Abstract
Background
Small bowel cancer is not a single entity. Population-based studies taking into account histological diversity are scarce. The aim of this study was to report on their trends in incidence by histology in France over the past 20 years.
Methods
All patients with a small bowel cancer diagnosed in 15 French administrative areas covered by a registry from the network of French cancer registries (FRANCIM) were included. Age-standardized incidence rates were estimated using the world standard population. Incidence rates were calculated by gender, age group, histology, and 5-year period.
Results
The overall age-standardized incidence rates were 1.46/100,000 inhabitants in men and 0.9/100,000 inhabitants in women. Adenocarcinoma was the most common histological type (38%), followed by neuroendocrine tumors (35%), lymphoma (15%) and sarcoma (12%). Age at diagnosis and tumor location differed between adenocarcinoma and neuroendocrine tumors. The incidence of all four tumor types increased significantly over the 20-year period, with the exception of lymphoma in men. The annual percentage change for neuroendocrine tumors was 3.89% in men and 3.61% in women; for sarcoma, it was 3.38% and 4.08%, respectively. The incidence of adenocarcinoma and lymphoma also increased in women with an annual percentage change of 3.05% and 3.32%, respectively.
Conclusion
Small bowel cancer incidence has increased over time. This increase occurred with different amplitudes and patterns in the four major histological types. The improvement in imaging techniques could partly explain this increase. It is necessary to determine whether predisposing conditions may contribute to this change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Information on incidence of small bowel cancer is scanty, mostly due to the fact that it is a rare oncologic condition especially considering the size of the organ, representing less than 2% of all digestive cancers. Recent data show that rates for newly diagnosed small bowel cancer cases have been rising over the last decades [1, 2]. Yet, epidemiological studies cannot consider small bowel cancers as a single entity due to clinical and histological diversity. Trends in incidence of the main histological types (adenocarcinoma, endocrine tumors, lymphoma and sarcoma) are thus not well known. Keeping track of trends in incidence can help scientists understand where additional research is needed to address challenges. Population-based studies, which accurately record all cases diagnosed in a well-defined population and thus provide unbiased and standardized measures, represent the best way to assess temporal changes in small bowel cancer incidence.
The objective of this study was to describe and model the evolution of small bowel cancer incidence according to histology, in France during the past 20 years.
Patients and methods
Data sources
The network of French population-based cancer registries FRANCIM, together with the biostatistics department of the Hospices Civils de Lyon, the French Institute for Public Health Surveillance (Santé Publique France, SPF), and the French National Cancer Institute (Institut national du cancer, INCa) manages a common database of all cancers diagnosed in well-defined administrative areas called ‘départements’. Quality checks are carried out at both the registry and at the common database level, according to the International Agency for Research on Cancer guidelines. The quality and exhaustiveness of these registries are certified every 4 years by an audit of the National Institute of Health and Medical Research (INSERM), the SPF and the INCa. Cases are notified by many sources: public and private pathology laboratories, regional databases of the National Health System, and public and private hospital discharge databases. French registries do not record incident cases that are notified by death certificates only. Death certificates mentioning small bowel cancer and which escaped the registration process during life were individually traced back. The few cases which were not traceable were not registered.
Malignant small bowel tumors (coded as C17 according to the International Classification of diseases in Oncology, third revision) diagnosed over the period 1996–2015 in 15 ‘départements’ representing 17% of the French population (Table 1) were extracted from the common French registries database (N = 2638 cases in men and N = 2095 in women). Small bowel cancers were categorized regarding to histology into adenocarcinoma (N = 1740), lymphoma (N = 671), sarcoma (N = 557 of which 80% were malignant GastroIntestinal Stromal Tumors) and neuroendocrine tumor (N = 1597). Rare histologies including eleven cases malignant melanoma were classified into ‘other’ histology (N = 28). Cases without histology were classified as ‘NOS’ histology (N = 140). Tumor location was coded as eventually described by the practitioners in the medical file. In case of overlapping tumor, the most involved location was assigned. Location was thus classified into: ‘duodenum’ (N = 1601), ‘jejunum’ (N = 468), ‘ileum’ (N = 1135) and NOS (N = 1529). Age was tabulated into “< 60 years”, “60–69 years”, “70–79 years”; and “> 79 years” classes and time period from 1996 to 2015 into four 5-year periods. Person-years at risk were estimated from the general population data provided by the French national institute of statistics (INSEE), by ‘département’, gender, calendar year and annual age from 1996 to 2015.
Methodology
The association between categorical covariates was analyzed using Chi2 test. The association between categorical and continuous covariates was analyzed using one-way ANOVA. Standardized incidence rates were estimated by the direct method using the world standard population. Incidence was modeled overall using a Poisson regression model adjusted on gender, age and period. It was also modeled separately by gender and histology except for the ‘other histology’ group. Annual percentage changes in age-standardized incidence (APCs) were estimated between 1996 and 2015 introducing year of diagnosis as a continuous covariate in the Poisson regression [3]. Among the 15 registries included in the study, 3 recent registries and their corresponding administrative population were added in 1998 and 2008. A sensitivity analysis was performed by removing these 3 registries. The results with or without the 3 recent registries provided the same patterns of evolution and the conclusions were similar (data not shown). The analyses were performed using STATA, release 15 (STATA, College Station, TX, USA) and a p value smaller than 0.05 was considered significant.
Results
Characteristics of patients according to histological type are given in Table 2. Adenocarcinoma was the most common histological type (37% of cases), preceding endocrine tumors (34%), lymphoma (14%), sarcoma (12%), not otherwise specified (3%) and ‘other’ histologic types (1%). Corresponding mean age at diagnosis was, respectively, 69.7 years (SD: 13.2), 67.0 years (SD: 12.7), 63.9 years (SD: 18.7), 64.3 years (SD: 14.5), 80.4 years (SD: 10.7), and 65.3 years (SD: 20.6, p < 0.001). The proportion of adenocarcinomas was higher among patients aged 70 years and over (41% vs 33%) whereas the proportion of malignant neuroendocrine tumors was higher among patients aged less than 70 years (36% vs 31%, p < 0.001). Not otherwise specified site accounted for 32% of small bowel cancers. When precise site was known, duodenum was the most common site (50% of cases), preceding ileum (35%) and jejunum (15%). The distribution of tumors by histological type strongly varied according to location. Among duodenal tumors 67% were adenocarcinoma, 14% malignant endocrine tumors, 8% lymphoma, and 5% sarcoma. Among ileal tumors, 61% were malignant endocrine tumors, 17% lymphoma, 14% adenocarcinoma and 7% sarcoma. The corresponding percentages for jejunum were 49% adenocarcinoma, 25% sarcoma, 12% for lymphoma and 11% for malignant endocrine tumors.
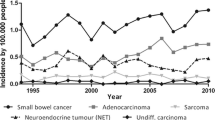
The overall age-standardized incidence rate was 1.46 per 100,000 in men and 0.90 per 100,000 in women. Age-specific incidence curves are presented in Fig. 1a. Trends over time of age-standardized incidence rates are provided for adenocarcinoma, NET, sarcoma and lymphoma, by 5-year period and by year (annual percentage of change or APC) according to histological type and gender in Table 3 and to histological type in Fig. 1b. The M/F sex ratio calculated on age-standardized incidence rates varied from 1.39 (sarcoma) to 2.20 (lymphoma). Incidence of malignant endocrine tumors and of sarcoma strongly increased in men and women over time with an APC varying from 3.38 to 4.08%. Adenocarcinoma and lymphoma similarly increased only in women. Incidence significantly increased for each age class for malignant endocrine tumors whereas it concerned only aged patients for other histological types (Table 4). For the 140 NOS histology cases, the overall incidence was 0.07 per 100,000 inhabitants in men and 0.04 in women. It remained stable over time in men and slightly increased in women (0.02 per 100 000 during the 1996–2000 period to 0.04 during the 2011–2015 period).
Discussion
Incidence of small bowel cancers increased over the 20-year period in France for all four major histological types, except for small bowel lymphoma in men. This trend concerned mostly patients aged 70 years and over. The patterns differed with histology: over the 20-year period, incidence of small bowel adenocarcinoma increased by 16% in men and by 38% in women and from 64% in men and 67% in women for neuroendocrine tumors. Information regarding trends in incidence of small bowel cancers by histology in the literature is scarce. To our knowledge, our results cannot be compared with similar recent published population-based European data. Data from the SEER program showed a slight decrease in incidence for small bowel adenocarcinoma between 1985 and 2005 [4] and a strong increase in incidence for neuroendocrine tumors between 1973 and 2012 [5]. The proportion of neuroendocrine tumors became the highest among small bowel cancers, representing 37% of incident cases during the last studied period. Similar patterns were observed in the UK, with an increasing neuroendocrine tumor incidence rates from 0 to 85 years of age and older for 8 time intervals from 1975 to 2012 [6].
Small bowel malignancies occur mainly in males after the age of 60 years [7, 8]. In our study, the proportion of adenocarcinoma was higher in patients aged 70 years and over, while the proportion of malignant neuroendocrine tumors was higher in patients aged under 70 years of age. Interestingly, after the age of 70 years, the incidence of adenocarcinoma increased steadily with increasing age while the incidence of neuroendocrine tumors decreased and the incidence of sarcoma and lymphoma stabilized. Our data show a slight increase over the study period in small bowel sarcoma, more pronounced in women. A study involving twenty-six population-based cancer registries in the United States between 1995 and 2008 showed a similar trend in incidence with an APC of 2.1% in men and 1.8% in women [9]. The incidence of small bowel lymphoma was stable over time in men, but increased in women without clear explanation concerning this gender discrepancy.
If increase in incidence was solely due to the spread of the improvement in diagnostic techniques, this increase should be uniform according to sex, histology and probably age group. Therefore, a more common use in everyday clinical practice of high-resolution imaging and endoscopy leading to “incidentally” recognized tumors may only partly explain this pattern. Although NOS sites accounted for one-third of the small bowel cancers in our study, we noted that the distribution of tumors by histological type varied considerably by location. Adenocarcinomas were predominant in the duodenum (67%) while endocrine tumors accounted for 61% of malignant tumors of the ileum. The cause of this propensity for adenocarcinoma to occur in the duodenum as opposed to the jejunum/ileum could be related to exposure to high levels of bile in the duodenum and proximal jejunum [10]. The intestinal microbiota may also play a role. In the large bowel, the microbiota can convert bile salts to carcinogenic deoxycholic acid [11]. However, the density of the microbiota is inversely correlated to the incidence of small bowel adenocarcinoma, suggesting other mechanisms of carcinogenesis [12], and due to the reduced transit time, the contact between intestinal cells and xenobiotics or dietary carcinogens is short. Some dietary factors such as consumption of red meat and smoked food may increase the risk of small bowel adenocarcinoma, while the consumption of fiber, fruit, vegetables and fish may reduce it [13, 14]. A meta-analysis suggested that alcohol consumption may increase the risk of small bowel adenocarcinoma [15].
Although small bowel adenocarcinomas are most often sporadic, predisposing diseases have been identified in up to 20% of cases [16], including genetic syndromes, Crohn’s disease and coeliac disease. Lynch syndrome is thought to be responsible for 5–10% of small bowel adenocarcinoma [17]. The relative risk of developing small bowel adenocarcinoma compared to the general population is between 25 and 300 and depends mainly on the type of mutation of the genes in the mismatch repair system [17]. The estimated cumulative lifetime risk varies between 1 and 4% depending on the studies [18, 19]. Familial adenomatous polyposis (FAP) is characterized by the appearance of multiple colonic but also duodenal adenomas, with a predilection for ampullary adenomas. The corresponding cumulative incidence of small bowel disease is 90%. These adenomas have a tendency to degenerate into adenocarcinoma in about 4% of cases. Peutz–Jeghers syndrome is characterized by the occurrence of multiple hamartomatous intestinal polyps, preferably located in the small bowel that can degenerate into adenocarcinoma with a relative risk of 520 compared to the general population [20, 21]. The increased relative risk of small bowel adenocarcinoma in Crohn’s disease has been estimated in population-based studies to range from 17 to 41 compared to the general population [22, 23]. In contrast to sporadic small bowel adenocarcinoma, in Crohn’s disease, this cancer appears in younger patients (fourth decade of life), and mainly in the ileal segment. The cumulative risk is estimated to be 0.2% after 10 years and 2.2% after 25 years of Crohn’s disease evolution [24, 25]. Coeliac disease is characterized by a lymphocytic infiltrate that induces immunological disruption and damage to the epithelial cells that can include premalignant changes and could increase the risk of both small bowel adenocarcinoma and T cell lymphoma. The site of these tumors is mainly jejunal. In a Swedish registry study, the estimated relative risk of small bowel cancer in patients with coeliac disease versus the general population was 10 [26]. The occurrence of small bowel lymphoma could be linked to poor adherence to the gluten-free diet [27].
Important progress has been made during the last decade in the histopathologic characterization of gut neuroendocrine tumors. Changes over time in coding with the introduction of ICD10 codes may partly explain that the increase in neuroendocrine tumors concerned all age classes. Despite the wide distribution of neuroendocrine cells in the normal intestine, some issues like tumor histogenesis and location needs further clarification. Special functional or pathologic conditions as chronic inflammation may locally influence the proliferative and differentiation state of the endocrine cells thus promoting tumor growth [28].
Lymphoma accounted for 14% of small bowel neoplasms in our series and was more frequent in the ileum. Almost 90% of the primary intestinal lymphoma is of B cell lineage with very few T cell lymphoma and Hodgkin lymphoma [29]. Certain histological subtypes have been noted to have a relative predilection site as mantle cell lymphoma in terminal ileum, jejunum, as well as enteropathy-associated T cell lymphoma in jejunum, and follicular lymphoma in duodenum [30]. Certain risk factors have been implicated in the pathogenesis of intestinal lymphoma including microbial pathogens human immunodeficiency virus, Campylobacter jejuni, Epstein–Barr virus, hepatitis B virus, human T cell lymphotropic virus-1, whereas patients with a history of celiac disease or inflammatory bowel disease and immunosuppression are at increased risk of T cell lymphoma [31].
In our study, sarcoma accounted for 12% of small bowel neoplasms. Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. They comprise 85% of all digestive sarcoma followed by leiomyosarcoma, and rarely liposarcoma, fibrosarcoma, Kaposi’s sarcoma, angiosarcoma, and clear cell sarcoma [32]. GIST and other sarcoma may be often incidentally diagnosed on CT scans. The increasing number of imaging techniques may partly explain their increasing incidence.
In conclusion, this study shows that small bowel cancers incidence increased over a 20-year period in France. This increase is in itself important public health information. However, further subtype-specific research is required and may need international multi-disciplinary collaborations to disentangle the respective roles of exposition to causative agents and of improvement in diagnostic techniques in this epidemiological pattern.
References
Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 Varying)—Linked To County Attributes—Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, Released April 2016, Based on the November 2015. https://seer.cancer.gov/statfacts/html/smint.html. Accessed 15 Feb 2019.
Defossez G, Le Guyader-Peyrou S, Uhry Z, et al. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Étude à partir des registres des cancers du réseau Francim. Rapport. Saint-Maurice (Fra): Santé publique France, 2019. 161 p. http://www.santepubliquefrance.fr. Accessed 15 Feb 2019.
Kotz SJN, Read CB. Encyclopedia of statistical sciences. New York: Wiley; 1988.
Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71.
Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42.
Shack LG, Wood HE, Kang JY, et al. Small intestinal cancer in England & Wales and Scotland: time trends in incidence, mortality and survival. Aliment Pharmacol Ther. 2006;23:1297–306.
Sakae H, Kanzaki H, Nasu J, et al. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer. 2017;117:1607–13.
Lepage C, Bouvier AM, Manfredi S, et al. Incidence and management of primary malignant small bowel cancers: a well-defined French population study. Am J Gastroenterol. 2006;101:2826–32.
Goodman MT, Matsuno RK, Shvetsov YB. Racial and ethnic variation in the incidence of small-bowel cancer subtypes in the United States, 1995–2008. Dis Colon Rectum. 2013;56:441–8.
Bernstein H, Bernstein C, Payne CM, et al. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:3329–40.
Delaunoit T, Neczyporenko F, Limburg PJ, et al. Pathogenesis and risk factors of small bowel adenocarcinoma: a colorectal cancer sibling? Am J Gastroenterol. 2005;100:703–10.
Raghav K, Overman MJ. Small bowel adenocarcinomas—existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534–44.
Chow WH, Linet MS, McLaughlin JK, et al. Risk factors for small intestine cancer. Cancer Causes Control. 1993;4:163–9.
Negri E, Bosetti C, La Vecchia C, et al. Risk factors for adenocarcinoma of the small intestine. Int J Cancer. 1999;82:171–4.
Bennett CM, Coleman HG, Veal PG, et al. Lifestyle factors and small intestine adenocarcinoma risk: a systematic review and meta-analysis. Cancer Epidemiol. 2015;39:265–73.
Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97–104.
Watson P, Vasen HFA, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–9.
Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10.
ten Kate GL, Kleibeuker JH, Nagengast FM, et al. Is surveillance of the small bowel indicated for Lynch syndrome families? Gut. 2007;56:1198–201.
Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–2.
Warth A, Kloor M, Schirmacher P, et al. Genetics and epigenetics of small bowel adenocarcinoma: the interactions of CIN, MSI, and CIMP. Mod Pathol. 2011;24:564–70.
Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62.
Jess T, Loftus EV Jr, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from Olmsted county, Minnesota. Gastroenterology. 2006;130:1039–46.
Palascak-Juif V, Bouvier AM, Cosnes J, et al. Small bowel adenocarcinoma in patients with Crohn’s disease compared with small bowel adenocarcinoma de novo. Inflamm Bowel Dis. 2005;11:828–32.
Bojesen RD, Riis LB, Hogdall E, et al. Inflammatory bowel disease and small bowel cancer risk, clinical characteristics, and histopathology: a population-based study. Clin Gastroenterol Hepatol. 2017;15(1900–7):e2.
Askling J, Linet M, Gridley G, et al. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428–35.
Howdle PD, Jalal PK, Holmes GK, et al. Primary small-bowel malignancy in the UK and its association with coeliac disease. QJM. 2003;96:345–53.
Solcia E, Vanoli A. Histogenesis and natural history of gut neuroendocrine tumors: present status. Endocr Pathol. 2014;25:165–70.
Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697–707.
Malamut G, Chandesris O, Verkarre V, et al. Enteropathy associated T cell lymphoma in celiac disease: a large retrospective study. Dig Liver Dis. 2013;45:377–84.
Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19:58–69.
Shenoy S. Small bowel sarcoma: tumor biology and advances in therapeutics. Surg Oncol. 2015;24:136–44.
Acknowledgements
Zoe Uhry (Biostatistique-Bioinformatique, Hospices Civils de Lyon, F-69003, Département des Maladies, Non Transmissible et des Traumatismes, Santé Publique France, France) for maintaining and extracting data from the common FRANCIM database.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflicts of interest to declare regarding this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouvier, AM., Robaszkiewicz, M., Jooste, V. et al. Trends in incidence of small bowel cancer according to histology: a population-based study. J Gastroenterol 55, 181–188 (2020). https://doi.org/10.1007/s00535-019-01636-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01636-z