Abstract
Background
The present study explored the treatment outcome of daclatasvir (DCV) and asunaprevir (ASV) therapy combining oral direct-acting antiviral agents (DAAs) for chronic hepatitis C (HCV) including liver cirrhosis according to resistance-associated variants (RAVs) in NS3/NS5A region.
Methods
Overall, 641 patients enrolled in Japan with HCV-1b received DCV and ASV for 24 weeks. Baseline drug-resistant mutations L31F/I/M/V, Q54H, P58S, A92K, and Y93H in the HCV NS5A region and V36A, T54A/S, Q80K/L/R, R155K/T/Q, A156S/V/T, and D168A/E/H/T/V in the HCV NS3/4A region were assessed by direct sequencing.
Results
Overall, 86.9 % (543/625) of patients had SVR12, which was significantly higher in NS5A 93Y (wild) (88.3 %) compared with NS5A 93H at baseline (48.0 %), indicating the SVR12 rate was significantly lower in patients with 93H mutations. Additionally, 66.7 % (18/27) of patients with prior triple therapy including simeprevir (SMV) failure had virological failure. The virological failure rate of DCV/ASV therapy after SMV failure was significantly higher in those with preexisting NS3/4A 168 substitutions compared with without substitutions at baseline [84.2 % (16/19) vs. 28.6 % (2/7), p = 0.014]. The number of patients with multiple RAVs or deletions in NS5A increased from 0 to 85 % in failed patients. Alanine aminotransferase elevation was a frequent adverse event causing discontinuation of DCV/ASV therapy, although 87.5 % (14/16) patients achieved SVR12, subsequently.
Conclusions
History of SMV therapy and pre-existing NS5A Y93H were associated with virological failure of DCV/ASV therapy, resulting in the emergence of multiple RAVs. Patients with RAVs at baseline should be assessed to optimize future DAA therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An estimated 170 million individuals worldwide are infected with hepatitis C virus (HCV) [1], of whom 1.5–2.0 million reside in Japan. Pegylated-interferon (PEG-IFN), ribavirin (RBV), and protease inhibitors such as telaprevir or simeprevir (SMV) are effective therapies for patients with HCV genotype 1 infection [2, 3], but HCV patients in Japan are not suitable for IFN regimens because there are many older patients as well as LC patients who might develop adverse events caused by PEG-IFN, RBV, or protease inhibitors. IFN-free regimens combining oral direct-acting antiviral agents (DAAs), are available for chronic hepatitis C patients, including those with LC, and IFN-free DAAs have achieved high rates of sustained virological response (SVR), even in LC patients with HCV. Daclatasvir (DCV) is a first-in-class NS5A replication complex inhibitor and asunaprevir (ASV) is a potent selective NS3 protease inhibitor. DCV and ASV became available for clinical use at the end of September 2014 in Japan. These regimens are particularly valuable for IFN intolerant or ineligible patients such as those with depression, LC, or advanced age as well as PEG-IFN/RBV-based regimen non-responders. Although IFN-free DCV/ASV therapy for 24 weeks improved the efficacy and safety outcomes for patients with HCV infection [4], virological responses of the current regimen were influenced by NS5A substitutions at baseline [5]. The aim of the present multicenter observation study in clinical practices was to assess the treatment outcome of DCV/ASV therapy according to resistance-associated variants (RAVs) in NS3/NS5A regions among patients with HCV genotype 1 (HCV-1) and determine the pattern of multiple NS5A RAVs that might affect future DAA therapies.
Materials and methods
Patients
Overall, 641 patients with HCV-1b were enrolled at multiple centers in Japan between September 2014 and October 2015. Treatment algorithms were based on Japanese guideline from JSH (http://www.jsh.or.jp/medical/guidelines/jsh_guidlines/hepatitis_c). According to this guideline, most patients with NS5A RAVs (except for LC patients, etc.) avoided the treatments of DCV/ASV due to a lower SVR rate and waited second-generation DAAs therapy. Exclusion criteria were patients with (1) clinical or biochemical evidence of decompensated cirrhosis (Child–Pugh classification B or C) and (2) the presence of HCC. The diagnosis of fibrosis was based on liver histopathology, and/or pathognomonic results utilizing ultrasound or computed tomography (CT). Patients received DCV and ASV for 24 weeks and were followed for an additional 12 weeks after treatment. DCV was administered orally at a dose of 60 mg once daily, and ASV was administered orally at a dose of 100 mg twice daily. The study protocol was approved by the Ethics Committees of the participating institutions in accordance with the 1975 Declaration in Helsinki and informed consent was obtained from each subject.
Laboratory investigations
Blood samples were obtained at baseline, at 1- or 2-week intervals from weeks 1 to 12, at 4-week intervals thereafter until the end of treatment, and then at 4-week intervals until 12 weeks after the end of treatment.
During DCV/ASV treatment, virological responses were defined as follows: sustained virological response (SVR) 12, HCV RNA undetectable at week 12 after the end of therapy; rapid virological response (RVR), HCV RNA undetectable at week 4 after the start of therapy, end of treatment response (ETR), and HCV RNA undetectable at end of therapy, using the Roche COBAS TaqMan HCV test (Roche Diagnostics, Tokyo, Japan). The dynamic range of this assay is 1.2–7.8 log IU/ml. A breakthrough was defined as a confirmed value greater than 1 log10 increase in HCV RNA over the nadir, or confirmed HCV RNA values less than the lower limit of quantitation (LLOQ). The IL28B genotype (rs8099917) was determined by TaqMan PCR using the ABI TaqMan allelic discrimination kit (7500 Real-Time PCR System; Applied Biosystems, Carlsbad, CA, USA).
Drug resistance testing
The drug-resistant mutations V36A, T54A/S, Q80K/L/R, R155K/T/Q, A156S/V/T, and D168A/E/H/T/V variant in the HCV NS3/4A region and L31F/I/M/V, Q54H, P58S, A92K, and Y93H in the HCV NS5A region were assessed by direct sequencing as shown in supplementary text.
Statistical analysis
Categorical variables were compared between groups by the χ 2-test or Fisher’s exact test and non-categorical variables by the Mann–Whitney U test. Variables with p < 0.05 in univariate analysis were evaluated using multivariate logistic regression to identify variables significantly associated with SVR12. The results are expressed as odds ratios (OR) and their 95 % confidence interval (CI). A value of p < 0.05 was considered statistically significant. Statistical analysis was performed with SPSSver.20 and Windows Excel 2010 software.
Results
Baseline characteristics of patients
Demographic baseline characteristics of patients are shown in Table 1. The median age of the patients (279 men and 362 women) was 71 years (range, 33–87 years). Of the 641 patients, 425 (66.3 %) were CH, 216 (33.7 %) were LC, 52.4 % (336/641) patients were treatment-naive and 38 had a history of PEG-IFN + RBV + PI treatment (including 27 with SMV therapy). Baseline polymorphisms at amino acid positions associated with resistance are shown in Table 1. In brief, 595 patients had no NS5A substitutions at baseline, but eight patients (1.2 %) with L31 M/V substitution and 25 patients (4.0 %) with Y93H substitution at baseline were initiated for treatment. Compared with the CH and LC group, there was no significant difference of baseline NS5A RAVs, but higher tendency was found in LC compared with CH [8.1 % (17/209) vs. 3.8 % (16/419), p = 0.069] (Table 1). Baseline characteristics of IFN-naive and IFN treatment-experienced patients were also shown in supplementary Table S1. IL28B favorable genotype, AFP and past HCC were higher in IFN treatment-experienced patients than IFN-naive patients.
Efficacy of antiviral therapy
The flowchart shows the process of the analysis (supplementary Figure S1). Overall, 641 patients started DCV/ASV therapy. Forty-three (6.7 %) patients stopped DCV/ASV treatment because of adverse events and 40 patients had virological failure. Overall, 75.7 % (485/641) patients had RVR. ETR, SVR4, SVR8, and SVR12 were achieved by 92.4 % (592/641), 89.5 % (574/641), 88.6 % (568/641), and 86.9 % (543/625) patients, respectively in the entire cohort (Fig. 1). The virological response rates were similar between CH and LC patients: 93.2 % (396/425) vs. 91.2 % (197/216) in ETR, 90.4 % (384/425) vs. 88.0 % (190/216) in SVR4, 89.9 % (382/425) vs. 86.0 % (186/216) in SVR8, and 88.1 % (364/413) vs. 84.4 % (179/212) in SVR12, respectively. Although RVR were higher in CH patients than in LC patients [78.1 % (332/425) vs. 70.8 % (153/216), p = 0.042]. No differences were observed for the undetectable HCV RNA rate between CH and LC (supplementary Figure S2).
HCV-RNA undetectable rates with and without NS5A 93H mutation at baseline. Overall, RVR, ETR, SVR4, SVR8, and SVR12 were achieved by 75.7, 92.4, 89.5, 88.6, and 86.9 % patients, respectively. For patients with NS5A 93Y at baseline, the percentage of undetectable HCV-RNA rates at week 4, ETR, SVR4, SVR8, and SVR12 were 77.1, 93.4, 90.7, 89.9, and 88.3 %, respectively. In contrast, patients with NS5A 93H at baseline, the percentage of undetectable HCV-RNA rates were 56.0, 68.0, 56.0, 52.0, and 48.0 %, respectively. SVR12 was significantly higher in NS5A 93Y (wild) compared with NS5A 93H at baseline. *Baseline NS5A 93 substitution could not be assessed in 13 patients
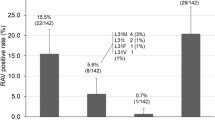
Resistance-associated variants (RAVs) at baseline and at time of failure of DCV/ASV therapy. Changes of RAVs in 52 failed patients were examined at baseline and at the time of failure of DCV/ASV therapy (including prior SMV therapy). The number of RAVs (0, 1, 2, 3) were 87, 13, 0, and 0 % at baseline, respectively, but were 9, 6, 52, and 27 %, respectively, and 6 % of deletion (29 or 32del) at the time of virological failure. Patients with multiple RAVs or deletion increased from 0 to 85 %
Next, univariate and multivariate analyses were performed to predict SVR12 by DCV/ASV therapy. Multivariate analysis showed that significant independent factors associated with SVR12 were previous triple therapy with simeprevir (OR 27.1; 95 % CI 8.64–84.9; p < 0.0001), NS5A 93H mutation at baseline (OR 6.21; 95 % CI 2.06–8.69; p = 0.0011), and non-RVR (OR 4.39; 95 % CI 2.22–8.69; p = 0.0002), respectively (Table 2).
Virological response rates with and without NS5A 93H mutation at baseline
Among patients with a NS5A 93Y at baseline, the percentage of undetectable HCV-RNA rates at week 4, ETR, SVR4, SVR8, and SVR12 were 77.1 % (465/603), 93.4 % (563/603), 90.7 % (547/603), 89.9 % (542/603), and 88.3 % (521/590), respectively. In contrast, among patients with NS5A 93H at baseline, the percentage of undetectable HCV-RNA rates at week 4, ETR, SVR4, SVR8, and SVR12 were 56.0 % (14/25), 68.0 % (17/25), 56.0 % (14/25), 52.0 % (13/25), and 48.0 % (12/25), respectively (Fig. 1). These results suggested that the SVR12 rate was significantly lower in patients with a 93H mutation.
Resistance-associated variants associated with virological failure of DCV/ASV therapy
Changes of RAVs in 52 failed patients were examined at baseline and at the time of failure of DCV/ASV therapy (including prior SMV therapy). Both NS5A and NS3/4A RAVs were detected in 40 patients with virological failure. The number of NS5A RAVs (0, 1, 2, 3) were 87, 13, 0, and 0 % at baseline, respectively, and were 9, 6, 52, and 27 %, respectively, and 6 % of deletion (del) at the time of virological failure. The number of patients with multiple RAVs or deletion increased from 0 to 85 %. Forty-one patients (79 %), one 29del patient (case 1), and two 32del patients (case 9, case 30) had more than 2 RAVs resistant to daclatasvir or Ledipasvir (LDV). Furthermore, 2 RAVs and a 32del emerged in one patient (case 31). Five patients had neither NS5A 31 nor 93 substitution after DCV/ASV failure. DCV/ASV treatment was stopped in two of five patients within 2 weeks because of severe eruptions (case 26, case 51), and other patient values were estimated using serum samples from 6 months after DCV/ASV failure (case 8, case 16, and case 39) (Fig. 2; supplementary Table S2).
NS3/4A 168 substitution pre-existing DCV/ASV therapy in the SMV failure group. NS3A 168 substitutions were assessed in 26 SMV failure patients before starting with DCV/ASV. Overall, 84.2 % (16/19) of patients with NS3/4A 168V/E/T mutations before starting DCV/ASV therapy had virologic failure, although 71.4 % (5/7) of patients with NS3/4A 168D achieved SVR12. The treatment outcome of DCV/ASV therapy was significantly different between those with and without preexisting NS3/4A 168 substitution after SMV failure (p = 0.014). *One of 27 patients could not be assessed
Other factors related to virological failure of DCV/ASV therapy
As shown in Fig. 3, among patients with prior triple therapy including SMV failure 66.7 % (18/27) had virological failure. The median period of starting DCV/ASV therapy after previous Peg-IFN/RBV/SMV treatment was 113 days (range, 21–561 days). NS3/4A 168 substitution was analyzed in 26 patients before starting DCV/ASV therapy. Sixteen (84.2 %) of 19 patients with NS3/4A 168V/E/T substitutions before starting DCV/ASV therapy had virological failure, although 71.4 % (5/7) patients with NS3/4A 168D (wild type) achieved SVR12. The treatment outcome of DCV/ASV therapy was significantly different between those with and without preexisting NS3/4A 168 substitutions after SMV failure (p = 0.014) (Fig. 4), although the treatment outcome was not related to the duration of SMV failure to the start of DCV/ASV therapy.
In addition, nine patients had genotype 2 or 3 despite being diagnosed with serotype 1 before DCV/ASV treatment. Another patient was diagnosed as genotype 1a. HCV-RNA levels decreased by 2–4 log IU/ml after 2 or 4 weeks of treatment, but immediately showed virological breakthrough. HCV-RNA have been detectable during the treatment in most genotype 2 patients (data not shown). After breakthrough, NS5A Q30E and NS3 Q80K, D168E were detected at the point of failure in genotype 1a patient.
Changes of liver function during DCV/ASV treatment
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels showed rapid improvement in most patients during the first 2–4 weeks of treatment. The AST and ALT levels were 46 (range, 11–298) and 39 (range, 6–352) at baseline, but were 24 (range, 4–186) and 17 (range, 4–195) at week 4, respectively. A significant reduction of both AST and ALT levels was observed at week 4 (p < 0.01), although some patients had an ALT flare-up during DCV/ASV therapy (Fig. 5). Additionally, AFP levels were significantly reduced during baseline and at 4 weeks of treatment [6.0 (range, 1–214.6) vs. 5.1 (range, 0.9–70.1) ng/ml] (p < 0.01).
Changes of AST and ALT levels during DCV/ASV treatment. Median AST: 46 (range, 11–298) at baseline, 24 (range, 4–186) at week 4. Median ALT: 39 (range, 6–352) at baseline, 17 (range, 4–195) at week 4. A significant reduction was observed at week 4 both ASL and ALT (p < 0.01). Sixteen cases stopped treatment because of grade 3 ALT elevation, and ALT normalized immediately after cessation
Adverse events leading to discontinuation
Overall, 6.7 % (43/641) patients discontinued the treatment because of adverse events. Sixteen patients (2.5 %) discontinued after 6–20 weeks of treatment because of ALT and AST elevations. All 16 cases stopped treatment because of grade 3 ALT elevations that were normalized immediately after cessation, and 14 of these (87.5 %) subsequently achieved SVR12. Fourteen cases continued treatment by a dose reduction of ASV to 100 mg/day, but two patients (No 7 and No 8) did not resolve despite the dose reduction of ASV and subsequently stopped the DCV/ASV therapy (Fig. 5 and supplementary Table S3). Twelve of 14 (85.7 %) patients with a dose reduction of ASV achieved SVR.
Three patients discontinued because of rash and high fever and four patients because of hepatic encephalopathy. Five patients developed HCC, six developed cardiovascular diseases and one developed ageusia. All patients who stopped treatment because of high fever, but not rash, achieved SVR12.
PEG-IFN and RBV therapy immediately after DCV/ASV failure
Three patients received additional PEG-IFN/RBV therapy immediately after DCV/ASV failure. All the patients were female, IFN treatment-naive and IL28B TT genotype (rs8099917). Because these three patients were old and had advanced fibrosis (70 years LC, 71 years CH, 65 years LC), we decided to start PEG-IFN/RBV therapy during low viral loads (1.9, 2.3, and 2.6 log IU/ml) after DCV/ASV failure. PEG-IFN-α2a (180 μg once a week) plus RBV (600–1000 mg daily according to body weight) were applied for 24 weeks. The doses of drugs were reduced according to the recommendations on the package inserts or the clinical conditions of the individual patients. HCV-RNA of all patients was undetectable within 4 weeks of treatment with PEG-IFN/RBV therapy, resulting in SVR12.
Discussion
In this study, baseline viral mutations conferring resistance to the NS5A replication complex inhibitor DCV were investigated by direct sequencing and the treatment outcome of DCV/ASV therapy was assessed according to RAVs in NS3/NS5A regions among patients with HCV-1. The SVR12 rate was significantly higher in the NS5A 93Y (wild) group (88.3 %) compared with the NS5A 93H group at baseline (48.0 %). After virological failure of DCV/ASV, patients with multiple NS5A RAVs and/or deletion (29del or 32del) that emerged at failure point, or were reported to be highly resistant to DCV or LDV [5, 6], increased from 0 to 85 %. Especially, because L31M-Q54H-Y93H in NS5A acquired both high replication levels and high an EC50 [7], such a clone might remain in patients resulting in poor treatment outcomes of future regimens including NS5A inhibitors. The frequency of 31V + 93H was the highest (30.8 %), followed by 31V + 54H/Y + 93H (19.2 %) and 31M + 93H (11.5 %). As these HCV strains with double or triple RAVs had much higher EC50 than that of single RAV in the HCV replicon system, our data support multiple RAVs are important for the efficacy of DCV/ASV therapy. For the other RAVs by DCV/ASV failure, NS5A 31F-93H/Y and 32del emerged in one patient, and those with NS5A 32 del had a high resistance for DCV (>390,000-fold) by in vitro replicon analysis. However, because 32del-93H did not replicate [8], both the P32del-Y93H and L31F-P32del strains appeared to be mixed in this patient. One patient showed the emergence of a NS5A 31I/M-A92K substitution, a rare mutation in DCV failure [9]. The prevalence of NS5A A92 K, R30Q, and Q54H variants at baseline were 7.4, 12.8, and 43.9 %, respectively, in Japanese patients [10]. These mutations by themselves do not affect DCV [9, 11], although R30Q and Q54H enhance the resistance phenotype of L31F mutants and Y93H, respectively [6].
Recently, an HCV NS5B nucleotide polymerase inhibitor, sofosbuvir (SOF), was approved in Japan. Clinical trials of SOF and LDV have been reported in patients with and without NS5A 93H substitution and have shown a high SVR rate, about 90 %, with only 12 weeks of therapy [12, 13]. However, the effect of SOF/LDV and future DAA treatments are unclear for drug resistance strains with multiple NS5A RAVs caused by DCV/ASV treatment failure. A recent in vivo study using chimeric mice with human hepatocytes showed that NS5A L31V plus Y93H variant upon treatment failure with DCV/ASV treatment was resistant to LDV [14], suggesting that the treatment response of SOF/LDV might be limited for patients with DCV/ASV failure.
For three patients with possible multiple NS5A RAVs caused by DCV/ASV treatment failure in this cohort, an additional PEG-IFN/RBV therapy was started immediately after DCV/ASV failure during low viral levels. Although NS5A Y93H substitution strains are more susceptible than wild-type strains, the mechanism involved is unclear. Previous studies revealed that Y93H RAV was less frequently detected in patients with the unfavorable IL28B genotype non-TT (rs8099917) [15], and Y93H in the NS5A region was linked to the IL28B TT genotype [16]. By starting PEG-IFN/RBV therapy under a low HCV-RNA load, patients with the IL28B TT genotype may achieve SVR, suggesting that PEG-IFN/RBV therapy is still a beneficial option for patients with DCV/ASV failure.
Because baseline ASV RAVs and NS3-D168 substitutions were rare [17], the baseline NS3 substitution was not examined at baseline in this cohort. However, in cases of treatment failure, baseline NS3-D168E and/or Q80K were prevalent with prior SMV treatment [18, 19]. Recently, Kan et al. reported that NS3-D168 showed low susceptibility to ASV treatment and failed to respond to DCV/ASV therapy in human hepatocyte chimeric mice [20]. NS3-168D substitutions generally decayed during off-drug follow-up periods, implying a lack of replicative fitness relative to the wild type in the absence of selective drug pressure [17, 21]. Although minor spices were detectable by deep sequence analysis after being undetectable by population sequences, it is not clear whether these minority species of substitutions effect the re-treatment by NS3 protease inhibitors [21, 22]. In this study, the treatment outcome of DCV/ASV therapy was significantly lower in those with preexisting NS3/4A 168 substitutions after SMV failure regardless of no NS5A substitutions at baseline, suggesting that DCV/ASV treatment should be avoided after SMV failure until NS3-168 substitutions are not undetectable by population sequences.
The rate of discontinuation of treatment with DCV/ASV because of adverse events was low; however elevated ALT and AST levels were the most frequent adverse events similar to previous reports [23, 24]. The causes of discontinuation for seven available patients were serum ASV concentrations and percentage of eosinophils 4 weeks before ALT elevation and ALT peak. Increased ASV concentrations or eosinophilia were reported in seven patients with ALT elevation during DCV/ASV therapy. Despite early cessation until 12 weeks, 14 of 16 patients who discontinued because of elevated levels of ALT and AST achieved SVR. A high concentration of ASV might be associated with ALT elevation [23]. Some patients continued DCV/ASV therapy and showed ALT improvement by a dose reduction of ASV, but the patients with ALT elevation caused by allergic factors (eosinophil elevation) [25] might be unable to continue the treatment.
ALT and AFP were rapidly improved by DCV/ASV treatment. Although HCV eradication with IFN-based therapy has been shown to prevent HCC [26, 27], it remains unknown whether the incidence of HCC can be reduced in patients who achieved SVR by IFN-free DAA combination therapy because of the relatively short duration of treatment and no evidence of anti-tumor effects by IFN therapy. Additionally, we have to treat patients with a high risk of HCC development such as the elderly and those with advanced fibrosis [28].
There were several limitations to this study. First, the observation period did not reach 12 weeks post-treatment follow-up in some patients. Second, baseline NS3/4A substitution has not been examined in this cohort except for those patients with previous SMV therapy. Baseline ASV RAVs, and NS3-D168 substitutions were rare [17]. According to a previous report, there was no apparent association between preexisting NS3 resistance-associated polymorphisms [4]. Third, the SVR12 rate among patients without Y93H mutation was relatively lower than that of a previous study [4]. One reason for this might be preexisting NS3-D168 substitutions after SMV failure. Our treatment algorithms were based on Japanese guidelines from JSH (http://www.jsh.or.jp/medical/guidelines/jsh_guidlines/hepatitis_c), but prior treatment-failed patients of SMV administered DCV/ASV therapy because there had not been evidence in 2014. Another reason might be the mismatch of HCV genotypes. Because HCV genotyping assay is not commercialized under medical service health insurance in Japan, many patients were started with DCV/ASV treatment by checking only HCV serotype. Fourth, there is substantial inclusion bias in this study. Most patients with NS5A RAVs avoided the treatments of DCV/ASV based on Japanese guidelines from JSH, though some patients with NS5A 93H were started with DCV/ASV therapy due to some risk factors for HCC development or decompensated cirrhosis. Fifth, in some cases we could not examine NS5A RAVs after DCV/ASV treatment failure. The reasons for this are a lack of serum samples after DCV/ASV failure, and some patients were started with PEG-IFN/RBV therapy under a low viral load. Finally, the investigation of the cause of elevated ALT (the main side effect of DCV/ASV therapy) was based on a small number of samples. Therefore, further examination with a large number of samples is required.
In conclusion, a history of SMV therapy and pre-existing NS5A Y93H were associated with virological failure during DCV/ASV therapy, resulting in the emergence of multiple RAVs. The treatment response of SOF/LDV might be limited for patients with DCV/ASV failure. Therefore, patients with RAVs at baseline should be required to undergo assessment to optimize future DAA therapies.
Abbreviations
- ASV:
-
Asunaprevir
- DAA:
-
Direct-acting antiviral agent
- DCV:
-
Daclatasvir
- HCV:
-
Hepatitis C virus
- IFN:
-
Interferon
- NS3:
-
Non-structural protein 3
- NS5A:
-
Non-structural protein 5A
- PCR:
-
Polymerase chain reaction
- RAV:
-
Resistance-associated variant
- SMV:
-
Simeprevir
- SNP:
-
Single nucleotide polymorphism
- SVR:
-
Sustained virological responses
References
Lavanchy D. The global burden of hepatitis C. Liver Int Off J Int Assoc Study Liver. 2009;29(Suppl 1):74–81.
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New Engl J Med. 2011;364(25):2405–16.
Ogawa E, Furusyo N, Dohmen K, et al. Effectiveness of triple therapy with simeprevir for chronic hepatitis C genotype 1b patients with prior telaprevir failure. J Viral Hepat. 2015;22(12):992–1001.
Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59(6):2083–91.
McPhee F, Hernandez D, Yu F, et al. Resistance analysis of hepatitis C virus genotype 1 prior treatment null responders receiving daclatasvir and asunaprevir. Hepatology. 2013;58(3):902–11.
Fridell RA, Qiu D, Wang C, et al. Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system. Antimicrob Agents Chemother. 2010;54(9):3641–50.
Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59(2):375–82.
Wang C, Sun JH, O’Boyle DR 2nd, et al. Persistence of resistant variants in hepatitis C virus-infected patients treated with the NS5A replication complex inhibitor daclatasvir. Antimicrob Agents Chemother. 2013;57(5):2054–65.
McPhee F, Hernandez D, Zhou N, et al. Virological escape in HCV genotype-1-infected patients receiving daclatasvir plus ribavirin and peginterferon alfa-2a or alfa-2b. Antivir Ther. 2014;19(5):479–90.
Krishnan P, Schnell G, Tripathi R, et al. Analysis of HCV genotype 1b resistance variants in Japanese patients treated with paritaprevir/ritonavir and ombitasvir. Antimicrob Agents Chemother. 2015;. doi:10.1128/AAC.02606-15.
McPhee F, Suzuki Y, Toyota J, et al. High sustained virologic response to daclatasvir plus asunaprevir in elderly and cirrhotic patients with hepatitis C virus genotype 1b without baseline NS5A polymorphisms. Adv Ther. 2015;32(7):637–49.
Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New Engl J Med. 2014;370(20):1879–88.
Kanda T, Nakamoto S, Nakamura M, et al. Direct-acting antiviral agents for the treatment of chronic hepatitis C virus infection. J Clin Transl Hepatol. 2014;2(1):1–6.
Kai Y, Hikita H, Tatsumi T, et al. Emergence of hepatitis C virus NS5A L31 V plus Y93H variant upon treatment failure of daclatasvir and asunaprevir is relatively resistant to ledipasvir and NS5B polymerase nucleotide inhibitor GS-558093 in human hepatocyte chimeric mice. J Gastroenterol. 2015;50(11):1145–51.
Itakura J, Kurosaki M, Higuchi M, et al. Resistance-associated NS5A variants of hepatitis C virus are susceptible to interferon-based therapy. PLoS One. 2015;10(9):e0138060.
Maekawa S, Sakamoto M, Miura M, et al. Comprehensive analysis for viral elements and interleukin-28B polymorphisms in response to pegylated interferon plus ribavirin therapy in hepatitis C virus 1B infection. Hepatology. 2012;56(5):1611–21.
McPhee F, Friborg J, Levine S, et al. Resistance analysis of the hepatitis C virus NS3 protease inhibitor asunaprevir. Antimicrob Agents Chemother. 2012;56(7):3670–81.
Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384(9941):403–13.
Lenz O, Verbinnen T, Lin TI, et al. In vitro resistance profile of the hepatitis C virus NS3/4A protease inhibitor TMC435. Antimicrob Agents Chemother. 2010;54(5):1878–87.
Kan H, Hiraga N, Imamura M, et al. Combination therapies with daclatasvir and asunaprevir on NS3-D168 mutated HCV in human hepatocyte chimeric mice. Antivir Ther. 2015;. doi:10.3851/IMP3009.
Karino Y, Toyota J, Ikeda K, et al. Characterization of virologic escape in hepatitis C virus genotype 1b patients treated with the direct-acting antivirals daclatasvir and asunaprevir. J Hepatol. 2013;58(4):646–54.
Lenz O, de Bruijne J, Vijgen L, et al. Efficacy of re-treatment with TMC435 as combination therapy in hepatitis C virus-infected patients following TMC435 monotherapy. Gastroenterology. 2012;143(5):1176-8; e1-6.
Akuta N, Sezaki H, Suzuki F, et al. Relationships between serum asunaprevir concentration and alanine aminotransferase elevation during daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. J Med Virol. 2015;. doi:10.1002/jmv.24360.
Kumada H, Suzuki F, Suzuki Y, et al. Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese HCV patients. J Gastroenterol Hepatol. 2015;. doi:10.1111/jgh.13073.
Fujii Y, Uchida Y, Mochida S. Drug-induced immunoallergic hepatitis during combination therapy with daclatasvir and asunaprevir. Hepatology. 2015;61(1):400–1.
Shiffman ML, Hofmann CM, Contos MJ, et al. A randomized, controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117(5):1164–72.
Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29(4):1124–30.
Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52(2):518–27.
Acknowledgments
The authors would like to thank Shintaro Ogawa, Kayoko Matsunami, and Shuko Murakami of Nagoya City University Graduate School of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yasuhito Tanaka is currently conducting research sponsored by Merck Sharp & Dohme, Corp., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb, and AbbVie Inc. The other authors declare no conflicts of interest.
Financial support
This research was (partially) supported by the Research Program on Hepatitis from Japan Agency for Medical Research and development, AMED.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iio, E., Shimada, N., Abe, H. et al. Efficacy of daclatasvir/asunaprevir according to resistance-associated variants in chronic hepatitis C with genotype 1. J Gastroenterol 52, 94–103 (2017). https://doi.org/10.1007/s00535-016-1225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1225-x