Abstract
Spatial expansion of root hemiparasitic Pedicularis kansuensis in Bayanbulak Grassland of Xinjiang Uygur Autonomous Region (China) has caused great loss of herbage yield and has threatened the local livestock industry. Current management practices using manual eradication and chemical control have been proved problematic. Arbuscular mycorrhizal (AM) fungi have been suggested to be potential biocontrol agents against a number of plant pests, but experimental evidence is lacking against weedy P. kansuensis. In this study, we tested the hypothesis that inoculation with AM fungi will cause growth depression in P. kansuensis and reduce its damage to host plants. Based on the confirmation of AM status and host community of the hemiparasite in the field, a pot cultivation experiment was conducted to test the influence of an AM fungus (Glomus mosseae) on growth of P. kansuensis and the parasitized host (Elymus nutans). AM colonization was observed in roots of P. kansuensis, but the levels were much lower than those of its adjacent host species. A negative correlation between AM levels and the numbers of haustoria was detected for the field samples of the hemiparasite. Strong suppression of haustorium formation, a significant reduction in plant dry weight (DW), as well as marked reduction in the survival rate of P. kansuensis after inoculation with AM fungi was observed. In contrast, inoculation with G. mosseae increased root DW and whole plant DW of parasitized host plants. Our findings demonstrated significantly repressive effects of AM fungi on growth performance of P. kansuensis with and without the presence of a host. The potential of AM fungi as biocontrol agents against the damaging hemiparasite was confirmed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root hemiparasitic plants are green and have photosynthetic capability, but depend at least partially on their host plants for acquisition of nutrients and water, via parasitic organs (known as haustoria) that connect the root xylem of the hemiparasite and that of its host plant (Irving and Cameron 2009). Infestation by root hemiparasitic plants often causes marked growth depression in their host plants by direct deprivation of nutrients and water (Jiang et al. 2003; Yoder et al. 2009; Hautier et al. 2010; Ren et al. 2010). Furthermore, they have strong influence on plant community structure and plant biodiversity in the ecosystems where they occur (Hedberg et al. 2005; Phoenix and Press 2005; Grewell 2008; Hellström et al. 2011; Borowicz and Armstrong 2012).
Pedicularis L. (Orobanchaceae) is a large genus of root hemiparasitic species widely distributed in the temperate zone of the Northern Hemisphere. There are at least 800 Pedicularis species worldwide (Mill 2001), with more than 352 species reported in China (Yang et al. 1998; Mill 2001). Pedicularis species are common flowering plants in alpine and subalpine grassland ecosystems. In most cases, they show patchy distribution patterns and do not exert significant influence on plant communities at a large scale. However, dramatic spatial expansion and damage to herbage yield have been reported for a few Chinese Pedicularis species (Song 2006; Bao and Wang 2011). Most notably, spatial expansion of Pedicularis kansuensis Maxim. in Bayanbulak Grassland of Xinjiang Uygur Autonomous Region of China has caused great loss of herbage yield and threatened the local livestock industry. This annual hemiparasite was previously misidentified as P. verticillata (Liu et al. 2008; Wang et al. 2010), but has been verified to be P. kansuensis from our personal observations and molecular evidence (Sui et al., unpublished). By 2008, the area severely affected by P. kansuensis reached 2.33 × 104 ha in Bayanbulak Grassland, with an annual spread rate of 3.3 × 103 ha (Liu et al. 2008; Wang et al. 2009). Chemical control and manual eradication have been used for the management of this damaging species (Wang et al. 2009, 2010). However, because root hemiparasitic plants fit into the life cycle of, and form direct connection with, their host plants, chemical control in parasitic plants has been problematic (Aly 2012). Damage to host plants and potential risk of environmental pollution has been suggested as a result of chemical control of P. kansuensis (Zhang et al. 2009; Wang et al. 2010). Due to the large scale (and high density) of infested areas and the low fodder quality of P. kansuensis (animals tend to avoid the root hemiparasite when grazing), cutting at peak juvenile stage, an option suggested for the management of an annual hemiparasitic weed Rhinanthus minor in Europe (Magda et al. 2004) is not feasible for P. kansuensis in Bayanbulak Grassland. More effective, sustainable, and environmental friendly control strategies against this fast spreading plant are in dire need.
Arbuscular mycorrhizal (AM) fungi are ubiquitous components of soil microflora in most terrestrial ecosystems and form mutualistic associations with the majority of land plants (Smith and Read 2008). These fungi have been suggested to be efficient biocontrol agents against soil-borne pathogens and nematodes (Veresoglou and Rillig 2012; Vos et al. 2012; Akhtar and Panwar 2013; Vos et al. 2013). AM fungi have also been observed to significantly reduce seed germination of a few obligate root parasitic plant species such as Striga hermonthica (Lendzemo et al. 2005, 2006; Hearne 2009; Gworgwor and Weber 2003) and Orobanche cumana (Louarn et al. 2012) and thus have been suggested to have a potential in biocontrol against obligate root parasitic weeds. Recently, Li et al. (2012b) observed that inoculation with AM fungi suppressed haustorium formation in facultative root hemiparasitic Pedicularis tricolor. In addition, they found that phosphorus transfer from the host plant into P. tricolor and Pedicularis rex was significantly reduced and growth of the hemiparasites was suppressed after AM inoculation, suggesting that AM fungi have a potential in the management of Pedicularis species (Li et al. 2013). However, effects of AM fungi on interactions between root hemiparasitic plants and their host plants vary greatly, depending at least partially on plant identities. For example, in contrast to the repressive effects of AM fungi observed for Hordeum vulgare–P. tricolor/rex interactions (Li et al. 2013), haustorium formation and growth performance of the root hemiparasite in the H. vulgare–R. minor association significantly increased after AM inoculation (Davies and Graves 1998). Thus, it is essential to gain a thorough knowledge of the influence of AM fungi on the specific interactions between the target root hemiparasite and its host species before we rush to the application of AM fungi as biocontrol agents. However, as far as we know, no effort has been taken from a biological perspective to control weedy Pedicularis despite the long efforts for its management. Little information is available regarding the parasitic habit and AM status of P. kansuensis, and no investigation has been done concerning the interactions between AM fungi and this plant.
In this study, the hypothesis was tested that AM fungi will suppress haustorium formation in P. kansuensis and alleviate its damage to host plants and thus have a potential as biocontrol agents. Firstly, AM colonization levels and haustorium formation in P. kansuensis and AM levels of its adjacent hosts were examined from two field sites that differ significantly in infestation levels by the hemiparasite, with the aim of gaining basic information about the AM status and parasitic habit of P. kansuensis. Secondly, a pot cultivation experiment was conducted investigating effects of AM fungi on growth of P. kansuensis and the parasitized host. Specifically, the following questions were addressed: (1) Do P. kansuensis and its dominant hosts associate with AM fungi in the field and do the AM levels differ between severely infested and poorly infested areas? (2) Does incidence of haustorium formation differ between severely infested and poorly infested areas? (3) Does inoculation with AM fungi have any influence on the growth of P. kansuensis and the parasitized host? Knowledge obtained will shed some light on the interactions among P. kansuensis, its host plants, and AM fungi. A better knowledge about the influence of AM fungi on the infestation of host plants by P. kansuensis will provide new clues for the potential use of AM fungi as biocontrol agents for a more effective and sustainable management of this weedy hemiparasite.
Material and methods
This study used two approaches. Firstly, field surveys were performed in two typical distribution areas (representing one severely infested and one poorly infested by P. kansuensis) to examine AM fungal colonization levels in roots of P. kansuensis and of the adjacent plant species. After that, the influence of AM fungi on growth of P. kansuensis and the parasitized host plant was tested in a pot cultivation experiment.
Field survey
Site description and sampling
P. kansuensis shows an aggregated distribution pattern and spreading tendency in severely infested areas of Bayanbulak Grassland. In contrast, it shows sporadical distribution in poorly infested areas. Two representative sites (Table 1), one for a severely infested area (with above 80 % coverage of P. kansuensis, site A) and the other for a poorly infested area (sporadically distributed with less than 1 % coverage of P. kansuensis, site B), were selected for field surveys. The concentrations of organic matter (OM), available nitrogen (AN), and available phosphorus (AP) were all much higher in soil samples from site B than those from site A, except that the concentrations of available potassium (AK) were very close between these two sites. In site A, we randomly collected 50 individual root systems of P. kansuensis, ten from each of five 10 × 10 m2 plots (four from the corners and one from the center of a 50 × 50 m2 area). In site B, 50 individual root systems were collected at much larger distance intervals due to the patchy distribution of the hemiparasite.
During root excavation, large clods of earth were dug out to reduce the damage to fine rootlets. Root samples of P. kansuensis and its adjacent host species were collected separately, carefully washed free of soil debris and kept in formalin–acetic acid–alcohol (FAA) fixative until further examination. Two dominant grass host species and one dominant forb host species from each of the sampling sites were chosen for AM examination to serve as a reference for general AM levels of the host species. Elymus nutans was the dominant host species for P. kansuensis in the severely infested site (site A), but was very patchy in the poorly infested site and seldom found in the sampled earth clods (site B). As a consequence, this host was not selected as a reference host in site B.
For assessment of AM colonization in P. kansuensis and its potential host species, as well as haustorium formation by the root hemiparasite, a weighed subsample of root material less than 1 mm in diameter was taken. The remainder was oven-dried at 75 °C for 48 h. Dry weight (DW) of the root subsample used for checking AM colonization and haustorium formation was obtained from the ratio between fresh weight (FW) and DW of the remainder and FW of the subsample. AM colonization in roots of P. kansuensis and its adjacent hosts was recorded according to the magnified intersections method (McGonigle et al. 1990), after clearing in 10 % KOH and staining in a 5 % ink–vinegar solution (Li et al. 2012b). The incidence of AM fungal structures was scored for hyphae, vesicles and arbuscules/coils. The percent incidence of each structure over total intersections (at least 150 intersections were observed for each sample) was calculated and the total percentage colonization was determined. The number of haustoria (Ha) formed by each P. kansuensis plant was counted after carefully separating rootlets of the hemiparasite from its host under a stereomicroscope. Ha tightly attached to a host root were cut off with as little host tissue as possible and pooled with P. kansuensis rootlets for calculation of Ha numbers. Numbers of Ha per gram dry root were used for further analysis.
Pot cultivation experiment
Experimental design
A factorial pot cultivation experiment was conducted using E. nutans Griseb. as host plant and Glomus mosseae (Nicol. and Gerd.) Gerdemann and Trappe (BGC YN05) to test the interactions among P. kansuensis, its host plant and the AM fungus. E. nutans was chosen as host because it was the most dominant species in the habitats of P. kansuensis, particularly in severely infested sites (Zhang et al. 2009). G. mosseae was used because it is one of the common fungal species observed in habitat soils of P. kansuensis in Bayanbulak Grassland (Li, unpublished data). The experimental design was as follows: (1) one P. kansuensis plant with one host and no AM fungus (+H-AM); (2) one P. kansuensis plant with one host and inoculated with AM fungus (+H + AM); (3) two P. kansuensis plants without host or AM fungus (−H-AM); and (4) two P. kansuensis plants without host but inoculated with AM fungus (−H + AM). Each treatment had ten replicated pots.
Growth medium, plant materials, and AM fungal inoculum
Growth medium
A mix of 10 % soil collected from Kunming Botanical Garden (Kunming, China), and 90 % fine sand was used as growth medium. Plant AN (by the Kjeldahl method), AP (by the phosphovanado-molybdate method), and AK (by the flame photometry method) concentrations of the soil mix were around 14.3, 2.7, and 62.4 mg kg-1 dry soil, respectively. The pH (in 0.01 M CaCl2 solution) of the growth medium was approximately 6.2. The soil and fine sand were sieved through a 0.83-mm sieve, then autoclaved at 121 °C for 2 h before use.
Plant materials
Seeds of E. nutans and P. kansuensis were collected from the severely infested area (site A) in late August 2010 and late September 2011, respectively. Seeds were surface-sterilized for 5–8 min in 4.5 % sodium hypochlorite and germinated on filter papers moistened with distilled water in Petri dishes at 18–25 °C. The photoperiod was 12 h light with 22.2 μmol m-2 s-1 irradiance and 12 h darkness.
AM fungal inoculum
The AM fungal inoculum consisted of colonized root fragments, soil, and spores, derived from pot cultures prepared with Sorgum bicolor in sandy soil, which was provided by Beijing Institute of Plant Nutrition and Resources, Academy of Agriculture and Forestry Sciences. Fungal inoculum (1 %, 21 g w/w) was added (through a tube to reduce seedling disturbance) into mycorrhizal pots at approximately 5-cm depth 2 weeks after seedlings of P. kansuensis were planted. The nonmycorrhizal treatments received equal amounts of the fungal inoculum that was sterilized at 121 °C for 30 min.
Planting and growth conditions
For both P. kansuensis and E. nutans, uniform newly germinated seeds with 2- to 5-mm-long radicles were used. One host seedling was planted into the center of each pot. After 2 weeks, five seedlings of P. kansuensis were planted around the host (or in the pot center in treatments without a host) at a fixed distance (1.5–2 cm). Seedlings of P. kansuensis were thinned to one (in the presence of a host) or two (in the absence of a host) per pot 8 weeks after planting, when establishment of functional connections between P. kansuensis and its host was confirmed based on faster growth rates of the attached root hemiparasite than the unattached ones.
All plants were grown under outdoor conditions in Kunming Botanical Garden (25°08′23″N, 102°44′24″E; altitude 1,990 m), but protected with a glass roof and a fly net to reduce the influence of rain water and insects. The experiment was conducted from mid-March to late August 2012 (24 weeks), with a temperature range from 10 to 30 °C. After 1 week of P. kansuensis planting, 20 ml modified Hoagland nutrient solution [0.08 mM Ca(NO3)2⋅4H2O; 0.81 mM KNO3; 0.16 mM NH4NO3; 0.02 mM KNO3; 0.08 mM MgSO4; 2.00 × 10-3 mM FeSO4⋅7H2O; 2.02 × 10-3 mM EDTA⋅2Na; 1.00 × 10-6 mM KI; 2.01 × 10-5 mM H3BO3; 2.64 × 10-5 mM MnSO4;1.07 × 10-5 mM ZnSO4; 2.07 × 10-7 mM Na2MoO4; 2.00 × 10-8 mM CuSO4; and 3.85 × 10-8 mM CoCl; pH 6.5] was supplied weekly. The pots were watered with tap water whenever necessary. Autoclaved polyethylene beads were put on the soil surface to retain moisture in the pots. All pots were fully randomized and re-randomized once a week to reduce position effects.
Harvest and sampling
Survival rates of P. kansuensis in the different treatments were recorded every week after planting. Root and shoot DW, total DW, and root:shoot ratio (R:S) of both P. kansuensis and host plants were determined as described by Li et al. (2012a). AM colonization levels of P. kansuensis and the host were examined using the method mentioned above for field samples. The number of Ha was also counted as described for the field samples. Functional haustoria (FHa) were determined under a stereomicroscope after staining with trypan blue (Li et al. 2012b). Numbers of Ha and FHa per gram dry root were used for further analysis.
Data analysis
For the field data, the two independent samples' t test was used to compare the differences between site A and B in terms of AM colonization levels (AMF%) and numbers of Ha per gram dry root of P. kansuensis. Correlation analysis was used to detect any correlation between AMF% and numbers of Ha. In the pot cultivation experiment, data for numbers of Ha and FHa, root DW and total DW per P. kansuensis plant, and shoot DW of the host plant were log-transformed before statistical analysis to meet the assumption of normality required by the analysis of variance (ANOVA) method. Two-way ANOVA was performed to examine the effects of AM fungi and host on plant DW, R:S ratios, and haustorium formation in P. kansuensis. The two independent samples' t test was used to examine the effects of AM fungi on shoot DW, root DW, total DW, and R: S ratio per plant of E. nutans. All the analyses were performed using the Statistical Product and Service Solution (SPSS) software (version 16.0; SPSS China, Shanghai, China).
Results
Field survey
AM fungal colonization in P. kansuensis and its adjacent hosts
P. kansuensis and the potential host plant species in its vicinity from both sampling sites were colonized by AM fungi (Table 2). AM colonization levels were generally higher for samples from site B than those from site A. Hyphae were the most common fungal structures observed in roots of P. kansuensis and its potential hosts. AM colonization level of P. kansuensis was significantly higher in site B than that in site A (t = −3.549, P < 0.01).
All the potential hosts examined had higher AM colonization levels than P. kansuensis in both sites. E. nutans (ca. 76 % root length colonized by AM fungi), the most dominant host species in the severely infested area, had nearly triple the AM levels of its adjacent P. kansuensis (ca. 28 % root length; Table 2).
Incidence of Ha produced by P. kansuensis and its correlation with AM colonization levels
P. kansuensis from both sampling sites produced Ha. Root samples from the severely infested site (site A) had significantly higher number of Ha (298.0 ± 37.5 g-1 dry root) than those from the poorly infested site (139.7 ± 14.9 g-1 dry root; t = 3.921, P < 0.001). AMF% in roots of P. kansuensis was found to be negatively correlated with numbers of Ha (r = −0.231, P < 0.05).
Pot cultivation experiment
Survival rate of P. kansuensis
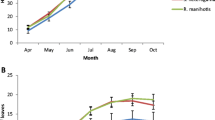
The growth performance of both host and hemiparasite in the + H + AM treatment was facilitated by G. mosseae to some extent in the first month after inoculation. However, significantly negative effects of G. mosseae on the survival and growth of P. kansuensis were observed 3 weeks after thinning (Fig. 1). In the absence of a host, seedling mortality of P. kansuensis was high, particularly when inoculated with G. mosseae. In the presence of a host, P. kansuensis grew much better when compared with those not attached to a host plant. However, severe defoliation was observed in mycorrhizal P. kansuensis but not in nonmycorrhizal plants in the presence of E. nutans 7 weeks after thinning. At harvest only three P. kansuensis seedlings (one for each pot) survived in -H + AM. The survival rates of the hemiparasite in other treatments were 40 % (+H + AM), 70 % (+H-AM), and 50 % (−H-AM), respectively.
Survival dynamics of Pedicularis kansuensis in different treatments after thinning Treatments: +H-AM, one P. kansuensis with one host (Elymus nutans) and no AM fungus; +H + AM, one P. kansuensis with one host and inoculated with AM fungus (Glomus mosseae); -H-AM, two P. kansuensis with neither host nor AM fungus; and -H + AM, two P. kansuensis without host but inoculated with AM fungus
AM fungal colonization levels and haustorium formation
P. kansuensis and E. nutans were all colonized by G. mosseae in mycorrhizal treatments. Negligible amounts of AM fungal structures were detected in nonmycorrhizal pots. The AM colonization level of E. nutans was 82.6 ± 1.4 % root length. Hyphae were the most common fungal structures observed, along with arbuscules and vesicles. In the presence of E. nutans, AMF% in roots of P. kansuensis was 49.5 ± 3.2 % root length. However, in the absence of a host plant, AMF% of P. kansuensis was 23.9 ± 3.3 % root length.
Ha were observed in all P. kansuensis plants except those from -H + AM treatment, where few living plants were obtained at harvest. AM fungal inoculation and host attachment showed significant interaction effects on the number of Ha, but not on the number of FHa (Table 3). The presence of E. nutans considerably facilitated the formation of both Ha and FHa (Table 3 and Fig. 2). In contrast, inoculation with G. mosseae strongly repressed haustorium formation in P. kansuensis, with or without the presence of E. nutans (Table 3 and Fig. 2).
Numbers of Haustoria (Ha) and functional haustoria (FHa) per gram dry root formed by Pedicularis kansuensis in different treatments. Data are presented as mean ± S.E. of seven (+H-AM), four (+H + AM), five (−H-AM), or three (−H + AM) replicated pots. Bars for Ha and FHa with different letters indicate statistically significant difference at P < 0.05 level. Treatments: +H-AM, one P. kansuensis with one host (Elymus nutans) and no AM fungus; +H + AM, one P. kansuensis with one host and inoculated with AM fungus (Glomus mosseae); -H-AM, two P. kansuensis with neither host nor AM fungus; and -H + AM, two P. kansuensis without host but inoculated with G. mosseae
Growth performance of P. kansuensis and its host
P. kansuensis benefited greatly from attachment to E. nutans. Shoot DW and root DW of P. kansuensis were both substantially increased when attached to E. nutans, regardless of the AM status (Fig. 3a and Table 3). In contrast, inoculation with G. mosseae showed a negative influence on the growth of P. kansuensis. In the absence of a host, the growth of the hemiparasite was severely inhibited by G. mosseae, as shown by the very low plant DWs (Fig. 3a). When grown with a host, shoot DW and total DW of P. kansuensis were also significantly suppressed by inoculation with G. mosseae (Fig. 3a and Table 3). However, the significant reduction in root biomass of P. kansuensis following inoculation of G. mosseae in the absence of a host was not observed in the hemiparasites grown with a host (Fig. 3a). Neither the host nor G. mosseae showed any effects on R:S ratios of P. kansuensis (Table 3).
Shoot dry weight [DW (g); above zero line], root DW (g; below zero line), and total DW (g) per plant of Pedicularis kansuensis (a) and its host Elymus nutans (b) in the pot cultivation experiment Data are presented as mean ± S.E. of seven (+H-AM), four (+H + AM), five (−H-AM) or three (−H + AM) replicated pots. Different letters indicate statistically significant difference at P < 0.05 level. Different letters in round brackets and square brackets indicate significant difference in total DW per plant and R:S ratio, respectively. Treatments: +H-AM, one P. kansuensis with one host (E. nutans) and no AM fungus; +H + AM, one P. kansuensis with one host and inoculated with AM fungus (Glomus mosseae); -H-AM, two P. kansuensis with neither host nor AM fungus; -H + AM, two P. kansuensis without host but inoculated with AM fungus; +P + AM, parasitized by P. kansuensis and inoculated with G. mosseae; +P-AM, parasitized by P. kansuensis and no AM fungal inoculation
Inoculation with G. mosseae showed a positive effect on biomass of E. nutans parasitized by P. kansuensis (Fig. 3b). The root DW (t = −2.843, P < 0.05) and total DW (t = −2.847, P < 0.05) of parasitized E. nutans were all significantly higher after inoculation with G. mosseae, when compared with the nonmycorrhizal hosts. However, influence of G. mosseae on shoot DW (t = −1.641, P = 0.135) and R:S ratio (t = −1.034, P = 0.333) of parasitized E. nutans was not significant.
Discussion
AM colonization levels in P. kansuensis roots from both field sites were much lower than those of its adjacent host species. This might be explained by its root hemiparasitic habit. As root hemiparasitic plants have the capability to extract nutrients from other plant species, which is doubtless a more effective way of nutrient acquisition, most root hemiparasites do not form AM associations (Atsatt 1973; Brundrett 2002). Although some Pedicularis species retain the capability for forming AM symbiosis, they tend to invest less in root biomass when successfully attached to appropriate host plants (Li et al. 2012a). As a consequence, the availability of carbon for AM fungal partners and hence AM colonization in roots of the root hemiparasites is likely to be much lower than in autotrophic plant species. Similarly, apart from different plant identity, significant reduction in plant community productivity (and hence reduced availability of plant carbohydrate for AM fungi) caused by heavy infestation of P. kansuensis (Liu et al. 2008; Wang et al. 2009; Zhang et al. 2009) may be one explanation for the overall lower AM colonization levels in the severely infested site than the poorly infested site.
Although a single site contrast for the field survey is far from sufficient to conclusively determine the effects of AM fungi on growth of P. kansuensis in the field, the significantly negative correlation between AM levels and haustorium formation incidence per gram dry root of P. kansuensis suggested functional interactions between the hemiparasite and AM fungi. Indeed in the pot cultivation experiment, inoculation with G. mosseae significantly reduced haustorium formation and plant DWs of the hemiparasite both in the presence and absence of the host. The results confirmed that G. mosseae had indirect (via host plant) as well as direct influence on P. kansuensis. Although a majority of AM fungi are mutualistic symbionts of most land plants (Smith and Read 2008), G. mosseae seemed to act towards the parasitism end in the parasitism–mutualism continuum when associated with P. kansuensis, as shown by the higher mortality level and lower DWs in the mycorrhizal plants when compared with the nonmycorrhizal ones. In view of its suppressive effects on the root hemiparasite and alleviation of host damage, this fungus may have a potential as a biocontrol agent against the weedy hemiparasite.
Plant DWs of P. kansuensis were significantly increased when attached to E. nutans, indicating strong host dependency of the hemiparasite. Because a control (nonmycorrhizal and nonparasitized) for host plant was not included in the pot cultivation experiment, the reduction in biomass of E. nutans caused by the infestation of P. kansuensis was not determined. However, a negative correlation between biomass of root hemiparasitic plants and that of their grass hosts has been reported for many parasite–host pairs (Matthies 1997; Hedberg et al. 2005; Hellström et al. 2011). The biomass of P. kansuensis decreased while that of E. nutans increased after AM inoculation. This suggested that AM fungi may have interfered with the interactions between the hemiparasite and its host and consequently alleviated the damage of the hemiparasite to its host.
Alleviation of host biomass reduction caused by root hemiparasitic plants upon AM inoculation has been observed previously in a few cases (Lendzemo and Kuyper 2001; Gworgwor and Weber 2003). However, the mechanisms involved may be very different between Pedicularis–host pairs and other parasite–host pairs due to their direct interactions with AM fungi (Li et al. 2013). The suppression of haustorium formation in P. kansuensis by AM fungal inoculation may at least partially account for the alleviation of biomass reduction of the parasitized host, since the available area for nutrient transfer from the host into the hemiparasite may have greatly declined as the number of Ha (particularly FHa) were reduced. However, more investigations are required to gain a better understanding of the underlying mechanisms for AM fungal-induced responses in the tripartite interactions.
This study investigated the influence of both host plants and AM fungi on the growth performance of the weedy P. kansuensis. AM fungal colonization and parasitic habit of P. kansuensis was experimentally confirmed. More importantly, the significant roles of AM fungi in regulation of the interactions between this hemiparasite and its host species were experimentally demonstrated for the first time. The findings support our initial hypothesis that inoculation with AM fungi will cause growth depression in P. kansuensis and reduce its damage to host plants. The AM fungal mediated alleviation of host biomass reduction caused by P. kansuensis, as well as growth depression in the hemiparasite, open up the possibility of using AM fungi as biocontrol agents for effective and sustainable management of this weedy hemiparasite.
References
Akhtar MS, Panwar J (2013) Efficacy of root-associated fungi and PGPR on the growth of Pisum sativum (cv. Arkil) and reproduction of the root-knot nematode Meloidogyne incognita. J Basic Microb 53:318–326
Aly R (2012) Advanced technologies for parasitic weed control. Weed Sci 60:290–294. doi:10.1614/ws-d-11-00066.1
Atsatt PR (1973) Parasitic flowering plants—how did they evolve. Am Nat 107:502–510. doi:10.1086/282853
Bao GS, Wang HS (2011) Allelopathic effects of Pedicularis kansuensis Maxim. on several graminaceous grass species on alpine meadow. Chin J Grassl 33:88–94 (in Chinese)
Borowicz VA, Armstrong JE (2012) Resource limitation and the role of a hemiparasite on a restored prairie. Oecologia 169:783–792. doi:10.1007/s00442-011-2222-7
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304. doi:10.1046/j.1469-8137.2002.00397.x
Davies DM, Graves JD (1998) Interactions between arbuscular mycorrhizal fungi and the hemiparasitic angiosperm Rhinanthus minor during co-infection of a host. New Phytol 139:555–563
Grewell BJ (2008) Parasite facilitates plant species coexistence in a coastal wetland. Ecology 89:1481–1488. doi:10.1890/07-0896.1
Gworgwor NA, Weber HC (2003) Arbuscular mycorrhizal fungi–parasite–host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum Sorghum bicolor (L.) Moench. Mycorrhiza 13:277–281. doi:10.1007/s00572-003-0238-5
Hautier Y, Hector A, Vojtech E, Purves D, Turnbull LA (2010) Modelling the growth of parasitic plants. J Ecol 98:857–866. doi:10.1111/j.1365-2745.2010.01657.x
Hearne SJ (2009) Control—the Striga conundrum. Pest Manage Sci 65:603–614. doi:10.1002/ps.1735
Hedberg AM, Borowicz VA, Armstrong JE (2005) Interactions between a hemiparasitic plant, Pedicularis canadensis L. (Orobanchaceae), and members of a tallgrass prairie community. J Torrey Bot Soc 132:401–410
Hellström K, Bullock JM, Pywell RF (2011) Testing the generality of hemiparasitic plant effects on mesotrophic grasslands: a multi-site experiment. Basic Appl Ecol 12:235–243. doi:10.1016/j.baae.2011.02.010
Irving LJ, Cameron DD (2009) You are what you eat: interactions between root parasitic plants and their hosts. Adv Bot Res 50:87–138. doi:10.1016/s0065-2296(08)00803-3
Jiang F, Jeschke WD, Hartung W (2003) Water flows in the parasitic association Rhinanthus minor/Hordeum vulgare. J Exp Bot 54:1985–1993. doi:10.1093/Jxb/Erg212
Lendzemo VW, Kuyper TW (2001) Effects of arbuscular mycorrhizal fungi on damage by Striga hermonthica on two contrasting cultivars of sorghum, Sorghum bicolor. Agric Ecosyst Environ 87:29–35. doi:10.1016/S0167-8809(00)00293-0
Lendzemo VW, Kuyper TW, Kropff MJ, van Ast A (2005) Field inoculation with arbuscular mycorrhizal fungi reduces Striga hermonthica performance on cereal crops and has the potential to contribute to integrated Striga management. Field Crop Res 91:51–61. doi:10.1016/j.fcr.2004.05.003
Lendzemo VW, van Ast A, Kuyper TW (2006) Can arbuscular mycorrhizal fungi contribute to Striga management on cereals in Africa? Outlook Agric 35:307–311
Li AR, Smith FA, Smith SE, Guan KY (2012a) Two sympatric root hemiparasitic Pedicularis species differ in host dependency and selectivity under phosphorus limitation. Funct Plant Biol 39:784–794. doi:10.1071/fp12159
Li AR, Guan KY, Stonor R, Smith SE, Smith FA (2013) Direct and indirect influences of arbuscular mycorrhizal fungi on phosphorus uptake by two root hemiparasitic Pedicularis species: do the fungal partners matter at low colonization levels? Ann Bot. doi:10.1093/aob/mct177
Li AR, Smith SE, Smith FA, Guan KY (2012b) Inoculation with arbuscular mycorrhizal fungi suppresses initiation of haustoria in the root hemiparasite Pedicularis tricolor. Ann Bot 109:1075–1080. doi:10.1093/aob/mcs028
Liu YY, Hu YK, Yu JM, Li KH, Gao GG, Wang X (2008) Study on harmfulness of Pedicularis verticillata and its control measures. Arid Zone Res 25:778–782 (in Chinese)
Louarn J, Carbonne F, Delavault P, Becard G, Rochange S (2012) Reduced germination of Orobanche cumana seeds in the presence of arbuscular mycorrhizal fungi or their exudates. Plos ONE 7(11):e49273. doi:10.1371/journal.pone.0049273
Magda D, Duru M, Theau JP (2004) Defining management rules for grasslands using weed demographic characteristics. Weed Sci 52:339–345
Matthies D (1997) Parasite–host interactions in Castilleja and Orthocarpus. Can J Bot 75:1252–1260
Mcgonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501
Mill RR (2001) Notes relating to the Flora of Bhutan: XLIII. Scrophulariaceae (Pedicularis) Edinburgh J Bot 58:57–98
Phoenix GK, Press MC (2005) Linking physiological traits to impacts on community structure and function: the role of root hemiparasitic Orobanchaceae (ex-Scrophulariaceae). J Ecol 93:67–78. doi:10.1111/j.1365-2745.2004.00950.x
Ren YQ, Guan KY, Li AR, Hu XJ, Zhang L (2010) Host dependence and preference of the root hemiparasite, Pedicularis cephalantha Franch. (Orobanchaceae). Folia Geobot 45:443–455. doi:10.1007/s12224-010-9081-6
Smith S, Read D (2008) Mycorrhizal symbiosis. Academic, London
Song ZS (2006) It is an urgent task to recovery and comprehensively manage the grassland ecology of Bayingbuluke. Chin J Agric Resour Reg 27:21–25 (in Chinese)
Veresoglou SD, Rillig MC (2012) Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol Lett 8:214–217
Vos C, Geerinckx K, Mkandawire R, Panis B, De Waele D, Elsen A (2012) Arbuscular mycorrhizal fungi affect both penetration and further life stage development of root-knot nematodes in tomato. Mycorrhiza 22:157–163. doi:10.1007/s00572-011-0422-y
Vos C, Schouteden N, van Tuinen D, Chatagnier O, Elsen A, De Waele D, Panis B, Gianinazzi-Pearson V (2013) Mycorrhiza-induced resistance against the root-knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol Biochem 60:45–54
Wang WL, Wang JY, Chen AL, Hu YK, Liu YY (2010) Study of Pedicularis verticillata's chemical control. Xinjiang Agric Sci 47:1242–1247 (in Chinese)
Wang WX, Sang GJ, Li L (2009) Study on the control techniques of poisonous grass Pedicularis in Xinjiang Bayanbulak Praire. Grass Feeding Livest 2:49–50
Yang HB, Holmgren NH, Mill RR (1998) Pedicularis L. In: Wu ZY, Raven PH (eds) Flora of China, vol 18. Beijing & Missouri Botanical Garden Press, St. Louis
Yoder JI, Gunathilake PC, Jamison-McClung D (2009) Hemiparasitic plants: exploiting their host's inherent nature to talk. In: Baluska F (ed) Plant–environment interactions: from sensory plant biology to active plant behavior. Signaling and Communication in Plants, pp 85–100. doi:10.1007/978-3-540-89230-4_5
Zhang XY, Hu YK, Ji CD, Guo ZG, Gong YM (2009) Studies of chemical control of Pedicularis verticilata with 2,4-d butyl ester and the effect on grassland vegetation. Acta Pratacult Sin 18:168–174 (in Chinese)
Acknowledgments
We thank Dr. Yongquan Ren for his inspiring discussion for writing up the paper. We are grateful to Prof. Sally Smith and Prof. Andrew Smith from the University of Adelaide, as well as the anonymous reviewers, for their valuable comments and suggestions to improve the paper. The research was financially supported by the Natural Science Foundation of China (grant nos. 30970288 and 31370512), Natural Science Foundation of Yunnan Province (grant no. 2009CD114), and Youth Innovation Promotion Association of Chinese Academy of Sciences (CAS), and a research fund (NO. P2012-KF03) from the Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, CAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui, XL., Li, AR., Chen, Y. et al. Arbuscular mycorrhizal fungi: potential biocontrol agents against the damaging root hemiparasite Pedicularis kansuensis?. Mycorrhiza 24, 187–195 (2014). https://doi.org/10.1007/s00572-013-0528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-013-0528-5