Abstract
Colonization by arbuscular mycorrhizal (AM) fungi was investigated in cucumber (Cucumis sativus), tomato (Lycopersicon esculentum) and Clethra barbinervis (Ericales) grown in field-collected soil known from previous studies to generate Paris-type arbuscular mycorrhizae in C. barbinervis. Spores of Paraglomus, Acaulospora, Glomus, and Gigaspora were found in the soil. Formation of hyphal coils and arbusculate coils of Paris-type mycorrhizae and of arbuscules of Arum-type mycorrhizae in roots raised in this soil in the growth chamber were compared with the detection of DNA of AM fungi from the same root systems using Glomales-specific primers. Only Paris-type mycorrhizae with extensive arbusculate coils developed in C. barbinervis, but cucumber and tomato developed both Paris- and Arum-types in the same root systems. Glomaceae and Archaeosporaceae and/or Paraglomaceae were detected strongly in the DNA from both cucumber and tomato roots, in which Arum-type mycorrhizae were observed. In contrast, DNA of Glomaceae was detected more sparingly in C. barbinervis, in which Paris-type mycorrhizae dominated. Acaulosporaceae and Gigasporaceae were strongly detected in the DNA from both C. barbinervis and tomato, whereas they were more weakly detected in cucumber. These results indicate that the morphology of colonization is strongly influenced by the selection of fungi to colonize the host plant from among those in the soil environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal (AM) fungi develop two morphological types of colonization, which were first described by Gallaud (1905). These are: (1) the Arum-type, defined by intercellular hyphal growth in the root cortex; and (2) the Paris-type, defined by cell-to-cell growth of intracellular hyphal coils (Smith and Smith 1997). A prominent feature of the Arum-type morphology is the intercellular growth of hyphae in a longitudinal manner through the root. Arbuscules arise on short side branches from these intercellular hyphae, typically at right angles to the main root axis (Smith and Smith 1997). Coils of the Paris-type of mycorrhiza often, but not invariably, become arbusculate, that is, they develop arbuscule branches from one or more loci on the coil (Gallaud 1905; Smith and Read 1997).
The Arum-type morphology is abundant in field crops (Smith and Smith 1997), whereas the Paris-type morphology has been more often seen in plants of natural ecosystems such as those occurring in herbaceous layers in temperate broadleaf forests (Brundrett and Kendrick 1988, 1990a, 1990b), various trees (Gerdemann 1965; Bonfante-Fasolo and Fontana 1985; Brundrett et al. 1990; Kubota et al. 2001), and plants of semi-arid systems (McGee 1986). These observations form part of a broader understanding that the plant species dictates the morphology of the mycorrhiza (Lackie et al. 1987).
Anatomical characteristics of host roots are thought to influence whether an Arum- or Paris-type of mycorrhiza forms. Specifically, the longitudinal hyphae of the Arum-type have been reported to follow air channels in roots in certain species of woodland herbs (Brundrett and Kendrick 1988, 1990a, 1990b). Cortical air spaces are common in transverse sections of the roots of species illustrated in standard anatomy texts (Esau 1965; Fahn 1990). Variations in longitudinal extent of the air spaces could influence mycorrhiza morphology. However, the identity of the AM fungus can also affect whether an Arum- or Paris-type morphology develops in a given host (Cavagnaro et al. 2001), thus further studies are necessary to more fully understand the controls on Paris- and Arum-morphology.
The Japanese tree Clethra barbinervis Sieb. et Zucc. was found to form AM associations of Paris-type morphology under field and laboratory conditions (Kubota et al. 2001). In the present study, we made use of soil collected from below C. barbinervis in the field as an inoculum to supply fungi known to be capable of forming abundant quantities of Paris-type mycorrhizae both in the field and under controlled conditions.
The aim of this study was to re-evaluate the influence of host on the formation of Paris- or Arum-type morphology. We raised cucumber (Cucumis sativus L.) and tomato (Lycopersicon esculentum L.), as well as Clethra barbinervis under identical conditions. Cucumber and tomato were selected as test species because growth systems for these plants were already well characterized in our laboratory.
Materials and methods
Plants and inoculum
Clethra barbinervis seeds were collected in 1999 from plants in Gifu Prefecture, Japan. Commercial supplies were used for seeds of Cucumis sativus cv. Jibai and Lycopersicon esculentum cv. House momotaro. Seeds were surface-sterilized with 70% ethanol for 1 min followed by 10% sodium hypochlorite for 15 min and rinsed three times in sterilized water. In preparation for use, surface-sterilized C. barbinervis seeds were sown on 0.7% water agar and incubated for 1 month in a growth chamber at 25°C with a light intensity of 120 μmol m−2 s−1 in a 14 h light/10 h dark daily cycle. Cucumber and tomato seeds for the experiment were pre-germinated on autoclaved Advantec No.1 filter paper (Toyo Roshi Kaisha, Japan) and kept moist with sterilized distilled water for 2 days in an incubator at 25°C in the dark.
A block of soil (30 cm ×30 cm ×5 cm deep) was collected from the base of each of several mature C. barbinervis trees in Ijira, Gifu, Japan. A detailed description of the field site is given in Kubota et al. (2001). Soils were collected from four sampling points within the site and were sieved through a 5 mm mesh. The soils were blended with an equal volume of an autoclaved mixture of volcanic soil and sand (1:1, v/v). Available P (Truog 1930) concentration and pH of the blended soils were 39 mg P kg−1 dry soil and 5.1, respectively.
To investigate AM spores in soil inoculum, the method described by Smith and Dickson (1997) was used with some modifications. Field-collected soils were suspended in distilled water and sieved through a 500 μm screen. The soil suspension was gently transferred to a tube containing 60% sucrose solution and centrifuged for 3 min at 1,600 g. Spores were collected, rinsed and divided into two sets. One set of spores was mounted on slides for microscopic observation; the other was used for DNA extraction. This procedure was repeated eight times. Spores of Paraglomus, Gigaspora, Glomus, and Acaulospora were observed in the sample soils (Fig. 1). DNA was extracted from the spores using an Isoplant DNA extraction kit (Nippon Gene, Toyama, Japan) and purified using a Geneclean Spin purification kit (BIO101 system, Qbiogene, Heidelberg, Germany). Nested-PCR was conducted using AM fungi Glomales-specific primers (Redecker 2000). The first amplification, with the universal primers NS5 and ITS4 (White et al. 1990), was performed as described by Redecker et al. (1997) with an annealing temperature of 51°C. Amplified products were diluted 1:10 and used as templates for the second round of PCR. The second PCR was conducted with various combinations of Glomales-specific primers (Redecker 2000), universal primers (White et al. 1990) and ITS1F, which is specific for fungi (Gardes and Bruns 1993). Primer combinations were ARCH1311, ACAU1660, GLOM1310 and LETC1670, each paired with ITS4, and a single paring of ITS1F and GIGA5.8R. These combinations are specific for Archaeosporaceae and/or Paraglomaceae, Acaulosporaceae, Glomaceae, and Gigasporaceae, respectively. Annealing temperatures were 61°C for 5 cycles then 60°C for 25 cycles as described by Redecker (2000). PCR products were electrophoresed in 2% agarose gels, and amplification by each primer pair of the expected size of fragment was confirmed. Spore family identities based on morphology were an identical match to those determined by DNA evaluation.
Growth conditions
Seedlings were transferred to pots of 6-cm diameter by 7.5-cm depth containing 150 ml blended soils (0.88 g/ml). Seedlings were grown in a growth chamber at 25°C with a light intensity of 300 μmol m−2 s−1 in a 14 h light/10 h dark daily cycle. Water (10 ml) was supplied to each pot every other day. Nutrient solution (10 ml of 1,000 times diluted 10-3-3; HYPONeX, Japan) was supplied every week after 2 weeks of growth. Mycorrhizal colonization of roots was evaluated after 8 weeks of growth in pots. The experiment was repeated twice using four replicates per plant species.
Determination of root colonization
Roots of each plant were cut into 5 mm segments and two sets of random sub-samples of roots were taken by dispersing the entire root system in excess water. One sub-sample of roots from each pair was freeze-dried and stored at −20°C until use for DNA extraction. The other sub-sample was fixed in formyl-acetic alcohol, cleared in 10% KOH at 90°C for 1 h, and stained with 0.05% chlorazol black E solution made up in 80% lactic acid, glycerin, and distilled water in a 1:1:1 ratio by volume (Brundrett et al. 1984). After clearing and before staining, C. barbinervis roots were bleached for 15 min with diluted alkaline peroxide solution comprising 30% hydrogen peroxide, 28% ammonium hydroxide solution, and distilled water in a 1:1:8 ratio by volume. Colonization was assessed according to McGonigle et al. (1990) at ×200 magnification to obtain the percentage of root length colonized by each of various types of fungal structure: hyphal coils, arbuscules, arbusculate coils, longitudinal hyphae, and vesicles. We refer throughout this work to arbuscules in reference to the Arum-type morphology and to arbusculate coils in reference to the Paris-type morphology. The percentage of the total root length that was colonized was determined separately for Arum-type arbuscules, hyphal coils and Paris-type arbusculate coils. All photographs were obtained from thin mounts of squashed roots with an Olympus BX50F-3 camera (Olympus, Tokyo).
Detection of AM fungi from roots
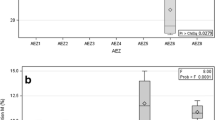
DNA was extracted from 200 mg freeze-dried root samples and PCR was conducted with the same procedure described above. The percent of detection was calculated as the number of PCR products obtained among samples. Degree of amplification was assessed on a scale from 0 to 4 where 0 = no amplification, 1 = amplification observed to the same degree as the marker, and 2–4 = amplification observed 2–4 times that of the marker. Initial work showed that the percent of detection was significantly (P<0.05) correlated with degree of amplification (Fig. 2), and so only percent of detection is presented.
Results
Morphology of colonization
The Paris-type morphology was observed consistently in C. barbinervis roots grown in field-collected soil in this study (Fig. 3a–d). The development of hyphal coils (Fig. 3a, c) and the formation of arbusculate hyphal coils (Fig. 3b, d) were typical of Paris-type mycorrhizae. Hyphal diameter and shape varied depending on position in the inner or outer cortex and cell size (Fig. 3a–d).
Morphological characteristics of the mycorrhizal association in roots of Clethra barbinervis (a–d), Lycopersicon esculentum (e–h), and Cucumis sativus (i–k), grown in field-collected soil. a, e, f Hyphal coils extend from cell to cell (arrow) intracellularly. b, g, i, j Coils are often arbusculate coils (AC) and extended from cell to cell intracellularly (arrow). c Compactness of hyphal coils varies. d Arbusculate coils (AC) are constrained by host cell size. h, k Longitudinal hyphae (LH) extended intercellularly and arbuscules (A) formed intracellularly. Bars 50 μm
On the other hand, both Arum- and Paris-type structures were observed in roots of cucumber and tomato (Fig. 3e–k) even within a single plant. In all cases, however, a single infection point gave rise to a body of infection that was consistent within itself as either Paris- or Arum-type. In some infection units, the formation of hyphal coils (Fig. 3e, f) and arbusculate hyphal coils (Fig. 3g, i, j) in the cortex were typical of Paris-type mycorrhizae. In others, the development of intercellular hyphae and intracellular arbuscules (Fig. 3h, k) were typical of Arum-type mycorrhizae.
Extent of colonization
Total colonization by AM fungi at 8 weeks was 23% in C. barbinervis, 19% in tomato, and 20% in cucumber. Colonization by hyphal coils and arbusculate coils in C. barbinervis was well represented (79% and 65% as a percentage of total colonization, respectively) (Fig. 4). Colonization with hyphal coils and arbusculate coils was also seen in tomato and cucumber. However, the formation of hyphal coils and arbusculate coils was less (44% and 21% as a percentage of total colonization in tomato, and 5% and 17% as a percentage of total colonization in cucumber, respectively) than that seen in C. barbinervis. On the other hand, formation of arbuscules was observed in tomato and cucumber (22% and 61% as a percentage of total colonization, respectively). The frequency of formation of arbuscules was high in cucumber (Fig. 4).
Types of AM fungi colonizing plant roots
Detection with the primer pairs LETC1670/GLOM1310 and ITS4 was 100% in cucumber and tomato with arbuscule formation (Fig. 4), whereas detection with these primers was 50% in C. barbinervis with no arbuscule formation (Fig. 4). Similarly, detection with the primer pair ARCH1331 and ITS4 was high in tomato and cucumber (100% and 63%, respectively) and low in C. barbinervis (25%). Detection with primer pair ITS1F and GIGA5.8 was 100% in C. barbinervis and tomato while it was 25% in cucumber. Similarly, detection with primer pair ACAU1660 and ITS4 was 100% and 75% in C. barbinervis and in tomato, respectively, while there was no detection in cucumber. These results indicate that Acaulosporaceae and Gigasporaceae are the dominant AM fungi in C. barbinervis, whereas Glomaceae and Archaeosporaceae and/or Paraglomaceae are dominant in cucumber. In contrast, tomato was colonized well by all of these families of AM fungi.
Discussion
We report here the simultaneous development of separate and internally consistent infection units of Paris-type and Arum-type mycorrhizae within the same root systems of tomato and cucumber. Bonfante-Fasolo and Fontana (1985) reported co-occurrence of Arum- and Paris-type morphologies in the same root system, but with only trace levels of Paris-type mycorrhiza. The data here (Figs. 3, 4) stand in contrast to that study, because the tomato and cucumber we studied had both morphological types well represented. Cavagnaro et al. (2001) demonstrated that both morphologies were formed in tomatoes grown in separate pots containing different single fungal species.
Formation of the Arum-type rather than the Paris-type morphology appears to be determined not only by the presence of air channels, which provide a pre-formed space through which the hyphae can pass, but also by the diameter and continuity of air spaces, as well as the hyphal diameter and the morphological plasticity of the AM fungus concerned, because both types can be seen in the same plant species (Cavagnaro et al. 2001) and even within the same root system (Fig. 3). In their review of AM infection strategies, Bonfante and Perotto (1995) concluded that colonization involves a combination of mechanical pressure at the penetration point and, when hyphae cross cortical cell walls, movement in air spaces, and production of weak or limited amounts of hydrolytic enzymes. C. barbinervis has only been seen to form Paris-type mycorrhizae, both here and previously (Kubota et al. 2001). Similar consistency was noted for Gentiana lutea in association with field soil and for isolated species of AM fungi that produced Paris-type mycorrhizae in pot trials (Jacquelinet-Jeanmougin and Gianinazzi-Pearson 1983). In contrast, Allium (Sanders and Tinker 1973; Jacquelinet-Jeanmougin and Gianinazzi-Pearson 1983; Brundrett et al. 1985) invariably shows Arum-type infection. These plant species could control AM morphology depending solely on the presence or absence of continuous air spaces.
In this study, AM fungi colonizing C. barbinervis, tomato and cucumber were detected using Glomales-specific primers, and these plants demonstrated different selectivities for AM fungi. Interestingly, a similar tendency was observed using the same test plants grown in field-collected soils from six different sites (Kubota and Hyakumachi 2004), showing host selectivity by AM fungi regardless of the soil source. Such selectivity by certain hosts for certain fungi could determine AM morphology as a reflection of the character of the AM fungus concerned. Our interpretation is broadly consistent with that of Cavagnaro et al. (2001), who demonstrated that Glomus intraradices, G. mosseae and G. versiforme formed Arum-type morphology whereas Gigaspora margarita and Scutellospora calospora formed Paris-type morphology in tomato. However, such selectivity by plants for AM fungus does not fully explain all of these results on AM morphology, because Glomus coronatum was also found to form Paris-type mycorrhizae in tomato (Cavagnaro et al. 2001). We conclude that the morphology of arbuscular mycorrhizae in the context of Arum- versus Paris-types is the result, at least in part, of the interplay between both the plant and the fungal species.
References
Bonfante P, Perotto S (1995) Tansley review no. 82. Strategies of arbuscular mycorrhizal fungi when infecting host plants. New Phytol 130:3–21
Bonfante-Fasolo P, Fontana A (1985) VAM fungi in Ginkgo biloba roots: their interactions at the cellular level. Symbiosis 1:53–67
Brundrett MC, Kendrick B (1988) The mycorrhizal status, root anatomy and phenology of plants in a sugar maple forest. Can J Bot 66:1153–1173
Brundrett MC, Kendrick B (1990a) The roots and mycorrhizas of herbaceous woodland plants. I. Quantitative aspects of morphology. New Phytol 114:457–468
Brundrett MC, Kendrick B (1990b) The roots and mycorrhizas of herbaceous woodland plants. II. Structural aspects of morphology. New Phytol 114:469–479
Brundrett MC, Piche Y, Peterson RL (1984) A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Can J Bot 62:2128–2134
Brundrett MC, Piche Y, Peterson RL (1985) A developmental study of early stages in vesicular-arbuscular mycorrhiza development. Can J Bot 63:184–194
Brundrett MC, Murase, G., Kendrick B (1990) Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can J Bot 68:551–578
Cavagnaro TR, Gao L-L, Smith FA, Smith SE (2001) Morphology of arbuscular mycorrhizas is influenced by fungal identity. New Phytol 151:469–475
Esau K (1965) Plant anatomy, 2nd edn. Wiley, New York
Fahn A (1990) Plant anatomy, 4th edn. Pergamon, Oxford
Gallaud I (1905) Etudes sur les mycorrhizes endotrophs. Rev Gen Bot 17:5–500
Gardes M, Bruns RD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gerdemann JW (1965) Vesicular-arbuscular mycorrhizae formed on maize and tuliptree by Endogone fasciculata. Mycologia 57:562–575
Jacquelinet-Jeanmougin S, Gianinazzi-Pearson V (1983) Endomycorrhizas in the Gentianaceae. I. The fungus associated with Gentiana lutea L. New Phytol 95:663–666
Kubota M, McGonigle TP, Hyakumachi M (2001) Clethra barbinervis, a member of the order Ericales, forms arbuscular mycorrhizae. Can J Bot 79:300–306
Kubota M, Hyakumachi M (2004) Morphology and colonization preference of arbuscular mycorrhizal fungi in Clethra barbinervis, Cucumis sativus and Lycopersicon esculentum. Mycoscience (in press)
Lackie SM, Garriock ML, Peterson RL, Bowley SR (1987) Influence of host plant on the morphology of the vesicular-arbuscular mycorrhizal fungus, Glomus versiforme (Daniels and Trappe) Berch. Symbiosis 3:147–158
McGee PA (1986) Mycorrhizal associations of plant species in a semi-arid community. Aus J Bot 34:585–593
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Redecker D (2000) Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 10:73–80
Redecker D, Thierfelder H, Walker C, Werner D (1997) Restriction analysis of PCR-amplified internal transcribed spacers of ribosomal DNA as a tool for species identification in different genera of the order Glomales. Appl Environ Microbiol 63:1756–1761
Sanders FE, Tinker PB (1973) Phosphate flow into mycorrhizal roots. Pestic Sci 4:385–395
Smith FA, Smith SE (1997) Tansley review No. 96. Structural diversity in (vesicular)-arbuscular mycorrhizal symbioses. New Phytol 137:373–388
Smith SE, Dickson S (1997) VA mycorrhizas: researcher’s manual. CRC for Soil and Land Management, Glen Osmond, Australia
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, San Diego
Truog E (1930) Determination of the readily available phosphorus of soils. J Am Soc Agron 874–882
White TJ, Bruns T, Lee S, Taylor (1990) Amplification and direct sequencing of fungal ribosomal RNA gene for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic Press, San Diego, pp 315–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubota, M., McGonigle, T.P. & Hyakumachi, M. Co-occurrence of Arum- and Paris-type morphologies of arbuscular mycorrhizae in cucumber and tomato. Mycorrhiza 15, 73–77 (2005). https://doi.org/10.1007/s00572-004-0299-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-004-0299-0