Abstract
Purpose
Measuring the neurotoxic effects of multiple anesthetic exposures during neurodevelopment is complex due to the numerous factors that can affect the outcome. While we recently discovered that the interval between multiple sevoflurane exposures can affect the level of neurotoxicity, the significance of interval for other anesthetic agents is unknown. Thus, we evaluated the significance of dosing interval in the neurotoxic effects of multiple ketamine injections in postnatal day (PND) 17 mice.
Methods
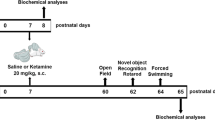
PND17 mice of both sexes were intraperitoneally injected with ketamine (35 mg/kg) three times at short (2 h) or long (24 h) intervals. Changes in synaptic transmission were measured in hippocampal pyramidal neurons 5 days after the last injection, and behavioral changes were assessed at the age of 8 weeks. Values are presented as mean ± SD.
Results
Whereas short-interval ketamine injections enhanced excitatory synaptic transmission, as evidenced by an increased frequency of miniature excitatory postsynaptic currents (mEPSCs; ketamine, 0.09 ± 0.07 Hz; control, 0.06 ± 0.03 Hz), long-interval ketamine injections did not; instead, they decreased the amplitude of miniature inhibitory postsynaptic currents (mIPSCs; ketamine, 47.72 ± 6.90 pA; control, 51.21 ± 7.65 pA,). However, only long-interval ketamine injections induced long-term changes in anxiety behavioral in the open-field test (decrease in center duration; ketamine, 400.1 ± 162.8 s; control, 613.3 ± 312.7 s).
Conclusions
Multiple ketamine injections induce interval-dependent, long-lasting synaptic changes and behavioral impairments. Future studies should carefully consider the dosing interval as a significant factor when studying the neurotoxic effects of multiple anesthetic exposures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The possibility of neurotoxicity from early general anesthesia exposure has attracted considerable attention owing to multiple reports of unanticipated neurological consequences in young animals [1]. While the issue is still a matter of debate, current evidence strongly suggests that a single, brief anesthetic exposure does not affect neurodevelopment [2]. However, preclinical studies have continuously shown that multiple exposures can be more neurotoxic [3,4,5], indicating that prolonged or multiple anesthetic exposures may still pose a risk. Recent clinical studies have also suggested that, unlike a single exposure, multiple anesthetic exposures may cause long-term behavioral consequences [6, 7].
Unfortunately, evaluating the long-lasting consequences of multiple anesthetic exposures is extremely complex, even in well-controlled preclinical animal studies, owing to the intrinsic heterogeneity of multiple anesthetic exposures, reflecting differences in the type, dose and duration of drug used, and the sex and age. Another unrecognized, but important factor is the interval between multiple exposures. While previous studies have reported that anesthetic exposure in late postnatal mice can increase dendritic spinogenesis and affect excitatory/inhibitory synaptic transmission [8,9,10], we have recently shown that the level of neurotoxicity from multiple sevoflurane exposures differs depending on the interval between exposures [11]. However, while a shorter interval may be more neurotoxic for sevoflurane, the significance of interval has not been evaluated with other anesthetic agents. Although it seems reasonable to assume a priori that multiple anesthetic exposures in a shorter interval would be more neurotoxic for most anesthetic agents, the significance of dosing interval may differ between anesthetics with distinct action targets.
To further determine the significance of dosing interval in multiple-exposure anesthesia protocols, we injected postnatal day 17 (PND17) mice with multiple ketamine injections at short [2 h] or long (24 h) intervals. Unlike sevoflurane, which targets GABAA receptors, ketamine is an intravenous agent that acts mainly by inhibiting NMDA receptors [12, 13]. Thus, in the present study, we further evaluated interval-dependent changes in synaptic transmission and long-term behavior after multiple ketamine injections. Both male and female mice were included because of possible sex-specific effects [9,10,11, 14]. Unexpectedly, multiple ketamine injections induced different long-lasting changes in synaptic transmission that depended on the dosing interval, with short-interval injections affecting excitatory synaptic transmission and long-interval injections affecting inhibitory synaptic transmission 5 days after the last injection. However, behavioral consequences were only observed in mice that received long-interval ketamine injections. While our results suggest that the interval between anesthetic exposures is indeed a significant factor, it also implies that a shorter interval does not necessarily lead to increased neurotoxicity depending on the anesthetic agent.
Methods
Animals
This study was conducted with the approval of the Committees on Animal Research at Chungnam National University Hospital (Daejeon, South Korea, CNUH-018-P0006). C57BL/6 mice were purchased from Damul Science (Daejeon, South Korea) and housed in normal cages at 22–4 °C (< 6 mice/cage) under a 12-h light/dark cycle.
Ketamine injections
Ketamine hydrochloride (Huons, South Korea) was diluted to 2.5 mg/ml with normal saline. In preliminary experiments designed to determine the appropriate dose of ketamine for use in late postnatal mice, which has not been well studied, we found that 35 mg/kg intraperitoneal injections induced a short immobilization period (5–10 min) without causing any mortality, while higher doses (> 40 mg/kg) caused occasional death (data not shown). At PND17, male and female mice were randomly divided into two groups: a short-interval group and a long-interval group. Mice in the short-interval group were intraperitoneally injected three times with either saline or ketamine (35 mg/kg, 14 ml/kg) at 2-h intervals. Mice in the long-interval group received the identical treatments, only at 24-h intervals. Mice were returned to their cages 30 min after each injection, and their full recovery was confirmed.
Electrophysiology
Whole-cell patch-clamp recordings were obtained from sagittal hippocampal slices as previously described [9, 10]. CA1 pyramidal neurons were examined 5 days after the last ketamine injection. In brief, sagittal hippocampal slices (300 µm) were prepared in ice-cold dissection buffer (212 mM sucrose, 25 mM NaHCO3, 5 mM KCl, 1.25 mM NaH2PO4, 10 mM D-glucose, 2 mM Na-pyruvate, 1.2 mM Na-ascorbate, 3.5 mM MgCl2, 0.5 mM CaCl2) using a VT1200S vibratome (Leica, Wetzlar, Germany). Brain slices were allowed to equilibrate for at least 30 min in a 32 °C chamber filled with artificial cerebrospinal fluid (aCSF; 125 mM NaCl, 25 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 10 mM d-glucose, 1.3 mM MgCl2, 2.5 mM CaCl2). All solutions were aerated with 5% CO2/95% O2. Internal solutions depended on the individual experiment. For miniature excitatory postsynaptic current recordings (mEPSCs), glass capillaries were filled with 117 mM CsMeSO4, 10 mM TEA-Cl, 8 mM NaCl, 10 mM HEPES, 5 mM QX-314-Cl, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 10 mM EGTA. For inhibitory postsynaptic current recordings (mIPSCs), glass capillaries were filled with 115 mM CsCl, 10 mM TEA-Cl, 8 mM NaCl, 10 mM HEPES, 5 mM QX-314-Cl, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 10 mM EGTA. Tetrodotoxin (0.5 μM) and picrotoxin (50 µM) were added during mEPSC recordings, and tetrodotoxin (0.5 µM), NBQX (10 µM) and D-AP5 (50 µM) were added during mIPSC recordings. All experiments were conducted under visual control (BX50WI; Olympus, Japan) using a MultiClamp 700A amplifier (Molecular Devices, CA, USA) and Clampex 9.2 software (Molecular Devices).
Behavioral tests
Behavioral tests (open-field, 3 chamber, and fear chamber tests) were performed on adult mice (8 weeks old) as previously described [9, 15]. All experiments were video recorded and subsequently analyzed using automated software (EthoVision XT; Noldus Information Technology, The Netherlands).
Open-field test
General activity was analyzed for 1 h after placing a mouse in the center of an open-field box (40 × 40 × 40 cm). Anxiety-like behavior was also evaluated by measuring the total time spent in the center region (20 × 20 cm).
Three-chamber test
Sociability was measured using the three-chamber apparatus as previously described [9]. The experiment consisted of three sessions: 2 habituation sessions (5 min each) and 1 interaction session (10 min). During the first habituation session, the mice were confined to the center chamber. In the second habituation session, the mice were allowed to explore all three chambers. After habituation, a novel object (blue cube, 3.0 × 3.0 × 4.0 cm) and a stranger mouse (same age, sex) were placed inside a small plastic cage in each side chamber. Subject mice were allowed to freely explore all 3 chambers for 10 min (interaction session). Sociability was measured using the preference index (PI). PI was calculated after measuring the time spent in each side chamber (Mo, time spent in the stranger mouse chamber; Ob, time spent in the novel object chamber). PI (%) = (Mo − Ob)/(Mo + Ob) × 100.
Fear chamber test
Fear chamber tests were performed for three consecutive days as previously described [9, 15, 16]. On day 1, fear conditioning took place in a fear chamber (Coulbourn Instruments, Allentown, PA, USA) enclosed in a sound-attenuating room. After a 5-min habituation period in the chamber, mice were exposed to a 20-s tone (3 kHz, 80 dB, conditioned stimulus) paired with a 1 mA, 1-s electrical shock (unconditioned stimulus) three times at 60-s intervals. On day 2 (context fear memory), mice were placed in the same chamber for 5 min and analyzed for freezing behavior. On day 3 (cue fear memory), mice were placed in a different context (white circular plastic chamber), and after a 5-min habituation period, were exposed to the same tone for 3 min while freezing behavior was measured using FreezeFrame software (Coulbourn Instruments).
Statistical analysis
Data were analyzed using the R statistical software package (3.1.2; R Core Team, Austria). Two-way analysis of variance (ANOVA) was used to evaluate interactions between the effects of anesthesia and sex. A nested model was performed to separately compare male and female results. The results of our statistical analyses are included as supplementary data.
Results
Short-interval (2 h) ketamine injections induce long-lasting changes in excitatory synaptic transmission
We first evaluated whether short-interval (2 h) ketamine injections caused long-lasting changes in excitatory/inhibitory synaptic transmission. mEPSCs and mIPSCs were measured in CA1 hippocampal pyramidal neurons 5 days after the last injection. Whereas ketamine-treated mice showed no change in mEPSC amplitude (two-way ANOVA with nested model), they exhibited a significant increase in mEPSC frequency (p = 0.015, two-way ANOVA) (Fig. 1a, b). These changes were not sex dependent and there was no significant difference according to sex when evaluated separately (two-way ANOVA with nested model) (Fig. 1a, b). In contrast to the long-lasting changes in excitatory synaptic transmission, there were no significant changes in the amplitude or frequency of mIPSCs (Fig. 1c, d; two-way ANOVA with nested model).
Multiple ketamine injections at a short (2 h) interval induce change in excitatory synaptic transmission in CA1 hippocampal pyramidal neurons 5 days after the last injection. a Representative image of mEPSCs (n = 18 cells from 5 control male mice; n = 18 cells from 5 ketamine-injected male mice; n = 17 cells from 5 control female mice; n = 20 cells from 5 ketamine-injected female mice). b Short-interval ketamine injections increased the frequency of mEPSCs, but did not affect their amplitude (*P < 0.05, n.s. not significant; two-way ANOVA with nested model). c Representative image of mIPSCs (n = 17 cells from 5 control male mice; n = 18 cells from 5 ketamine-injected male mice; n = 20 cells from 5 control female mice; n = 20 cells from 5 ketamine-injected female mice). d Short-interval ketamine injections did not affect mIPSCs 5 days after the last injection (n.s. not significant; two-way ANOVA with nested model). Values are presented as means ± SD
Long-interval (24 h) ketamine injections induce long-lasting changes in inhibitory synaptic transmission
To determine the significance of interval duration between multiple ketamine injections, we next evaluated changes in synaptic transmission after injections performed at a longer interval (24 h). Interestingly, unlike mice that received short-interval (2 h) ketamine injections, there was no change in mEPSC amplitude or frequency in mice that received long-interval (24 h) injections (Fig. 2a, b; two-way ANOVA with nested model). However, we did find that mIPSC amplitude was significantly decreased without a change in frequency (Fig. 2c, d; p = 0.038, two-way ANOVA). Although this decrease was not sex dependent (two-way ANOVA, p = 0.2), only female mice displayed a significant change when separately analyzed (nested model; male p = 0.605, female p = 0.018). These results suggest that multiple ketamine injections can lead to distinct changes in synaptic transmission that depend on the interval between injections.
Multiple ketamine injections at a long (24 h) interval induce changes in inhibitory synaptic transmission in CA1 hippocampal pyramidal neurons 5 days after the last injection. a Representative image of mEPSCs (n = 18 cells from 5 control male mice; n = 18 cells from 5 ketamine-injected male mice; n = 17 cells from 5 control female mice; n = 15 cells from 5 ketamine-injected female mice). b Long-interval ketamine injection did not affect mEPSCs 24 h after the last injection (n.s. not significant; two-way ANOVA with nested model). c Representative image of mIPSCs (n = 17 cells from 5 control male mice; n = 20 cells from 5 ketamine-injected male mice; n = 19 cells from 5 control female mice; n = 22 cells from 5 ketamine-injected female mice). d Long-interval ketamine injection decreased the amplitude, but not the frequency, of mIPSCs 5 days after the last injection. When separately evaluated, mIPSC amplitude was decreased only in female mice (*P < 0.05, n.s. not significant; two-way ANOVA with nested model). Values are presented as means ± SD
Long-interval (24 h) ketamine injections at PND17 increase anxiety-like behavior in adulthood
Excitatory/inhibitory imbalance during neurodevelopment has been shown to have long-term consequences [17, 18]. Thus, to measure the significance of changes in synaptic transmission in terms of long-lasting effects of ketamine injections, we performed behavioral studies at the age of 8 weeks. The long-lasting changes in excitatory synaptic transmission after short-interval ketamine injections did not lead to subsequent changes in activity or the duration of time spent in the open-field test (Fig. 3a–c). However, mice that received long-interval ketamine injections spent significantly less time in the center region of the open-field box without displaying a change in overall activity (Fig. 3d–f; p = 0.006, two-way ANOVA). These changes in anxiety-like behavior (center duration) were not sex dependent (two-way ANOVA, p = 0.868), but only male mice showed significant differences when separately evaluated (nested model; male p = 0.035, female p = 0.059).
Multiple ketamine injections induce interval-dependent, anxiety-like behavior in the open-field test. a Representative image of the open-field test. Red lines indicate mice movement routes for 1 h. b, c Short-interval ketamine-injected mice exhibited normal activity and spent a similar amount of time in the center region of the open field as control mice (n = 12 control male mice; n = 12 ketamine-injected male mice; n = 11 control female mice; n = 12 ketamine-injected female mice). *P < 0.05, n.s. not significant (two-way ANOVA with nested model). d Representative image of the open-field test. Red lines indicate mice movement routes for 1 h. e, f Long-interval ketamine-injected mice exhibited normal activity, but spent significantly less time in the center region of the open field. When separately evaluated, anxiety level was increased only in male mice (n = 12 control male mice, n = 12 ketamine-injected male mice; n = 12 control female mice, n = 12 ketamine-injected female mice.) **P < 0.005, *P < 0.05, n.s. not significant (two-way ANOVA with nested model). Values are presented as means ± SD
We next evaluated whether early ketamine injections could affect sociability by performing the three-chamber test (Fig. 4), since previous studies have shown that early anesthesia can also affect sociability [19]. Ketamine exposures did not affect sociability regardless of interval, as mice that had received ketamine injections preferred the chamber containing the stranger mice similar to control mice (two-way ANOVA) (Fig. 4b, c). Unexpectedly, when male and female mice were separately evaluated, female mice that had received short-interval ketamine exposures showed increased sociability (Fig. 4b) (two-way ANOVA with nested model, p = 0.046).
Multiple ketamine injections do not induce social impairment regardless of dosing interval. a Representative heat map images of the three-chamber test. Sociability was measured by calculating the preference index (PI). b Mice that received short-interval ketamine injections showed comparable sociability to control mice. However, when separately evaluated, ketamine injections increased sociability only in female mice (n = 12 control male mice, n = 12 ketamine-injected male mice; n = 12 control female mice, n = 12 ketamine-injected female mice). *P < 0.05 and n.s. = not significant. (two-way ANOVA and nested model). c Mice that received long-interval ketamine injections also displayed normal sociability, as there was no significant difference in PI compared to control mice (n = 12 control male mice, n = 12 ketamine-injected male mice; n = 12 control female mice, n = 12 ketamine-injected female mice. two-way ANOVA and nested model, n.s. not significant). Values are presented as mean ± SD
Multiple ketamine injections at PND17 do not affect learning and memory in adult mice
Finally, we assessed changes in learning and memory after multiple ketamine injections using the fear chamber test (Fig. 5a). First, mice were placed in the fear chamber and conditioned to a foot shock at the end of a tone. The following day (day 2), mice were again placed in the same chamber for 5 min. Mice that received short- or long-interval ketamine injections displayed freezing behavior similar to that of control mice (context fear memory) (Fig. 5b; two-way ANOVA with nested modeling). The next day (day 3), mice were placed in a different context and exposed to the same tone they received on day 1. Again, ketamine-injected mice showed no difference compared with control mice regardless of dosing interval (cue fear memory) (Fig. 5c; two-way ANOVA with nested modeling). These results suggest that early ketamine injections do not induce long-lasting changes in learning and memory.
Multiple ketamine injections do not affect fear memory regardless of dosing interval. a Schematic images of the fear chamber test procedure. b Both short- and long-interval ketamine-injected mice displayed similar context memory (freezing behavior) compared with control mice. c Cued memory was also unaffected by ketamine injections regardless of dosing interval (n = 12 control male mice; n = 12 ketamine-injected male mice; n = 12 control female mice, n = 12 ketamine-injected female mice). n.s. not significant (two-way ANOVA with nested model). Values are presented as means ± SD
Discussion
Our group has previously shown that neurotoxicity from multiple sevoflurane exposures is significantly affected by the interval between exposures, and that neurotoxicity can be reduced by simply increasing the interval [11]. To confirm whether interval also has a similar affect with different anesthetic agents, we evaluated the changes after multiple ketamine injections. Ketamine is still widely used in pediatric patients owing to its analgesic properties and minimal effects on cardiovascular and pulmonary functions. While our ketamine results are in line with our recent sevoflurane study, as they both indicate the interval between exposures as significant factor, there are several important differences. While long-lasting changes in synaptic transmission and behavioral deficits were induced only after short-interval sevoflurane exposures, multiple ketamine injections led to distinct changes in synaptic transmission depending on the interval. Whereas ketamine delivered at a short, 2-h interval increased excitatory synaptic transmission (mEPSC frequency), when delivered at a relatively longer 24-h interval it decreased inhibitory synaptic transmission (mIPSC amplitude). Importantly, only long-interval ketamine-injected mice displayed behavioral deficits in adulthood.
Altered excitatory/inhibitory synaptic balance during the critical period has been suggested as an important mechanism of neurodevelopmental disorders in mice [17, 18]. Previous studies also show that anesthetic exposure in young animals can also induce similar synaptic changes [9, 10, 20], which may manifest as anesthetic neurotoxicity and long-term behavioral impairments. We recently reported that short-interval sevoflurane exposures induce long-lasting decrease in mIPSC frequency, which leads to hypoactivity and anxiety-related behavior [11]. Our present results also suggest that reduction in inhibitory synaptic transmission (decrease in mIPSC amplitude) after long-interval ketamine injections can induce anxiety-like behavior without affecting learning and memory. However, it should be noted that the long-lasting increase in excitatory synaptic transmission (increase in mEPSC frequency) following short-interval ketamine injections did not induce long-term behavioral impairments. Thus, although both short- and long-interval exposures lead to synaptic changes that can increase neuronal excitability, only direct changes in inhibitory synaptic transmission induced behavior impairment. This may be related with the location of inhibitory synapses, which are located not only on dendrites but also on the cell soma and axon [21]. Due to the proximity of inhibitory synapses to the axon initial segment (AIS), where action potential is generated, it is possible that changes in GABAergic synaptic transmission have a greater affect [21]. Previous studies have also shown that altered GABAergic transmission during neurodevelopment can significantly affect synaptic circuits [22, 23]. Another interesting difference between short- and long-interval ketamine exposures regarding synaptic transmission is that short-interval injections induced changes in frequency (mEPSC), while long-interval injections induced change in amplitude (mIPSC) of miniature postsynaptic currents. Change in frequency usually suggests a change in the number of synapses or presynaptic vesicle release probability, while change in amplitude usually suggests a change in the function or number of postsynaptic receptors [24]. Thus, it is possible that ketamine injections may cause different pre- and post-synaptic changes depending on the interval between injections. However, additional studies measuring the actual changes in neuronal excitability and separately evaluating pre- and post-synaptic changes are necessary to further understand the interval-dependent effects ketamine injections.
Previous studies have also shown that multiple ketamine injections during neurodevelopment (neonatal and late postnatal mice) can induce long-term behavior consequences [3, 4]. In one study that examined the effects of multiple anesthetic exposures (ketamine at 2-day intervals) in mice of a similar age (PND14) [4], it was reported that early, multiple anesthetic exposures impaired motor learning and learning-dependent dendritic spine plasticity. Although experimental details of this study (e.g., combined use of xylazine, behavioral studies performed at a younger age, different behavioral assays) differ from ours in many ways, the study itself supports the conclusion that multiple ketamine injections delivered at relatively long intervals may significantly affect neurodevelopment in late postnatal mice.
Sex-dependent differences are known to contribute to neurodevelopmental disorders and neurotoxicity [25]. Although often neglected, sex has been repeatedly shown to contribute to anesthesia-induced neurotoxicity [14]. Although we previously reported sex-dependent changes in synaptic transmission after sevoflurane exposure [9, 10], we did not detect sex-dependent changes after ketamine injections in the present study. One possible reason for this lack of difference is that sex differences with respect to anesthesia-induced neurotoxicity are agent dependent. The majority of studies reporting sex differences have employed inhalation agents [14]. However, ketamine exposure during the fetal period has also been shown to induce sex-dependent changes [26]. Thus, it is possible that not only the agent but also the neurodevelopmental stage may be important for the manifestation of sex differences.
While our study provides novel insights regarding the significance of interval regarding the long-lasting effects of multiple ketamine injections, it is not without limitations. First, we are unable to translate 2-h and 24-h intervals in mice to humans. It is unlikely that a child would receive multiple anesthetic exposures in a 2- or 24-h interval. However, since neurodevelopment is much faster in mice, it is possible that this duration may represent a longer period in humans. Second, synaptic transmission was measured at different ages due to difference in interval between injections (PND22 in short-interval ketamine-injected mice and PND24 in long-interval ketamine-injected mice). Although we compared the effects of ketamine using control mice of the same age to overcome such differences, it is possible that the 2-day difference may have affected the results due to the rapid neurodevelopment in mice. Third, although we did not discover any long-term behavioral changes accompanied with the changes in excitatory synaptic transmission after short-interval ketamine exposures, this may be due to the limited number of behavioral assays. Another limitation is the short anesthetic duration after intraperitoneal ketamine injection. Before conducting our study, we performed preliminary experiments to establish an ideal ketamine dose for intraperitoneal injection in PND17 mice. The dose used (35 mg/kg) was chosen because it caused no deaths and effectively induced immobilization. However, the immobilization period was only 5–10 min, which is significantly shorter than that observed in most studies that have evaluated the neurotoxic effects of anesthesia.
In conclusion, the interval between multiple ketamine exposures is a significant factor that can affect the level of neurotoxicity. Importantly, unlike sevoflurane, increasing the interval between ketamine injections lead to a reduction in inhibitory synaptic transmission and long-term behavioral deficits. Our results agree that the neurotoxic effects from multiple anesthetic exposures can be affected by the dosing interval, but the effects may differ between distinct anesthetic agents. Future studies should acknowledge these factors when considering to measure the neurotoxic effects of multiple anesthetic exposures.
Change history
12 May 2023
Supplementary information file was missing and included in this version
References
Lee JR, Loepke AW. Does pediatric anesthesia cause brain damage? Addressing parental and provider concerns in light of compelling animal studies and seemingly ambivalent human data. Korean J Anesthesiol. 2018;71(4):255–73.
Vutskits L, Culley DJ. GAS, PANDA, and MASK: no evidence of clinical anesthetic neurotoxicity! Anesthesiology. 2019;131(4):762–4.
Huang L, Hayes S, Yang G. Long-lasting behavioral effects in neonatal mice with multiple exposures to ketamine-xylazine anesthesia. Neurotoxicol Teratol. 2017;60:75–81.
Huang L, Yang G. Repeated exposure to ketamine-xylazine during early development impairs motor learning-dependent dendritic spine plasticity in adulthood. Anesthesiology. 2015;122(4):821–31.
Raper J, De Biasio JC, Murphy KL, Alvarado MC, Baxter MG. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2018;120(4):761–7.
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, VoigtRG Paule MG, Chelonis JJ, Flick RP. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring GeneralAnesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiol. 2018;129(1):89–105.
Zaccariello MJ, Frank RD, Lee M, Kirsch AC, Schroeder DR, Hanson AC, Schulte PJ, Wilder RT, Sprung J, Katusic SK, Flick RP, Warner DO. Patternsof neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. British J Anaesthesia. 2019;122(5):671–81.
De Roo M, Klauser P, Briner A, Nikonenko I, Mendez P, Dayer A, Kiss JZ, Muller D, Vutskits L. Anesthetics rapidly promote synaptogenesis during acritical period of brain development. PloS one. 2009;4(9):e7043.
Chung W, Ryu MJ, Heo JY, Lee S, Yoon S, Park H, Park S, Kim Y, Kim YH, Yoon SH, Shin YS, Lee WH, Ju X, Kweon GR, Ko Y. SevofluraneExposure during the Critical Period Affects Synaptic Transmission and Mitochondrial Respiration but Not Long-term Behavior in Mice. Anesthesiol. 2017;126(2):288–99.
Ju X, Jang Y, Heo JY, Park J, Yun S, Park S, Huh YH, Kim HJ, Lee Y, Kim YH, Lim CS, Lee SY, Ko Y, Kweon GR, Chung W. Anesthesia affectsexcitatory/inhibitory synapses during the critical synaptogenic period in the hippocampus of young mice: Importance of sex as a biological variable. Neurotoxicol. 2019;70:146–53.
Ju X, Cui J, Lee Y, Park S, Hong B, Yoo S, Kim YH, Ko Y, Lim C, Lee SY, Kweon GR, Heo JY, Chung W. Increasing the interval between repeated anesthetic exposures reduces long-lasting synaptic changes in late postnatal mice. J Neurochem. 2020.
Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABA(A) receptors. Current Neuropharmacol. 2010;8(1):2–9.
Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD. Ketamineand Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. 2018;70(3):621–60.
Cabrera OH, Gulvezan T, Symmes B, Quillinan N, Jevtovic-Todorovic V. Sex differences in neurodevelopmental abnormalities caused by early-life anaesthesia exposure: a narrative review. Br J Anaesth. 2020;124(3):e81–91.
Chung W, Park S, Hong J, Park S, Lee S, Heo J, Kim D, Ko Y. Sevoflurane exposure during the neonatal period induces long-term memory impairmentbut not autism-like behaviors. Paediatric Anaesthesia. 2015;25(10):1033–45.
Lee S, Chung W, Park H, Park H, Yoon S, Park S, Park J, Heo JY, Ju X, Yoon SH, Kim YH, Ko Y. Single and multiple sevoflurane exposures duringpregnancy and offspring behavior in mice. Paediatric Anaesthesia. 2017;27(7):742–51.
Lee E, Lee J, Kim E. Excitation/Inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry. 2017;81(10):838–47.
Meredith RM. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci Biobehav Rev. 2015;50:180–8.
Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors anddeficits in fear conditioning in mice. Anesthesiol. 2009;110(3):628–37.
Woodward TJ, Timic Stamenic T, Todorovic SM. Neonatal general anesthesia causes lasting alterations in excitatory and inhibitory synaptic transmission in the ventrobasal thalamus of adolescent female rats. Neurobiol Dis. 2019;127:472–81.
Contreras A, Hines DJ, Hines RM. Molecular specialization of GABAergic synapses on the soma and axon in cortical and hippocampal circuit function and dysfunction. Front Mol Neurosci. 2019;12:154.
Chattopadhyaya B, Cristo GD. GABAergic circuit dysfunctions in neurodevelopmental disorders. Front Psychiatry. 2012;3:51.
Ramamoorthi K, Lin Y. The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol Med. 2011;17(8):452–62.
Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–19.
Torres-Rojas C, Jones BC. Sex differences in neurotoxicogenetics. Front Genet. 2018;9:196.
Aligny C, Roux C, Dourmap N, Ramdani Y, Do-Rego JC, Jégou S, Leroux P, Nicollet L. Ketamine alters cortical integration of GABAergic interneurons and induces long-term sex-dependent impairments in transgenic Gad67-GFP mice. Cell Death Dis. 2014;5(7):e1311.
Acknowledgement
This research was supported by a special Research Grant funded by the Korean Society of Neuroscience in Anesthesiology and Critical Care (KSNACC-2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lee, Y., Youn, A.M., Ju, X. et al. Interval-dependent neurotoxicity after multiple ketamine injections in late postnatal mice. J Anesth 35, 93–101 (2021). https://doi.org/10.1007/s00540-020-02876-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02876-7