Abstract
Microfluidics devices have attracted increasing interest over the last decade. Glass was initially the materials of choice for these devices but polymers such as polymethylmethacrylate (PMMA) have a great potential to be used for these devices because of their low cost, ease of fabrication and chemical properties. A key step in fabrication of these microfluidic devices is the enclosing of microchannels by cover plate, i.e., layer to layer bonding. This investigation focused on the thermal bonding of PMMA layers of different molecular weights. The bond strength and the effect of temperature and pressure on bond strength between various PMMA pairs of different molecular weights were studied. Thermal bonding was realized using a hot embossing system. PMMA strips made from predefined parameters were prepared and a customized CNC machine mold was used to determine the optimized parameters of the thermal bonding. The PMMA pairs investigated are of molecular weights 96.7, 120, 350 and 996 kDa using Instron machine; the shear strength of the thermally bonded specimens was determined. For the PMMA pairs investigated, the greatest shear strength of 1.589 ± 0.286 MPa was observed between molecular weights of 350 and 996 kDa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
During last decade, field of microfluidics has emerged into one of the most dynamic field in analytical chemistry (McDonald et al. 2000; Boone et al. 2002; Soper et al. 2000). It has the potential benefits of reduced size, disposability, improved performance, low cost, reduced sample and reagent volume, and reduced power consumption. However, the choice of materials and fabrication procedure are critical aspects of commercial microfluidic devices. Polymer materials have become the most promising materials for microfluidics devices due to the advantages of low cost, ease of fabrication, biocompatibility and higher flexibility over their traditional counterpart like glass and silicon. Different polymer materials like poly(dimethylsiloxane) (PDMS), poly(methyl methacrylate) (PMMA) or polycarbonate (PC) have become the most promising materials. PMMA, owing to the advantage of low cost, high transparency and excellent dielectric as well as mechanical properties, has become one of the dominant materials for fabrication of microfluidic devices.
Due to growing interest in disposable microfluidic devices, several plastic-chip manufacturing processes have been developed in recent years, including X-ray photolithography, hot embossing or imprinting, laser ablation, injection molding, etc. With all these methods, the fabricated microchannels are sealed on the top with a cover plate of the same plastic material to form enclosed microstructure.
Thus the key step in the fabrication of these microfluidic devices is the enclosing of microchannel by cover plate. This layer to layer bonding is a very important procedure and challenging issue in the design and fabrication of polymer based microfluidic devices. Several methods for sealing of polymeric substrates have been demonstrated, including thermal bonding in convention oven or in hot embossing machine (Kelly and Woolley 2003; Fiorini and Jeffries 2003) gluing such as SU-8 resist, PDMS films (Han et al. 2003), laser lamination (Robert et al. 1997) and surface modification associated with other bonding methods (Lee et al. 2003; Zhu et al. 2004). Of these methods, thermal bonding approaches are especially desirable since they allow the formation of a uniform channel surface comprised entirely of the same polymeric material. Other sealing approaches unavoidably introduce multiple materials to form the microchannels which cause inhomogeneities in the ζ-potential along the channels and cause undesired effects on the electrodynamics of the microsystem such as Taylor dispersion and reduced electrophoretic separation efficiencies (Kelly and Woolley 2003). However, conventional thermal bonding approaches still faces the sever challenges of microchannel deformation, low reproducibility and poor yield, which warrants continuing research to improve the thermal bonding methods. Recently Zhu et al. (2007) has investigated the traditional thermal bonding as well as the surface modification bonding with respect to temperature, surface modification, pressure and aging effect with PMMA.
This investigation focused on the thermal bonding of PMMA layers of different molecular weights. The bond strength and the effects of temperature and pressure on bond strength between various PMMA pairs of different molecular weights were studied.
2 Experimental
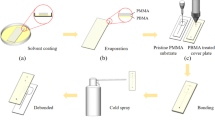
Polymethylmethacrylate of molecular weights 96.7, 120, 350 and 996 kDa were purchased from Sigma-Aldrich. The pellets were molded to sheets of 1 mm thickness by using a laboratory scale hydraulic hot press. The test specimens were cut into sizes. The samples were bonded in a lap shear joint geometry with contact area of 15 mm × 11 mm × 1 mm using the Carver Laboratory press. The PMMA substrates were put together and heated to the bonding temperature before bonding pressure were applied in the hot embossing system. After bonding for a given time, the samples were cooled to room temperature under pressure.
The methodology adopted is depicted in Fig. 1. The bonding pairings were selected with low T g PMMA at the top and high T g PMMA on the bottom and the bonding temperature selected were just above the T g of the low T g PMMA.
The bond strength and its dependence on bonding temperature and pressure were measured by tensile testing in an Instron-5569 model. An experimental speed of 1 mm/min was used in this study. The maximum force was divided by the bonded area to calculate the bond strength before failure. For each measurement, five sample specimens were taken. Surface profile was analyzed using a JEOL JSM-5600LV scanning electron microscope.
3 Results and discussions
The types of different molecular weights of PMMA combinations and heating temperature, heating time, cooling time, cooling load, etc. used for this study are depicted in Tables 1 and 2, respectively.
Figure 2 shows a general plotting of the data of force against extension collected from the Instron machine. In all the cases show a linear increment of shear force with respect to the extension of the specimen. The specimen portrayed Hooke’s law of elasticity by showing perfect elastic deformation before the fracture. The threshold force and the extension at which it occurred were obtained. Together with the dimensions of the bonded area measured before each testing, the shear strength was obtained. The results for bonding between 96.7 and 120 kDa, 96.7 and 350 kDa, 96.7 and 996 kDa, 120 with 996 kDa and 350 and 996 kDa are obtained. Using the calculated shear strength of each specimen for each bonding type, the mean shear strength was calculated. Table 3 shows the mean shear strength of different types of bonding tested. It is observed that the bond strength increases with increasing of molecular weight pairs for constant bonding load of 3 metric ton and heating time of 5 min. It is observed that the bond strength increases with increasing of molecular weight pairs for constant bonding load of 3 metric ton and heating time of 5 min.
When two compatible polymer surfaces are brought into contact at elevated temperatures, adhesion occurs at the interface. A high strength is developed above the glass transition temperature (T g) of amorphous polymers where the molecular mobility is high and the process is diffusion controlled (Kline and Wool 1988; Jud et al. 1981). That means that there is diffusion of segments of the macromolecular chains across the interface. It is observed that the mean shear strength of bonding between molecular weight 350 and 996 kDa is the maximum. This can be attributed to the interdiffusion of large number of macromolecular chain segments at the interface. We have concentrated our study for the effect of temperature and pressure for the pair of bonding between the molecular weights 350 and 996 kDa.
Figure 3 shows the effect of bonding temperature on bond strength at a bonding pressure of 1.225 MPa for the bonding between 350 and 996 kDa pair. It is observed that the bond strength increases with increase of bonding temperature. Higher temperature will damage the specimen although it can heighten the bond strength. Beyond the temperature of 145°C, the specimen deformed and so the data were not taken into consideration.
Figure 4 shows the bonding pressure’s effect of bond strength of the bonding between 350 and 996 kDa PMMA. Though the bond strength increases with increasing bonding pressure, the specimens get deformed beyond 2.225 MPa.
Figure 5 shows a laser cut microchannel of 996 kDa PMMA is covered with 350 kDa PMMA at bonding pressure of 1.225 MPa and temperature of 135°C having no deformation of the microchannel.
Figure 6a and b shows the surface morphology of the PMMA after fracture of the bonded pairs as observed by scanning electron microscopy. It is seen that the fractured surface shows a specific morphology. This morphology (and rugosity) indicates that there is some molecular mobility at the PMMA/PMMA interfaces. When two amorphous polymer surfaces are brought into contact at elevated temperatures (above T g) where the molecular mobility is high, adhesion occurs at the interface. The chain ends penetrate to the opposite side of the interface in the surface layer, leading to high strength. It has also been proposed that the polymer surface layer is more mobile than the chains in the bulk since very significant decrease of T g have been observed in that layer of the polymer (Mayes 1994; Kajiyama et al. 1995).
These studies indicate that the conformational structure and molecular mobility at the polymer surface differ from those of the bulk; molecules and specially the chain ends are less entangled and more mobile. Therefore there is possibility of chain ends to penetrate to the opposite side of the interface in the surface layer leading to the interdiffusion at the surface of glassy polymer.
4 Conclusions
The bonding strength increases with increase in molecular weights of the PMMA. The bonding between the molecular weights 350 and 996 kDa has maximum strength. The bond strength increases with increase in bonding temperature and pressure. At bonding pressure of 1.225 MPa and temperature of 135°C, the bonding between 350 with 996 kDa shows bond strength of 1.91 MPa without any deformation of the specimen. SEM micrographs show the bonding is due to interdiffusion of polymer chains at the interfaces.
References
Boone TD, Fan ZH, Hooper HH, Ricco AJ, Tan H, Williams SJ (2002) Plastics advances microfluidic devices. Anal Chem 74:78A–86A. doi:10.1021/ac021943c
Fiorini GS, Jeffries GDM (2003) Fabrication of thermoset polyester microfluidic devices and embossing masters using rapid prototyped polydimethylsiloxane molds. Lab Chip 3:158–163
Han A, Wang O, Graff M (2003) Multilayer plastic/glass microfluidic systems containing electrical and mechanical functionality. Lab Chip 3:150–157
Jud K, Kaush HH, Willams JG (1981) Fracture mechanics studies of crack healing and welding of polymers. J Mater Sci 16:204–210. doi:0022-2461/81/010204-07$02.70/0
Kajiyama T, Tanaka K, Takahara A (1995) Depth dependence of the surface glass transition temperature of a poly(styrene-block-methyl methacrylate) diblock copolymer film on the basis of temperature dependent X-ray photoelectron spectroscopy. Macromolecule 28:3482–3484. doi:10.1021/ma00113a059
Kelly RT, Woolley AT (2003) Thermal bonding of polymeric capillary electrophoresis microdevices in water. Anal Chem 75:1941–1945. doi:10.1021/ac0262964
Kline DB, Wool RP (1988) Polymer welding relations investigated by a lap shear joint method. Poly Eng Sci 28:52–57. doi:10.1002/pen.760280109
Lee HS, Kim DS, Kwon TH (2003) A novel low temperature bonding technique for plastic substrates using X-ray irradiation. In. Digest. Tech papers transducers 03 conference, Boston, 8–12 June, pp 1331–1334. ISBN: 0-7803-7731-1, INSPEC accession number: 7913799
Mayes AM (1994) Glass transition of amorphous polymer surfaces. Macromolecule 27:3114–3115. doi:10.1021/ma00089a033
McDonald JC, Dufy DC, Anderson JR, Chiu DT, Wu H, Schueller OJA, Whiteside GM (2000) Fabrication of the microfluidic systems in poly(dimethyl-siloxane). Electrophoresis 21:27–40. doi:10.1002/(SICI)1522-2683(20000101)21:1<27:AID-ELPS27>3.0.CO;2-C
Robert MA, Rossier JS, Bercier P, Giurault HH (1997) UV laser machined polymer substrates for the development of microdiagnostics systems. Anal Chem 69:2035–2042. doi:10.1021/ac961038q
Soper SA, Ford SM, Qi S, McCarley RL, Kelly K, Murphy MC (2000) Micro-electromechanical systems fabricated in polymeric materials: applications in chemistry and life sciences. Anal Chem 72:643A–651A
Zhu X, Liu G, Tian Y (2004) A new method of layer-to layer bonding of PMMA. In: Proceedings of the 4th international workshop on microfactories (IWMF), Shanghai, 15–17 October
Zhu X, Liu G, Guo Y, Tian Y (2007) Study of PMMA thermal bonding. Microsyst Technol 13:403–407. doi:10.1007/s00542-006-0224-x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nayak, N.C., Yue, C.Y., Lam, Y.C. et al. Thermal bonding of PMMA: effect of polymer molecular weight. Microsyst Technol 16, 487–491 (2010). https://doi.org/10.1007/s00542-009-0926-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-009-0926-y