Abstract
Background
Colorectal Cancer (CRC) has been molecularly classified into several subtypes according to tumor, stromal, and immune components. Here, we investigated whether the preventive effect of vitamin D on CRC varies with subtypes defined by Vitamin D receptor (VDR) expression in tumors and their surrounding stroma, along with the association of somatic mutations in CRC.
Methods
In a population-based prospective study of 22,743 Japanese participants, VDR expression levels in tumors and their surrounding stroma were defined in 507 cases of newly diagnosed CRC using immunohistochemistry. Hazard ratios of CRC and its subtypes according to dietary vitamin D intake were estimated using multivariable Cox proportional hazards models.
Results
Dietary vitamin D intake was not associated with CRC or its subtypes defined by VDR expression in tumors. However, an inverse association was observed for CRC with high VDR expression in the stroma (the highest tertile vs the lowest tertile: 0.46 [0.23–0.94], Ptrend = 0.03), but not for CRC with low VDR expression in the stroma (Pheterogeneity = 0.02). Furthermore, CRC with high VDR expression in the stroma had more somatic TP53 and BRAF mutations and fewer APC mutations than those with low VDR expression in the stroma.
Conclusions
This study provides the first evidence that the preventive effect of vitamin D on CRC depends on VDR expression in the stroma rather than in the tumors. CRC with high VDR expression in the stroma is likely to develop through a part of the serrated polyp pathway, which tends to occur with BRAF but not with APC mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer, with more than 1.9 million new cases worldwide in 2020 [1]. Given that the consequences of this disease confer a substantial burden on patients, including intestinal obstruction and adverse effects of treatment, the prevention of CRC is a pivotal issue for public health. The improvement in vitamin D status is expected as a potential strategy for preventing CRC [2,3,4], because vitamin D has been shown to possess anti-cancer functions such as inhibition of cell proliferation and angiogenesis in many laboratory studies [5,6,7]. Nevertheless, in epidemiological studies, including our previous studies of vitamin D intake or plasma concentration [8, 9], the preventive potential of vitamin D on CRC remains controversial.
CRC has been believed to develop through several distinct pathways, resulting in tumor subtypes with different molecular features [10]. For example, the adenoma-carcinoma pathway tends to occur with APC mutations, while the serrated pathway likely involves BRAF mutations. Interestingly, recent prospective studies [11,12,13,14,15] of adenomas and serrated polyps suggested that vitamin D intake may be differentially associated with precursor lesions of CRC with different molecular features. Therefore, tumor heterogeneity resulting from tumor molecular subtypes is one explanation for the controversial results regarding the preventive potential of vitamin D on CRC.
The preventive effects of vitamin D against tumorigenesis are primarily mediated by the binding of the vitamin D metabolite (1α,25-dihydroxy vitamin D3, 1α,25(OH)2D3) to the vitamin D receptor (VDR), a member of the steroid hormone receptor superfamily [2, 16]. In epithelial cells, 1α,25(OH)2D3-activated VDR regulates the expression of genes related to the cell cycle, apoptosis, and differentiation [3], which helps prevent carcinogenesis [17]. Additionally, the vitamin D/VDR axis also exerts anticancer effects on the fibroblasts in the stroma. In fibroblasts of the tumor stroma, 1α,25(OH)2D3-activated VDR inhibited tumor-supporting secretomes, such as exosomal miRNAs, and thus prevented cancer development [18, 19]. Therefore, the evidence from laboratory studies suggests that the preventive effects of vitamin D on tumorigenesis may depend on VDR in these tissue components.
VDR is stably expressed in normal large intestinal epithelial cells, but repressed by the SNAIL transcription factor in tumors [20]. Meanwhile, Stromal VDR is not expressed in normal tissue [21, 22], but upregulated by stroma activation such as cytokine induction and changes in the extracellular matrix [18]. Therefore, the expression levels of VDR vary across tumors [23] and their surrounding stroma [24], which may be one of the tumor molecular features that differ between CRC subtypes arising from several distinct pathways. Collectively, it is hypothesized that the preventive effects of vitamin D may be restricted to certain CRC subtypes with high VDR expression in the tumors and/or stroma. However, no studies have examined the association between vitamin D and CRC risk in relation to VDR expression levels in tumors and their surrounding stroma.
In this population-based prospective study with up to 15 years of follow-up, we collected CRC tissues and investigated the association between pre-diagnosed dietary vitamin D intake and CRC risk in relation to VDR expression levels in the tumors and stroma. Furthermore, typical molecular features of CRC, namely APC, TP53, KRAS, and BRAF mutations [10], were examined in relation to VDR expression in tumors and stroma to unravel the characteristics of CRC subtypes with different expression levels of VDR.
Methods
Study population
This study is part of the Japan Public Health Center-based Prospective (JPHC) study examining lifestyle and other factors related to cancer and other disease risks using self-administered questionnaires. In brief, participants of the JPHC study were recruited from 11 public health centers across Japan between 1990 and 1994 and asked about their dietary and lifestyle habits as well as medical history every five years for 15 years.
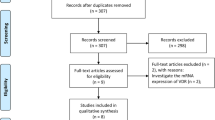
In the JPHC study area, we collected tumor tissues stocked at the department of pathology, regional core hospitals in Akita (the Hiraka General Hospital and the Yokote Municipal Hospital) and Okinawa (the Okinawa Prefecture Chubu Hospital and the Nakagami Hospital), where 29,988 participants were registered at the initiation of the JPHC study. For the present study, we excluded participants who were ineligible, moved out, died or were with a history of cancer before the commencement of this study, or those who did not respond to any questionnaires. Finally, 22,743 participants in Akita and Okinawa were followed up (Fig. 1).
Assessment of exposure and covariates
Daily food intake was calculated using the food frequency questionnaire (FFQ) of the 10-year follow-up survey conducted in 2000. This FFQ included 159 food and beverage items and enquired about nine categories of eating frequency and three categories of portion size for each food item of the previous year [25, 26]. The daily intake of energy, vitamin D, and other nutrients was estimated according to the Standard Tables of Food Composition in Japan (seventh version) [27]. Participants with extreme daily energy intake in the highest or lowest 2.5th percentile (< 971 or > 4377 kcal/day for men and < 755 or > 3892 kcal/day for women) were excluded. The validity of these intakes is described in Supplementary Methods. In addition to dietary habits, information on lifestyle and anthropometric data were gathered using the 10-year follow-up questionnaire.
Follow-up and outcome assessment
Participants in Akita were followed up from January 2004, while those in Okinawa were followed up from the date of their response to the 10-year survey questionnaire (January–November 2000). This is because we were able to collect tumor tissues from CRC cases diagnosed after 2003 in Akita and after 1999 in Okinawa. In both areas, the end of the follow-up period was December 2014. During these follow-up periods, newly diagnosed CRC cases were mainly ascertained by record linkage with population cancer registries and active patient notifications from local hospitals. The site and histological features of each cancer case were coded according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [28]. In terms of the cancer site, C18 was designated for the colon, and C19–C20 for the rectum.
At the end of the follow-up period, 646 CRC cases were reported. Among these, after 65 cases without histological confirmation or at hospitals other than the regional core hospitals were excluded, we found 561 CRC tissues with more than 95% of the retrieval rate. Finally, we obtained 507 CRC tissues with vitamin D and covariate information for analysis (approximately 80% of all incident cases, Fig. 1).
Definition of VDR expression using immunohistochemistry
The expression of the VDR protein in tissues was detected by immunohistochemistry. For this analysis, formalin-fixed paraffin-embedded tumor tissues were prepared at 5 μm thickness, and the tissue sections were then dewaxed and rehydrated. Next, we performed antigen retrieval with a high-pH buffer and quenched endogenous peroxidase activity with H2O2 solution. After preparation for immunohistochemistry, the tissue sections were incubated with 1/400 diluted Anti-VDR Rabbit Monoclonal Antibody (D2K6W, #12550, Cell Signaling Technology, Massachusetts, USA) at 37 °C for 32 min, followed by incubation with linker antibody (OptiView HQ Universal Linker, Ventana Medical Systems, Inc., Tucson, Arizona USA) for 8 min, and subsequently with the labelled polymer horseradish peroxidase (OptiView HRP multimer, Ventana Medical Systems, Inc.) for 8 min. Finally, the tissue sections were stained with 3,3V-diaminobenzidine (DAB) chromogen (OptiView DAB and OptiView H2O2, Ventana Medical Systems, Inc.). The dewax-to-DAB reaction procedures were performed using an auto-strainer (VENTANA BenchMark GX, Ventana Medical Systems, Inc.). We assessed VDR expression in the nucleus, where VDR predominantly functions as a transcription factor. Since there are no clinical cut-off points for staining range and intensity, cells with a stained nucleus were regarded as positive cells regardless of staining intensity by microscopy. The percentage of immunoreactive nuclear cells was rated from 0–100% in 10% increments. Tumors and stroma with 50% or more positive cells were categorized as tumors with high VDR expression levels and those with less than 50% as low VDR expression levels. The assessment was conducted by a laboratory technician. A pathologist assessed 112 randomly selected cases. The concordance of these assessments between technician and pathologist were high, with Kappa coefficients of 0.97 and 0.90 for tumors and their surrounding stroma, respectively. Both analysts were blinded to the patients’ lifestyles and medical histories. All processes used to define VDR expression were conducted at GeneticLab Co. Ltd. (Japan).
Definition of somatic TP53, APC , KRAS, and BRAF mutations using target sequencing
DNA was extracted from formalin-fixed paraffin-embedded tumor tissue sections using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). The purified DNA was amplified by multiplex PCR using the Ion AmpliSeq Cancer Hotspot Panel v2 covering TP53, APC, KRAS, and BRAF regions (Thermo Fisher Scientific, Massachusetts, USA) to generate an amplicon library. The obtained library was sequenced using the Ion Proton platform (Thermo Fisher Scientific). Next, we applied sequencing data to variant calls using the Torrent Suite Software v5.0.4 (Thermo Fisher Scientific, Massachusetts, USA) and performed quality control for the samples (a base quality score of 20 > 90% and median and mean depth > 1000) using cisCall [29]. If they failed to pass quality control, the samples were sequenced again. However, due to poor quality or an insufficient amount of DNA, we successfully obtained sequencing data from 399 samples. In addition to quality control for the samples, we also conducted quality control for called variants (variant frequency in this analysis > 7%, minor allele frequency based on 1000 Genomes Project and Japanese Multi Omics Reference Panel < 1%, and pathogenic or oncogenic variants registered in Annovar and OncoKB). Eventually, among the 399 cases, we identified TP53, APC KRAS, and BRAF mutations in 228, 194, 197, and 19 cases, respectively. The details of these mutations are presented in Supplementary Table S1.
Statistical analyses
Vitamin D intake was divided into tertiles based on the distribution of dietary vitamin D intake by sex. Similarly, other nutrients were divided into tertiles by sex. As a sensitivity analysis, to assess vitamin D status in the body, we utilized plasma 25-hydroxyvitamin D concentration in the first survey of the JPHC study, which was measured in the participants of a previous case-cohort study [8]. Considering the relatively small numbers of participants with available plasma samples (N = 916), plasma 25-hydroxyvitamin D concentrations were divided into high and low based on the median of the concentrations adjusted for sex, season, and study area. Missing dietary vitamin D intake and covariate values in the 10-year survey were imputed from the 5-year survey data.
Person-years of follow-up for each participant were calculated from the starting point of this study (January 2004 in Akita and January–November 2000 in Okinawa) until the date of diagnosis of cancer, death, moving away from the study areas, loss to follow-up, or the end of follow-up (December 2014), whichever came first. The median follow-up period was 11 years. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using Cox proportional-hazards models. Crude models were stratified by study area and minimally adjusted for sex and age. Multivariable models were further adjusted for potential confounding factors, including smoking status, alcohol consumption, body mass index, physical activity (metabolic equivalent of tasks, METs), history of diabetes, intake of calcium, fiber, and red and processed meats, vitamin D or multivitamin supplement use, and CRC screening. The associations according to CRC subtypes defined by VDR expression levels in the tumors and stroma were analyzed using a duplication method [30]. The heterogeneity of the associations between the CRC subtypes was assessed to test the null hypothesis that vitamin D effects are common among CRC subtypes with high and low VDR expression [30]. Fisher’s exact test was used to compare the prevalence of mutations in CRC cases with high and low VDR expression. The significance level was set at a two-sided P-value of < 0.05. Statistical analyses were conducted using SAS ver 9.4(SAS Institute, NC, USA).
Results
Characteristics of study participants
Table 1 shows the characteristics of participants according to dietary vitamin D intake. Participants with higher intake of vitamin D were likely to drink more alcohol than those with lower vitamin D intake, and those with higher vitamin D intake took more colorectal screening, fiber, calcium, and less meats than lower vitamin D consumers. The clinical characteristics of CRC cases were relatively similar, regardless of with or without tumor tissue, except for stage (Supplementary Table S2). However, this may be because of the difference in the frequency of missing information on the stage between with (2.0%) and without (14.0%) tumor tissue. Among the CRC cases with tumor tissues, 82.3% and 12.1% cases showed high VDR expression levels in the tumors and stroma, respectively (Fig. 2). Except for one case, high VDR expression in the stroma was observed simultaneously with high VDR expression in the tumors. Although CRC cases with tumor tissue did not differ largely in their characteristics according to VDR expression status, CRC cases with high VDR expression in stroma tended to be women, right-sided, and middle grade of differentiation compared with other CRC subtypes (Supplementary Table S3).
Vitamin D intake and CRC defined by VDR expression
Table 2 presents the associations between dietary vitamin D intake and overall CRC and its subtypes, defined by VDR expression levels in the tumors and stroma. Without considering VDR expression levels, higher dietary vitamin D intake was not associated with the risk of CRC. Similarly, no apparent association was observed with CRC, regardless of VDR expression levels in the tumors. After adjusting for potential confounders, the HRs were consistent. Compared to participants with the first tertile of dietary vitamin D intake, the multivariable HRs (95% CI) for the second and third tertile were 0.95 (0.73–1.23) and 1.03 (0.78–1.35) for high VDR expression, and 0.53 (0.30–0.94) and 0.78 (0.43–1.43) for low VDR expression, respectively. In terms of the stroma, the corresponding HRs for dietary vitamin D intake were 0.71 (0.36–1.38) and 0.46 (0.23–0.94) for high VDR expression, and 0.87 (0.68–1.11) and 1.07 (0.83–1.39) for low VDR expression, respectively. An inverse association was found only for the CRC subtype with high VDR expression in the stroma (Ptrend = 0.03). Simultaneously, heterogeneity between CRC with high and low VDR expression in the stroma was indicated (Pheterogeneity = 0.02). Although sensitivity analyses of plasma 25-hydroxyvitamin D concentrations in a relatively small number of participants (N = 916) did not show statistically significant results, similar heterogeneity was observed between CRC subtypes with high and low VDR expression in the stroma (Supplementary Table S4).
Molecular features of CRC defined by VDR expression
Table 3 shows the typical molecular features of CRC according to VDR expression levels in the tumors and stroma. VDR expression in tumors was not associated with any molecular features. In contrast, CRC with higher VDR expression levels in the stroma was likely to encompass somatic TP53 (P = 0.04) and BRAF (P = 0.02) mutations and had fewer APC mutations (P = 0.01) compared to CRC with lower expression levels in the stroma.
Discussion
This prospective study found that dietary vitamin D intake was inversely associated with CRC with high VDR expression in the stroma but not CRC with low VDR expression in the stroma. However, there was no association between dietary vitamin D intake and CRC overall, and irrespective of VDR expression in tumors. Sensitivity analysis of plasma 25-hydroxyvitamin D concentrations showed similar results, albeit with no statistical significance. Our study might provide the first line of population-based evidence that the preventive effect of vitamin D on CRC risk might depend on VDR expression in the stroma rather than in the tumors.
The evidence for vitamin D as a protective factor against CRC is obscure, although it has been investigated in many types of epidemiological studies. For the relation between circulating 25-hydroxyvitamin D and CRC risk, a recent pooling project of 17 cohorts [31] and meta-analyses from only Asian countries [32] or across ethnicity [33] consistently showed an inverse association. In contrast, a meta-analysis examining dietary vitamin D intake and CRC risk presented an inverse association in case–control studies, including 47,540 CRC cases, but not in prospective studies, including 14,676 CRC cases [34]. Moreover, several clinical randomization trials did not indicate the preventive effect of vitamin D supplements on colorectal cancer and adenoma risk [35,36,37]. Notably, in a trial, daily vitamin D3 supplementation (1000 IU) and/or calcium (1200 mg) did not decrease the risk of recurrent adenoma [35], and the decreased risk was observed only in individuals with specific genotypes of two VDR polymorphisms (rs7968585 and rs731236) [38]. Given that these VDR polymorphisms affect the mRNA and protein levels, the effect of vitamin D on CRC may vary according to individual VDR expression in tissues, as suggested by the present study.
By focusing on VDR expression in CRC tumors, one US study examined the association between the predicted vitamin D levels and CRC risk by VDR expression in tumors [39]. However, predicted vitamin D scores were associated with decreasing CRC risk regardless of VDR expression in tumors. In accordance with the results of this study, our study did not suggest a heterogeneous association of dietary vitamin D intake with CRC risk by VDR expression status in tumors, despite several VDR-dependent effects of vitamin D, including cell growth arrest [40, 41]. These findings may be attributed to the diverse effects of vitamin D independent of VDR expression in tumors.
Recently, accumulating evidence has shown that the stroma surrounding tumor cells, including cancer-associated fibroblasts (CAF), influences CRC development. VDR in CAFs was activated by vitamin D and suppressed the secretion of exosomal miRNAs, promoting tumor-stroma crosstalk [18, 19]. This novel evidence motivated our analysis of CRC subtypes defined by VDR expression levels in the stroma. In a CRC survival study, higher VDR expression in the stroma was associated with better overall and progression-free survival, regardless of its expression in carcinoma cells [24]. In the present study, higher dietary vitamin D intake was associated with a lower risk of CRC with high VDR expression in the stroma, and no apparent association was observed for CRC with low VDR expression in the stroma. These findings supported the evidence that VDR in CAFs prevents CRC development by interfering with pro-tumor miRNAs [18, 19] and could help disentangle controversial results regarding the association between vitamin D and CRC risk.
Furthermore, we found that CRC with high VDR expression in the stroma was associated with APC wild type as well as somatic TP53 and BRAF mutations. Interestingly, similar findings were observed in an Australian study reporting an inverse association between 25-hydroxyvitamin D concentrations and CRC with BRAF mutations but no obvious association for CRC with KRAS mutations [42]. Meanwhile, one study reported that cytoplasmic VDR overexpression in tumors was correlated with KRAS and PIK3CA mutations [43]. However, the antibody against VDR, staining pattern of VDR, and evaluation of VDR expression in the previous study were different from those in this study; therefore, we could not compare the results. Additionally, no studies have examined the relationship between VDR expression in stroma and these somatic mutations, which presumably provides some clues for understanding the link between VDR expression status in the stroma and pathways of CRC development.
While CRC arising from a conventional adenoma is likely to possess APC mutations, CRC arising from a serrated polyp tends to have BRAF mutations. In contrast, TP53 and KRAS mutations were common in both CRC subtypes [44]. Therefore, CRC with high VDR expression in the stroma is likely to occur through part of the serrated polyp pathway rather than the adenoma–carcinoma pathway. Moreover, CRC with high VDR expression in the stroma which harbored somatic TP53 and BRAF mutations may be classified as stem/serrated/mesenchymal transcriptional subtype [45,46,47]. Of note, this subtype was associated with stroma activation [48, 49], which may upregulate VDR expression in stroma during tumorigenesis. Considering a poor prognosis and a resistance to immune checkpoint blockade treatment of this subtype [50, 51], these findings may shed light on CRC prevention and therapy through vitamin D. Although further supportive studies are needed, VDR expression status in the stroma may be a unique molecular feature in CRC development and is useful for promoting prevention and therapy of CRC by vitamin D.
Strengths and limitations
This study had several strengths. First, we collected tumor tissues with a high retrieval rate and performed immunohistochemistry on each tissue sample with a larger size than tissue micro-array. Large tissues can help prevent bias arising from the misclassification of CRC subtypes. In addition, these tissues allowed us to examine VDR expression not only in the tumors, but also in the stroma of CRC. Second, our prospective and population-based design was less likely to cause recall or selection bias than the hospital-based case–control design commonly adopted in pathological studies. Third, validated dietary exposure and detailed covariate data would lead to more precise estimates, although residual confounding could not be ruled out because of the observational nature of the study. This study had several limitations. Dietary intake of vitamin D may not accurately reflect vitamin D status in the body, which is provided by both diet and ultraviolet irradiation exposure. However, to weaken the influence of ultraviolet irradiation, the study areas were stratified for all the analyses. Furthermore, we observed similar results in a sensitivity analysis using plasma 25-hydroxyvitamin D concentrations, albeit in a limited number of participants. Finally, the small number of CRC cases with high VDR expression in the stroma and the VDR expression defined by single immunohistochemistry warrants caution in interpretation, and further studies are needed to confirm these findings.
In summary, this longitudinal study demonstrated that the association of vitamin D intake with CRC risk differed according to VDR expression in the stroma rather than in tumors. Although higher vitamin D intake is likely associated with a lower risk of overall CRC, the magnitude of the association was the largest for CRC with high VDR expression in the stroma. Our findings may support the preventive effect of vitamin D on CRC and the importance of tumor stroma features in response to the effect of vitamin D.
Abbreviations
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- DAB:
-
3,3V-diaminobenzidine
- FFQ:
-
Food Frequency Questionnaire
- HR:
-
Hazard ratio
- JPHC:
-
Japan Public Health Center-based Prospective
- METs:
-
Metabolic equivalent of task score
- VDR:
-
Vitamin D receptor
- 1α,25(OH)2D3 :
-
1α,25-Dihydroxy vitamin D3
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700.
Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57.
Peixoto RD, Oliveira LJC, Passarini TM, et al. Vitamin D and colorectal cancer - a practical review of the literature. Cancer Treat Res Commun. 2022;32: 100616.
Mantell DJ, Owens PE, Bundred NJ, et al. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20.
Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76.
Fleet JC, DeSmet M, Johnson R, et al. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61–76.
Budhathoki S, Hidaka A, Yamaji T, et al. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ. 2018;360: k671.
Ishihara J, Inoue M, Iwasaki M, et al. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr. 2008;88:1576–83.
Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60.
Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017;152:92–104.
He X, Wu K, Ogino S, et al. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. 2018;155:355-73.e18.
Kim H, Lipsyc-Sharf M, Zong X, et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology. 2021;161(1208–17): e9.
Crockett SD, Barry EL, Mott LA, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. 2019;68:475–86.
Song M, Lee IM, Manson JE, et al. No association between vitamin D supplementation and risk of colorectal adenomas or serrated polyps in a randomized trial. Clin Gastroenterol Hepatol. 2021;19(128–35): e6.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87.
Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93.
Kong F, Li L, Wang G, et al. VDR signaling inhibits cancer-associated-fibroblasts’ release of exosomal miR-10a-5p and limits their supportive effects on pancreatic cancer cells. Gut. 2019;68:950–1.
Palmer HG, Larriba MJ, Garcia JM, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–9.
Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–33.
Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Kane KF, Langman MJ, Williams GR. 1,25-Dihydroxyvitamin D3 and retinoid X receptor expression in human colorectal neoplasms. Gut. 1995;36:255–8.
Ferrer-Mayorga G, Gomez-Lopez G, Barbachano A, et al. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–62.
Sasaki S, Kobayashi M, Ishihara J, et al. Self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study: questionnaire structure, computation algorithms, and area-based mean intake. J Epidemiol. 2003;13:S13-22.
Tsugane S, Kobayashi M, Sasaki S, et al. Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: comparison with dietary records for main nutrients. J Epidemiol. 2003;13:S51–6.
Japan Ministry of Education, Culture Sports, Science and Technology. Standard Tables of Food Composition in Japan. Official Gazette Cooperation of Japan. 2015.
WHO Organization. International classification of diseases for oncology (ICD-O), 3rd ed, 1st revision. 2013.
Kato M, Nakamura H, Nagai M, et al. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin-embedded samples in cancer clinical sequencing. Genome Med. 2018;10:44.
Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800.
McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. 2019;111:158–69.
Zhang L, Zou H, Zhao Y, et al. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: a systematic review and dose-response meta-analysis. BMJ Open. 2019;9: e030513.
Ma Y, Zhang P, Wang F, et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82.
Boughanem H, Canudas S, Hernandez-Alonso P, et al. Vitamin D intake and the risk of colorectal cancer: an updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers. 2021;13:2814.
Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin d for the prevention of colorectal adenomas. N Engl J Med. 2015;373:1519–30.
Lappe J, Watson P, Travers-Gustafson D, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317:1234–43.
Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44.
Barry EL, Peacock JL, Rees JR, et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol. 2017;3:628–35.
Jung S, Qian ZR, Yamauchi M, et al. Predicted 25(OH)D score and colorectal cancer risk according to vitamin D receptor expression. Cancer Epidemiol Biomarkers Prev. 2014;23:1628–37.
Eelen G, Verlinden L, van Camp M, et al. The effects of 1alpha,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–46.
Wu W, Zhang X, Zanello LP. 1alpha,25-Dihydroxyvitamin D(3) antiproliferative actions involve vitamin D receptor-mediated activation of MAPK pathways and AP-1/p21(waf1) upregulation in human osteosarcoma. Cancer Lett. 2007;254:75–86.
Heath AK, Hodge AM, Ebeling PR, et al. Circulating 25-Hydroxyvitamin D concentration and risk of breast, prostate, and colorectal cancers: the Melbourne collaborative cohort study. Cancer Epidemiol Biomarkers Prev. 2019;28:900–8.
Kure S, Nosho K, Baba Y, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2765–72.
Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394:1467–80.
Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6.
Sadanandam A, Wang X, de Sousa EMF, et al. Reconciliation of classification systems defining molecular subtypes of colorectal cancer: interrelationships and clinical implications. Cell Cycle. 2014;13:353–7.
Marisa L, de Reynies A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 2013;10: e1001453.
Isella C, Terrasi A, Bellomo SE, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47:312–9.
Calon A, Lonardo E, Berenguer-Llergo A, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320–9.
Zhou YJ, Lu XF, Chen H, et al. Single-cell transcriptomics reveals early molecular and immune alterations underlying the serrated neoplasia pathway toward colorectal cancer. Cell Mol Gastroenterol Hepatol. 2023;15:393–424.
Utsumi T, Yamada Y, Diaz-Meco MT, et al. Sessile serrated lesions with dysplasia: is it possible to nip them in the bud? J Gastroenterol. 2023;58:705–17.
Acknowledgements
We thank the participants and all the staff members in this study. We also thank Ms. Yoko Shimada, Ms. Maiko Matsuda, Ms. Sachiyo Mitani, and Dr. Hitoshi Ichikawa at the Division of Genome Biology, National Cancer Center Research Institute and the Department of Clinical Genomics, Fundamental Innovative Oncology Core, National Cancer Center Research Institute, Dr. Makoto Niwa at the Yokote Municipal Hospital, Mr. Taizan Miyazato at the Department of Diagnostic Pathology, Okinawa Prefecture Chubu Hospital, and Drs. Zenji Miyazato and Tsutomu Shimoji at the Nakagami Hospital for their assistance and support. We are indebted to the Akita and Okinawa Cancer Registries for providing their incidence data and staff at the Hiraka General Hospital, the Yokote Municipal Hospital, the Okinawa Prefecture Chubu Hospital and the Nakagami Hospital for collecting tumor tissues. The Japan Public Health Center-based Prospective Study members are listed at the after site: http://epi.ncc.go.jp/en/jphc/781/7951.html.
Funding
This study was supported by the National Cancer Center Research and Development Fund (23-A-31 [toku], 26-A-2, 29-A-4,2020-J-4), and NCC Core Facility was supported by the National Cancer Center Research and Development Fund (2020-J-2). A Grant-in-Aid for Cancer Research from the Ministry of Health, Labour, and Welfare of Japan (from 1989 to 2010), and the Practical Research for Innovative Cancer Control (JP19ck0106266 and JP22ck0106551) from the Japan Agency for Medical Research and Development. The funders had no role in the study design, data collection, and analysis, decision to publish, or the preparation of the study.
Author information
Authors and Affiliations
Contributions
Concept and design: Nakano, Yamaji, and Iwasaki. Acquisition or interpretation of data: All authors. Statistical analysis: Nakano and Yamaji. Drafting of the manuscript: Nakano drafted the first manuscript with Yamaji’s support. Critical revision of the manuscript for important intellectual content: All authors. Administrative, technical, or material support: Yamaji, Sawada, Inoue, Tsugane, and Iwasaki. Obtained funding: Tsugane, Iwasaki, and Sawada. Supervision: Tsugane and Iwasaki.
Corresponding author
Ethics declarations
Competing interests
None of the authors report conflicts of interest related to this article. Full disclosures are as follows; Kohno has received personal fees outside of this work from Chugai Pharmaceutical, Eli Lilly Japan, and Sysmex Corporation; and a patent fee from ThermoFisher Scientific.
Ethics approval and consent to participate
Participants were informed of the JPHC study objectives, and completing the questionnaire indicated consent to participate in the JPHC study. Before the initiation of tissue collection, information regarding this study was posted on our center’s website to provide participants with the opportunity to opt out at any time. The comprehensive study protocol, including this study, was approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan (Approval No. 2014-214). All the procedures complied with the principles of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakano, S., Yamaji, T., Hidaka, A. et al. Dietary vitamin D intake and risk of colorectal cancer according to vitamin D receptor expression in tumors and their surrounding stroma. J Gastroenterol 59, 825–835 (2024). https://doi.org/10.1007/s00535-024-02129-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-024-02129-4