NM plants, however, sharp decreases in the expression of this gene under high Ni concentrations of 90 and 180 ppm, by 41 and 59 % respectively, were observed (Fig. 6).
Relative expression of ABC (a, b) and MET (c, d) genes in roots and shoots of non-mycorrhizal (NM) and mycorrhizal (M) plants of tall fescue after 90 days treatment with Ni. The data are expressed as relative expression to the actin gene. Bars represent standard errors and different letters indicate significant differences between means (LSD test at p < 0.05, n = 3)
Similar to ABC, the MET gene was upregulated in roots of M tall fescue plants compared to the NM ones. In NM plants, there was a significant difference between 0, 30, and 90 ppm concentrations of Ni in the expression of this gene. In the shoots, similar to ABC, M plants showed no significant difference in MET gene expression in all Ni treatments compared to the Ni 0 treatment. However, as shown in Fig. 6d, expression of the MET gene was upregulated at the Ni concentration of 30 ppm compared to Ni 0 in NM tall fescue plants.
Discussion
It has been previously reported that plants undergo growth reduction under nickel stress (Amir et al. 2013; Doubkova and Sudova 2014). Both root and shoot growth of F. arundinacea plants also significantly decreased with rising Ni concentration in soil, but the detrimental effect on root growth was more pronounced. Mycorrhizal symbiosis has long been identified as a key factor for plant growth and development under stress environments (Smith and Read 2008). AMF contribute not only to plant uptake of macronutrients and micronutrients but also to metal stress alleviation (Gamalero et al. 2009). For example, Latef (2011) reported that AMF inoculation promoted the biomass production of mycorrhizal pepper plants as compared to non-mycorrhizal ones in response to increasing soil Cu concentrations. In the present study, mycorrhizal tall fescue plants inoculated with F. mosseae also showed higher growth indices compared to NM plants, and these were higher at lower concentrations of nickel.
It is known that changes in the content of chlorophyll and carotenoids, and the proportion of pigments, are important indicators of environmental stress. Gajewska and Skłodowska (2007) reported that chlorophyll a and b, total chlorophyll, and the ratio of chlorophyll a/b decreased in the leaves of wheat under Ni stress. High concentrations of Ni could decrease the absorption of essential nutrients such as iron and manganese in plants, resulting in the reduction in chlorophyll concentration (Yusuf et al. 2011). Singh and Pandey (2011) reported that the carotenoid content of lettuce was reduced under increased nickel concentrations, while the carotenoid content of safflower was reported to increase in response to the application of 100 mM copper (Ahmed et al. 2013). A carotenoid increase represents an increase in the non-enzymatic antioxidant defense of a plant. In the current study, F. mosseae not only improved nutrition and water absorption but there was also a significant increase in the content of leaf pigments (chlorophyll, carotenoid) under Ni stress, which is consistent with the previous observations on other plants (Latef 2011; Baslam et al. 2013). Increased amounts of carotenoids in mycorrhizal tall fescue plants could be effective in reducing the impact of nickel stress since, as antioxidants, carotenoids have the ability to quench singlet oxygen and to scavenge free radicals (Kagan et al. 2002).
Heavy metals may interfere with plant nutrient uptake by altering plasma membrane permeability and by affecting element transport processes across the membrane (Gussarsson 1994). The increased root P concentration of F. arundinacea plants under metal exposure is consistent with results reported by other workers showing enhanced P concentrations with increasing levels of cadmium in Sedum alfredii and maize (Yang et al. 2004; Aghababaei et al. 2014). Increased levels of root P in tall fescue in the presence of Ni could result from damage in the plasma membrane of root cells by metal exposure and altered membrane permeability, resulting in facilitated P uptake by roots. F. mosseae further improved the P nutritional status of tall fescue plants exposed to Ni stress which is consistent with the previous study of Amir et al. (2013) on other plant species. Mycorrhizal colonization can increase the P concentration in plants by enhancing facilitated uptake through an extensive extraradical hyphal network allowing the exploration of more volume of soil for minerals compared to NM plants (Smith and Read 2008).
Stresses such as salinity, drought, low temperature, and toxicity of heavy metals may all directly or indirectly lead to the accumulation of reactive oxygen species, and may cause the accumulation of soluble sugars in response to stress conditions as one of the possible mechanisms of adaptation (Roitsch and Ehneß 2000). However, Alaoui-Sosse et al. (2004), for example, showed that copper stress increased the amount of soluble sugars in the leaves of cucumber, while Alsokari (2009) reported that total soluble carbohydrates and chlorophyll decreased in leaves of Sorghum under Cd stress during the growth period. In the present study, the presence of Ni in soil increased the level of soluble carbohydrates in roots of NM tall fescue plants for all concentrations of nickel, but soluble carbohydrates remained unchanged in roots of plants exposed to Ni stress in the presence of the AMF F. mosseae. Contrary to these results, Demir (2004) reported that inoculation of pepper plants with Glomus intraradices increased fructose, glucose, and sucrose as well as the phosphorus content, dry matter, and chlorophyll a and b in shoots of plants. Considering the involvement of soluble carbohydrates in vacuoles as antioxidants (Van den Ende and Peshev 2013), the increased sugar content in NM tall fescue plants could contribute to the reduction of oxidative stress caused by Ni. Reduction of oxidative stress could also result from the improved phosphorus content and therefore improved photosynthesis in NM plants. The decrease in sugar content in NM plants at the highest Ni concentration (180 ppm) was probably due to the decrease in photosynthesis, which was also evident by the reduction in total chlorophyll content.
The concentration of Ni in both roots and shoots of tall fescue plants inoculated with F. mosseae was lower than in non-inoculated plants. Decreased Ni concentrations in mycorrhizal plants have been well documented (e.g., Vivas et al. 2006; Amir et al. 2013). Since AMF inoculation increased shoot dry weight of tall fescue, decreased Ni concentrations could be due to a biomass dilution effect, as observed for arsenic or Ni in other plants by Dong et al. (2008), Jianfeng et al. (2009), and Orlowska et al. (2011). Previous studies have also shown that AMF could improve plant tolerance to Ni stress by decreasing metal accumulation and translocation to shoots (Vivas et al. 2006; Amir et al. 2013). In tall fescue plants, translocation of Ni from roots to shoots was inhibited by F. mosseae (M plants), and the translocation factor was decreased under all levels of Ni. Hildebrandt et al. (2007) also showed that roots of mycorrhizal plants retained toxic metals so that metal transfer to the shoots was restricted, and suggested that AMF could filter out toxic metals by accumulating them in their mycelia. However, a positive effect of mycorrhiza on Ni uptake and its translocation to shoots has on the contrary been reported in other plants by Turnau and Mesjasz-Przybylowicz (2003) and Lagrange et al. (2011). It is known that metal uptake rates directly influence metal tolerance and sensitivity (Clemens 2001). The tufted root system in tall fescue may help to immobilize soil pollutants (Soleimani et al. 2009), accumulate Ni, and therefore contribute to tolerance of high metal concentrations (180 ppm).
In higher plants, several specialized peptides are involved in heavy metal ion homeostasis and detoxification. Gene expression of several ABC transporters, such as At-ATM3, At-PDR8, or At-PDR12, in Arabidopsis is stimulated by lead (Lee et al. 2005; Kim et al. 2006, 2007). Transient expression of the Arabidopsis metallothioneins At-MT2a and At-MT3 has been shown to enhance the resistance of Vicia faba guard cells to cadmium (Lee et al. 2004). Also, the expression of Brassica juncea BjMT2 in Arabidopsis thaliana seedlings increased plant tolerance against cadmium (Zhigang et al. 2006). Kim et al. (2007) proposed that At-PDR8 confers cadmium resistance in Arabidopsis by pumping the metal out of the roots. However, there have been few reports concerning the effects of Ni on ABC and MET gene expression in plants. It was found that MET could be induced by Ni in some ecotypes of Arabidopsis (Murphy and Taiz 1995). In the present study, expression of the putative tall fescue ABC transporter and MET type II genes in roots significantly increased in the presence of Ni (except at 180 ppm) compared to the Ni 0 treatment. In shoots, transcript accumulation of these two genes increased only at 30 ppm Ni but decreased in the two other treatments (Ni 90 and Ni 180 ppm) for the ABC gene. High concentrations of heavy metals may reduce the protein pool (Singh et al. 2010), as a result of modifications in gene expression (Kovalchuk et al. 2005), increase in ribonuclease activity (Gopal and Rizvi 2008), and stimulation of protease activity (Jana and Choudhuri 1982). However, the appearance of necrosis on the leaf tips and margins at concentrations of 90 and 180 ppm Ni asserts that these concentrations were toxic for tall fescue plants, or that leaves were more sensitive than roots.
AMF colonization of roots has been reported to have a significant impact on the expression of several plant genes coding for proteins presumably involved in heavy metal tolerance/detoxification (Rivera-Becerril et al. 2005; Hildebrandt et al. 2007; Cicatelli et al. 2014). Cicatelli et al. (2010) showed that inoculation of Populus alba with F. mosseae or G. intraradices caused induction of MET genes in Cu- and Zn-polluted soil, while the mycorrhizal symbiosis attenuated Cd stress in Pisum sativum by increasing transcripts of the PsMTA MET gene (Rivera‐Becerril et al. 2002). The current research is the first attempt to study the role of Ni and AMF in the expression of putative ABC transporter and MET type II genes in tall fescue plants. Transcript accumulation of these two genes was unrelated to the presence of F. mosseae in tall fescue, since it was similar in roots and shoots of both M and NM plants in the absence of Ni. However, transcript levels of ABC and MET in roots of tall fescue plants were higher under Ni stress in M plants than in non-inoculated plants, although an obvious increase did not occur in the shoots of M plants as compared to NM counterparts.
In conclusion, Ni entrance into roots and translocation to the shoots of F. arundinacea was reduced by F. mosseae at concentrations of Ni greater than 30 ppm. Reduction in Ni translocation to the shoots may be related to the activation of mechanisms in M roots, viz chelation of Ni and/or compartmentation within vacuoles. While F. mosseae increased levels of ABC and MET transcripts in the roots of tall fescue, its reduction of Ni translocation probably made metal levels insufficient for the expression of these genes in the shoots. Many metal transporters in organisms are regulated at the transcriptional level by extracellular metal concentrations via transcription factor proteins (Radisky and Kaplan 1999). The ratio of ABC to MET expression was about 2–3 in the mycorrhizal roots of tall fescue, reflecting a relatively higher transcription rate of the ABC gene and suggesting that these genes are transcriptionally controlled, with their regulation probably mediated by Ni-sensitive transcriptional factors. The identical response of the two genes in roots to Ni could be explained by the interaction of the same regulatory protein, or proteins, with their promoter elements, while their dose-dependent expression in shoots may reflect the involvement of F. mosseae in Ni partitioning between the roots and shoots, so that transcript accumulation is indirectly related to the presence of the mycorrhizal fungus. The results presented here highlight the importance of mycorrhizal colonization in the reduction of Ni transport from roots to shoots of tall fescue, which alleviates Ni-induced stress in this plant.
References
Ahmed A, Hasnain A, Akhtar S, Hussain A, Yasin G, Wahid A, Mahmood S (2013) Antioxidant enzymes as bio-markers for copper tolerance in safflower (Carthamus tinctorius L.). Afr J Biotechnol 9:5441–5444
Aghababaei F, Raiesi F, Hosseinpur AR (2014) The significant contribution of mycorrhizal fungi and earthworms to maize protection and phytoremediation in Cd-polluted soils. Pedobiologia 57:223–233
Alaoui-Sosse B, Genet P, Vinit-Dunand F, Toussaint M-L, Epron D, Badot P-M (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Allen SE (1989) Chemical analysis of ecological materials. Blackwell, London
Alsokari S (2009) Modulatory role of kinetin on photosynthetic characteristics, yield and yield attributes of cadmium-treated Sorghum bicolor plants. J Appl Sci Res 5:2383–2396
Amir H, Lagrange A, Hassaϊne N, Cavaloc Y (2013) Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23:585–595
Arnon DI (1949) Cooper enzymes in isolated chloroplasts. Phenol-oxidase in Beta vulgaris. Plant Physiol 24:1–15
Auge RM, Moore JL, Sylvia DM, Cho K (2004) Mycorrhizal promotion of host stomatal conductance in relation to irradiance and temperature. Mycorrhiza 14:85–92
Baslam M, Esteban R, García-Plazaola JI, Goicoechea N (2013) Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl Micobiol Biotechnol 97:3119–3128
Cammack R, Fernandez VM, Schneider K (1988) Nickel in hydrogenases from sulphate reducing, photosynthetic, and hydrogen oxidizing bacteria. In: Lancaster JR jr (ed) The bioorganic chemistry of nickel. Verlag-Chemie, Weinheim, pp 167–190
Carpio LA, Davies FT, Arnold MA (2005) Arbuscular mycorrhizal fungi, organic and inorganic controlled-release fertilizers: effect on growth and leachate of container-grown bush morning glory (Ipomoea carnea ssp. fistulosa) under high production temperatures. J Am Soc Hortic Sci 130:131–139
Cicatelli A, Lingua G, Todeschini V, Biondi S, Torrigiani P, Castiglione S (2010) Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot 106:791–802
Cicatelli A, Torrigiani P, Todeschini V, Biondi S, Castiglione S, Lingua G (2014) Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: biochemical and molecular aspects. iForest 7:333–341
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Davies F, Olalde-Portugal V, Aguilera-Gomez L, Alvarado M, Ferrera-Cerrato R, Boutton T (2002) Alleviation of drought stress of Chile ancho pepper (Capsicum annuum L. cv. San Luis) with arbuscular mycorrhiza indigenous to Mexico. Sci Hortic 92:347–359
Davies FT, Puryear JD, Newton RJ, Egilla JN, Grossi JAS (2001) Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J Plant Physiol 158:777–786
Demir S (2004) Influence of arbuscular mycorrhiza on some physiological growth parameters of pepper. Turk J Biol 28:85–90
Dong Y, Zhu Y-G, Smith FA, Wang Y, Chen B (2008) Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil. Environ Pollut 155:174–181
Doubkova P, Sudova R (2014) Nickel tolerance of serpentine and non-serpentine Knautia arvensis plants as affected by arbuscular mycorrhizal symbiosis. Mycorrhiza 24:209–217
Gajewska E, Skłodowska M (2007) Relations between tocopherol, chlorophyll and lipid peroxides contents in shoots of Ni-treated wheat. J Plant Physiol 164:364–366
Gamalero E, Lingua G, Berta G, Glick BR (2009) Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Can J Microbiol 55:501–514
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gohre V, Paszkowski U (2006) Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremedialion. Planta 223:1115–1122
Gonzalez-Chavez M (2000) Arbuscular mycorrhizal fungi from As/Cu polluted soils. Dissertation, University of Reading, UK
Gopal R, Rizvi AH (2008) Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 70:1539–1544
Gussarsson M (1994) Cadmium-induced alterations in nutrient composition and growth of Betula pendula seedlings: the significance of fine roots as a primary target for cadmium toxicity. J Plant Nutr 17:2151–2163
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68:139–146
Jana S, Choudhuri MA (1982) Senescence in submerged aquatic angiosperms: effects of heavy metals. New Phytol 90:477–484
Jianfeng H, Xiangui L, Rui Y, Jiang Q, Yufang S (2009) Effects of arbuscular mycorrhizal fungi inoculation on arsenic accumulation by tobacco (Nicotiana tabacum L.). J Environ Sci 21:1214–1220
Kagan VE, Kisin ER, Kawai K et al (2002) Towards mechanism based antioxidant interventions. Ann NY Acad Sci 959:188–198
Kim DY, Bovet L, Kushnir S, Noh EW, Martinoia E, Lee Y (2006) AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol 140:922–932
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218
Kovalchuk I, Titov V, Hohn B, Kovalchuk O (2005) Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat Res 570:149–161
Kramer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature 379:635–638
Lagrange A, Ducousso M, Jourand P, Majorel C, Amir H (2011) New insights into the mycorrhizal status of Cyperaceae from ultramafic soils in New Caledonia. Can J Microbiol 57:21–28
Latef AAHA (2011) Influence of arbuscular mycorrhizal fungi and copper on growth, accumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuum L.). Mycorrhiza 21:495–503
Lee J, Donghwan S, Won-yong S, Inhwan H, Youngsook L (2004) Arabidopsis metallothioneins 2a and 3 enhance resistance to cadmium when expressed in Vicia faba guard cells. Plant Mol Biol 54:805–815
Lee M, Lee K, Lee J, Noh EW, Lee Y (2005) AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol 138:827–836
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Röber B, Schulz B (2002) Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214:345–355
Murphy A, Taiz L (1995) Comparison of metallothionein gene expression and nonprotein thiols in ten Arabidopsis ecotypes (correlation with copper tolerance). Plant Physiol 109:945–954
O’Halloran TV, Culotta VC (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275:25057–25060
Orlowska E, Przybylowicz W, Orlowski D, Turnau K, Mesjasz-Przybylowicz J (2011) The effect of mycorrhiza on the growth and elemental composition of Ni-hyperaccumulating plant Berkheya coddii Roessler. Environ Poll 159:3730–3738
Perotto S, Martino E (2001) Molecular and cellular mechanisms of heavy metal tolerance in mycorrhizal fungi: what perspectives for bioremediation? Minerva Biotechnol 13:55–63
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55:158–161
Poorter H, Villar R (1997) The fate of acquired carbon in plants: chemical composition and construction costs. In: Bazzaz FA, Grace J (eds) Plant resource allocation. Academic, San Diego, pp 39–72
Porcel R, Barea JM, Ruiz‐Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157:135–143
Radisky D, Kaplan J (1999) Regulation of transition metal transport across the yeast plasma membrane. J Biol Chem 274:4481–4484
Rivera-Becerril F, van Tuinen D, Martin-Laurent F, Metwally A, Dietz KJ, Gianinazzi S, Gianinazzi-Pearson V (2005) Molecular changes in Pisum sativum L. roots during arbuscular mycorrhiza buffering of cadmium stress. Mycorrhiza 16:51–60
Rivera‐Becerril F, Calantzis C, Turnau K, Caussanel JP, Belimov AA, Gianinazzi S, Strasser RJ, Gianinazzi‐Pearson V (2002) Cadmium accumulation and buffering of cadmium‐induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. J Exp Bot 53:1177–1185
Roitsch T, Ehneß R (2000) Regulation of source/sink relations by cytokinins. Plant Growth Regul 32:359–367
SAS Institute Inc., (1999) SAS/STAT User's Guide. SAS Institute, Inc, Cary, NC
Singh K, Pandey S (2011) Effect of nickel-stresses on uptake, pigments and antioxidative responses of water lettuce, Pistia stratiotes L. J Environ Biol 32:391–394
Singh R, Tripathi R, Dwivedi S, Kumar A, Trivedi P, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour Technol 101:3025–3032
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Soleimani M, Hajabbasi M, Afyuni M, Charkhabi A, Shariatmadari H (2009) Bioaccumulation of nickel and lead by Bermuda grass (Cynodon dactylon) and tall fescue (Festuca arundinacea) from two contaminated soils. Caspian J Environ Sci 7:59–70
Turnau K, Mesjasz-Przybylowicz J (2003) Arbuscular mycorrhiza of Berkheya codii and other Ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza 13:185–190
Van den Ende W, Peshev D (2013) Sugars as antioxidants in plants. In: Tuteja N, Gill SS (eds) Crop improvement under adverse conditions. Springer, pp 285–307
Vivas A, Biró B, Németh T, Barea JM, Azcón R (2006) Nickel-tolerant Brevibacillus brevis and arbuscular mycorrhizal fungus can reduce metal acquisition and nickel toxicity effects in plant growing in nickel supplemented soil. Soil Biol Biochem 38:2694–2704
Williams LE, Pittman JK, Hall J (2000) Emerging mechanisms for heavy metal transport in plants. Biochim Biophys Acta 1465:104–126
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. B Environ Contam Toxicol 86:1–17
Zhigang A, Cuijie L, Yuangang Z, Yejie D, Wachter A, Gromes R, Rausch T (2006) Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J Exp Bot 57:3575–3582
Acknowledgments
This project was supported by the research council of University of Shahrekord, SKU (Grant No. 160/12).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shabani, L., Sabzalian, M.R. & Mostafavi pour, S. Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea . Mycorrhiza 26, 67–76 (2016). https://doi.org/10.1007/s00572-015-0647-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0647-2