Abstract
Background
Helicobacter pylori induces chronic inflammation and intestinal metaplasia (IM) through genetic and epigenetic changes and activation of intracellular signaling pathways that contribute to gastric carcinogenesis. However, the precise mechanism of IM in gastric carcinogenesis has not been fully elucidated. We previously found that intestine-specific homeobox (ISX) mRNA expression increased in organoids cultured from Helicobacter-infected mouse mucosa. In this study, we elucidate the role of ISX in the development of IM and gastric carcinogenesis.
Methods
ISX expression was assessed in Helicobacter-infected mouse and human gastric mucosa. MKN45 gastric cancer cells were co-cultured with H. pylori to determine whether Helicobacter infection induced ISX expression. We established stable MKN45 transfected cells expressing ISX (Stable-ISX MKN45) and performed a spheroid colony formation assay and a xenograft model. We performed ISX immunohistochemistry in cancer and adjacent gastric tissues.
Results
ISX expression was increased in mouse and human gastric mucosa infected with Helicobacter. The presence of IM and H. pylori infection in human stomach was correlated with ISX expression. H. pylori induced ISX mRNA and protein expression. CDX1/2, cyclinD1, and MUC2 were upregulated in Stable-ISX MKN45, whereas MUC5AC was downregulated. Stable-ISX MKN45 cells formed more spheroid colonies, and had high tumorigenic ability. ISX expression in gastric cancer and adjacent mucosa were correlated.
Conclusions
ISX expression induced by H. pylori infection may lead to IM and hyperproliferation of gastric mucosa through CDX1/2 and cyclinD1 expression, contributing to gastric carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori is classified as a class 1 carcinogen by IARC. H. pylori infection in the stomach results in gastric inflammation, which leads to stepwise changes, such as chronic gastritis, intestinal metaplasia, and gastric cancer [1]. Gastric cancer is the third leading cause of cancer-related deaths worldwide; thus, therapies targeting this cancer are needed [2]. H. pylori-induced signaling pathways contribute to intestinal metaplasia and gastric carcinogenesis [3–5]. CDX1/2 is a well-known intestine-specific homeobox gene, and CDX transgenic mice develop gastric cancer, suggesting that CDX plays a key role in intestinal metaplasia and carcinogenesis [6]. Although eradication for H. pylori is now covered by national health insurance in Japan, the reduction of cancer risk after eradication seems to be limited to 75 % of “ideal” cases (observed risk reduction was about 40 %) [7, 8]. The relationship between CDX expression and gastric carcinogenesis has been called irreversible “field cancerization”, in which the progression of carcinogenesis will not stop [9], and “field cancerization” may become a target for the development of novel therapeutics in gastric cancer.
We previously found that ISX mRNA expression increased in organoids cultured from Helicobacter-infected mouse gastric mucosa (unpublished observation). ISX is a 245-amino-acid protein located in the nucleus that contains one homeobox DNA-binding domain and maps to human chromosome 22. ISX shows an intestine-specific expression pattern in adult and fetal intestine [10]. The homeodomain transcription factor plays a critical role in differentiation, proliferation, and organogenesis [11]; however, ISX has not been associated with these roles in intestine [12, 13]. ISX is an important regulator of hepatocellular carcinoma (HCC) progression with significant potential as a prognostic and therapeutic target in HCC [14]. ISX expression enhances the proliferation of HCC by directly binding to cyclinD1 promoter in vitro and in vivo. Since we have previously found the increase of ISX expression in Helicobacter-infected metaplastic mucosa (unpublished observation), we hypothesized that ISX in gastric cancer cells was an important regulator of gastric cancer progression with significant potential as a prognostic and therapeutic target.
Recent evidence in vivo suggests that tumors originate from cancer stem cells [15, 16]. Cancer stem cells are defined as “cells within a tumor that possesses the capacity for self-renewal and that can cause the heterogeneous lineages of cancer cells that constitute the tumor” [17]. Several studies have demonstrated possible interactions among H. pylori, stem cells, and gastric cancer [18–21]. Other studies suggest that cancer stem cells exist in gastric cancer and that these cells possibly originated from resident stem cells, differentiated epithelial cells, or stem cells derived from bone marrow-derived cells [22, 23]. CD44 was proposed as a marker for gastric cancer stem cells and for gastric tissue stem cells [24]. Takaishi et al. reported that gastric antral stem cells could be the origin of some types of gastric cancer [25]. Wada et al. suggested that CD44 expression in a mouse model promoted the survival and proliferation of basal cryptic progenitor-like cells that gave rise to spasmolytic polypeptide-expressing metaplasia, resulting in progression of the metaplasia-carcinoma sequence in the stomach [26]. Our previous observation on organoids from mouse gastric mucosa infected with Helicobacter suggested that ISX expression was restricted to stem or progenitor cells. Thus we hypothesized that H. pylori-induced ISX expression in stem/progenitor cells contributed to the progression of cancer stem cells.
The aim of this study was to investigate our hypothesis that H. pylori-induced ISX expression contributed to intestinal metaplasia, cell proliferation, and the acquisition of cancer stem cell characteristics, resulting in gastric carcinogenesis.
Methods
Patients
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee/Institutional Review Board of Yokohama City University Hospital, Japan (no. B130307022). The subjects gave written informed consent and patient anonymity was preserved. We used 12 endoscopic gastric biopsy samples from 12 patients with gastritis and 28 patients with early gastric cancer resected by endoscopic submucosal dissection. Patients with gastritis were recruited consecutively at Yokohama City University Hospital, Kanagawa, Japan. Two biopsy specimens were taken from greater curvature of pyloric zone. One biopsy sample was immediately stored in RNAlater (Qiagen, Hilden, Germany) at −20 °C until mRNA measurements. The other biopsy sample was fixed with 10 % neutral formalin and embedded in paraffin for immunohistochemical staining.
Gastric cancer tissue microarray
We used tissue microarrays for immunohistochemical analysis in advanced gastric cancer, consisting of 55 cases/100 cores of gastric adenocarcinoma with matched adjacent tissue and normal tissue. Pathological diagnosis was defined as manufacturer’s instructions (BC01114, US Biomax, Rockville, MD, USA).
Chronic Helicobacter felis infection model
Helicobacter felis (H. felis) strain (ATCC 49179) used in this study has been described previously [27]. Mice were inoculated with H. felis or with sterile broth as a control. Inocula (0.2 ml H. felis, 1010 colony-forming units/ml) were delivered by oral gavage three times per week using a sterile gavage needle. Infection status was confirmed by gastric antrum histology. Animals were killed at 6 and 12 months post-infection by CO2 asphyxiation and necropsied as described previously [27].
Reagents
Recombinant human tumor necrosis factor (TNF)-α (10 ng/ml) and interleukin (IL)-1β (10 ng/ml) were used to stimulate the cells (R&D Systems, Minneapolis, MN, USA). We used well-characterized inhibitor NEMO-binding domain (NBD) peptide as a nuclear factor kappa B (NF-κB) signaling inhibitor, as described previously [28].
Establishing ISX stable transfectant cells and ISX knockdown cells
To establish gastric cells that stably express ISX, MKN45 cells were transfected with myc-tagged human ISX (ORIGENE, Rockville, MD, USA). To establish the ISX knockdown cells, MKN-74 cells were transfected with pEGFP-H1/ISX short hairpin RNA interference vector (shISX vector), which was kindly provided by Shin-Hsien Hsu [14]. All plasmid transfections were performed with X-tremeGENE HP DNA transfection reagent (Roche, Basel, Switzerland). These transfectants were cultured with 150 μg/ml G418 at least 3 weeks, and selected clones were subjected to immunoblot analysis with anti-myc antibody (Cell Signaling Technology, Danvers, MA, USA) or anti-ISX antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). GFP fluorescence observation was also used to confirm the presence of transgene (Stable-shISX MKN74).
Luciferase assay
MKN45 were seeded in six-well plates and cultured under standard conditions. When about 80 % confluent, cells were transfected with DNA mix in 200 μl of Opti-MEM media (ThermoFisher Scientific, Waltham, MA, USA), free of antibiotic and antimycotic. In all, 0.2 μg of reporter plasmid, pGL3-[−760 ± 261]CDX2-Luc (kindly provided by Makoto Saegusa [29]) was used for cell transfection with X-tremeGENE HP DNA transfection reagent (Roche). Cells were co-transfected with 0.8 μg of either human ISX vector (ORIGENE), human CDX2 vector (ORIGENE), or the corresponding empty vector and also of the 0.01 μg Renilla luciferase reporter vector. Twenty-four hours post-transfection, total extracts were prepared using the Dual-Luciferase Reporter Assay System Kit (TOYO INK, Tokyo, Japan) according to the manufacturer’s instructions, and luciferase activity was measured in Luminometer Model TD-20/20 (Promega, Madison, WI, USA). Each experiment was carried out at least three times. Results were expressed as mean ± SD and were expressed as fold induction compared with values obtained for the empty vector.
CDX2 knockdown experiments
MKN45 cells were seeded in six-well plates and at about 80 % confluence, cells were transfected with a mix of three double-stranded small interfering RNA (siRNA) directed to different sequences of CDX2 mRNA (siCDX2) (Santa Cruz Biotechnology) or scrambled controls in a total concentration of 40 nM. The siRNA duplexes were used in a ratio of 1:10 relatively to X-tremeGENE siRNA Transfection Reagent (Roche).
Spheroid colony formation assay
Cells were inoculated into each well (10 cells or 25 cells/well) of ultra-low-attachment 96-well plates (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 100 μl serum-free RPMI-1640 medium (ThermoFisher Scientific), 10 mM HEPES, 20 ng/ml human recombinant epidermal growth factor (ThermoFisher Scientific), and 10 ng/ml human recombinant basic fibroblast growth factor (ThermoFisher Scientific). All wells were examined under a light microscope after 2 weeks. The visualized spheroid bodies were dissociated and seeded in each of the wells, as described above, and the wells were examined again 2 weeks later. Images of the spheroid colonies were recorded using a light microscope (Leica Microsystems, Wetzlar, Germany) and a 600ES-CU CCD camera (Pixera Corp. Santa Clara, CA, USA). The images were converted to JPEG files using Pixera viewfinder 3.0.1. Areas of emerging spheroid colonies were assessed with the public domain Java image-processing software ImageJ 1.48. (http://rsb.info.nih.gov/ij/index.html).
In vivo anti-tumor study (xenograft model)
Approximately 107 stably expressing ISX gastric cancer cells (Stable-ISX MKN45) and parent MKN45 cells in the logarithmic growth phase were suspended in 0.2 ml phosphate-buffered saline and injected subcutaneously into the flanks of immunodeficient BALB/c Nude mice (Charles River Laboratories Japan, Yokohama, Japan) (n = 8 each). Tumor diameters were measured weekly using electronic calipers and converted to volume (V) using the formula V = d × d × d. Tumor weights were also measured at 5 weeks.
Statistical analysis
Data were analyzed using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA) and expressed as mean ± standard error. Student’s t test was used to detect significant differences between two groups. Multiple comparisons were performed with one-way analysis of variance. Spearman’s ρ and Kendall’s τ were used to assess correlations. A value of p < 0.05 was considered to indicate significance.
All protocols for culturing H. pylori (TN2 [30] and TN2-ΔcagPAI [31] at MOI of 100), cell culture (MKN-45 and MKN-74 human gastric cancer cell lines), real-time qRT-PCR analysis, immuno-histochemical staining, immuno-blotting analysis, and immuno-fluorescence were detailed in supplementary methods.
Results
ISX expression was increased in H. pylori-infected human gastric mucosa
ISX has been reported to be specifically expressed in the intestine [10]. Initially, we analyzed ISX expression in human gastric mucosa with or without H. pylori infection. Immunohistochemical analysis showed that ISX expression increased significantly in gastric mucosa infected with H. pylori compared with that of uninfected gastric mucosa (Fig. 1a). ISX mRNA expression was upregulated and significantly correlated with protein expression after Helicobacter infection (Fig. 1b). We also analyzed expression of well-known intestinal markers CDX2 and MUC2, and found that both markers were expressed more frequently in H. pylori-infected gastric mucosa than in uninfected mucosa (Fig. 1a, b). The correlation between mRNA expression and immunohistochemical staining of each molecule was significant (ISX: Spearman’s ρ = 0.648, p = 0.023; CDX2: Spearman’s ρ = 0.693, p = 0.013), and MUC2: Spearman’s ρ = 0.821, p = 0.001). We also confirmed a correlation between ISX and CDX2 mRNA expression (Fig. 1c), or CDX2 and MUC2, as described previously [32, 33]. These results indicated that ISX mRNA and protein expression were induced by H. pylori infection and were strongly correlated with the expression of intestinal metaplasia markers.
ISX expression induced by Helicobacter infection in human and mice gastric mucosa. a Representative immunohistochemical staining of ISX, CDX2, and MUC2 in human gastric mucosa. b qRT-PCR analysis of ISX, CDX2, and MUC2 expression in H. pylori-infected human gastric mucosa (*indicates a statistically significant difference p ≤ 0.05. Original magnification ×100). c Correlation of ISX expression with CDX2 expression, and CDX2 expression with MUC2 expression in human gastric mucosa. d qRT-PCR analysis of ISX expression in mice infected with Helicobacter felis for 6 or 12 months (*indicates a significant statistically difference p < 0.05). e Representative immunohistochemical staining of ISX in mice infected with Helicobacter felis. Original magnifications, ×100 (top) and ×400 (bottom). f Rate of ISX-positive cells in mice infected with Helicobacter felis (*indicates a significant difference p < 0.05)
ISX expression was induced by Helicobacter infection in mouse stomach
To clarify whether ISX expression is induced by Helicobacter infection, we used a chronic mouse gastritis model infected with H. felis. The results showed that ISX mRNA expression increased significantly at 6 and 12 months post-infection compared with uninfected mice (Fig. 1d). ISX expression at 12 months was significantly higher than that of 6 months. In addition, the number of ISX-positive gastric epithelial cells increased significantly in H. felis-infected stomachs, as confirmed by immunohistochemical analysis (Fig. 1e, f). These results suggest that Helicobacter infection induces ISX expression in gastric epithelial cells in mice.
H. pylori induced ISX expression in vitro
To confirm whether ISX expression was induced by H. pylori infection in vitro, we performed quantitative real-time PCR and immunoblot analyses of ISX expression. ISX mRNA expression increased significantly in MKN45 cells 3 h after co-culturing with H. pylori (Fig. 2a). ISX protein expression in MKN45 cells was also detected at 12, 18, and 24 h after co-culturing with H. pylori, then decreased thereafter until 36 h after stimulation (Fig. 2b). Since proinflammatory cytokines, such as TNF-α and IL-1β, are known as ISX inducers in HCC cells [14], and these cytokines were also induced by H. pylori infection in the stomach, we investigated whether TNF-α, IL-1β, or H. pylori could induce ISX expression in gastric cancer cells. As shown in Fig. 2c, stimulation with TNF-α, IL-1β, or H. pylori induced ISX protein expression, as confirmed by the immunoblot analysis. Numerous signaling pathways, such as NF-κB, are activated by H. pylori and most of activation depends on the presence of the H. pylori major virulence factor, cagPAI [34, 35]. To assess whether ISX expression was cag-dependent and to determine whether ISX expression was induced by activating NF-κB signaling, we infected MKN45 cells with either wild-type or cagPAI-negative isogenic mutant of H. pylori, and performed qRT-PCR analysis for ISX mRNA expression. As shown in Fig. 2d, ISX mRNA expression induced by H. pylori was cagPAI dependent, and NF-κB signaling was involved in ISX expression in gastric cancer cells as assessed by NF-κB inhibitor NBD.
ISX expression induced by H. pylori infection in vitro. a qRT-PCR analysis of ISX expression with H. pylori infection. Gastric cancer cells (MKN45) were cultured with H. pylori (TN2) for 8 h at a multiplicity of infection of 100 (M.O.I.100) (*p < 0.05). b Immunoblot analysis of ISX expression (GAPDH as internal control) in MKN45 cultured with H. pylori (TN2, M.O.I.100) for 12, 18, 24, 30, or 36 h. c Immunoblot analysis of ISX stimulated with TNF-α (20 ng/ml), IL-1β (10 ng/ml), or H. pylori (TN2, M.O.I.100) for 24 h in MKN45. d qRT-PCR analysis of ISX mRNA expression in MKN45 cells co-cultured with wild-type or ΔPAI H. pylori (TN2-WT or TN2-ΔPAI). To assess the contribution of NF-κB signaling, MKN45 cells were treated with an NF-κB inhibitor, NBD peptide (200 μM). After 1 h, cells were co-cultured with wild-type H. pylori (TN2-WT, M.O.I.100) for 24 h, RNA was extracted, and qRT-PCR was performed (TN2-WT + NBD)
ISX expression enhanced cell proliferation and induced biological markers for intestinal metaplasia and cell stemness
To investigate the biological function of ISX in vitro, we established stably expressing ISX gastric cancer cells (Stable-ISX MKN45). We performed real-time qRT-PCR and immunoblot analysis to determine whether ISX contributed to cell proliferation, intestinal metaplasia, or stemness. As expected, mRNA expression of MUC5AC, a gastric marker, was down-regulated, whereas intestinal markers, such as MUC2 and CDX1/2, were up-regulated in Stable-ISX MKN45 cells compared to those in parent MKN45 cells (Fig. 3a). In addition, protein expression of cyclinD1, a cell-cycle regulator, CDX1/2, a key intestinal metaplasia transcription factor, and CD44, a gastric cancer stem cell marker, were upregulated by overexpressing ISX (Fig. 3b), suggesting that ISX plays key roles in proliferation, intestinal metaplasia, and stemness in gastric epithelial cells. To confirm whether ISX induced transcriptional activity of CDX2, we performed CDX2 dual-luciferase reporter assay with transient expression of ISX (Fig. 3c). After co-transfection of ISX and CDX2 promotor construct, transcriptional activity of CDX2 was significantly enhanced with overexpression of ISX. We also performed immunofluorescence to confirm the nuclear localization of ISX upon the transfection of ISX expressing vector (Fig. 3d).
a, b mRNA/protein expression analysis in stably expressing ISX gastric cancer cells (Stable-ISX MKN45). a qRT-PCR analysis of MUC5AC, MUC2, CDX1, CDX2, CyclinD1, or CD44 mRNA expression in Stable-ISX MKN45 or parent MKN45 were performed. b Immunoblot analysis of ISX, CDX1, CDX2, CyclinD1, and CD44 in Stable-ISX MKN45 or parent MKN45. GAPDH was used as internal control. c, d Luciferase reporter assay and immunofluorescence analysis. c CDX2 dual-luciferase reporter assay showing ISX-induced CDX2 upregulation in MKN45 cells. After co-transfection of ISX and CDX2 promotor construct, transcriptional activity of CDX2 was significantly enhanced. CDX2 expression vector was used as a positive control. The values obtained were corrected for transfection efficiency with Renilla luciferase activity, and results were expressed as fold induction compared with values obtained for the empty vector (here normalized to 1) (*p ≤ 0.05). d Immunofluorescence microscopy showing nuclear localization of ISX (green, arrowhead) upon ISX transfection in MKN45 cells
ISX as a key molecule for intestinal phenotype
To further investigate the role of ISX in intestinal phenotype, we established ISX knockdown cells that stably transfected shISX vector in MKN74 cells (Stable-shISX MKN74). We confirmed the significant decrease of CDX2 in both mRNA and protein level in Stable-shISX MKN74 (Fig. 4a, b), indicating that CDX2 was regulated, at least in part, by ISX expression. Another intestinal marker, MUC2, was also decreased in Stable-shISX MKN74. On the other hand, when we performed knockdown of CDX2, decreased expression of MUC2 did not affect the expression level of ISX, suggesting that ISX shares the upstream signaling pathway of CDX2 regulation (Fig. 4c) [32, 36].
Knockdown analysis of ISX or CDX2. a mRNA expression analysis in established ISX-knockdown cells with stably expressing of shISX (Stable-shISX MKN74). ISX knock down showed decreased expression of CDX2 and MUC2 (**p ≤ 0.001, *p ≤ 0.05). b Immunoblot analysis of ISX, CDX1, CDX2, and CyclinD1 in Stable-shISX MKN74, or Mock. GAPDH was used as internal control. c Knock down of CDX2 by siRNA CDX2 (p ≤ 0.05) showing decreased expression of MUC2 (p ≤ 0.05) without affecting the expression of ISX. (*p ≤ 0.05)
ISX expression in human gastric cancer
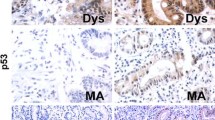
To investigate whether ISX expression in the stomach contributes to gastric carcinogenesis, we assessed positive rate of ISX in gastric cancer tissues. In early gastric cancer, 12/28 (42.9 %) samples were positive for ISX protein expression (Fig. 5a), whereas in advanced gastric cancer, 19/45 (42 %) were positive for ISX protein expression (Fig. 5b). Clinical characteristics of early or advanced gastric cancer were shown in supplementary Tables (ST1 and ST2). In advanced gastric cancer, the ISX-positive group was more progressed compared to ISX-negative group (ST2). Other parameters were not statistically significant regardless of the status of ISX expression. We also performed immunohistochemical staining to clarify ISX expression in gastric cancer and its adjacent tissues. Interestingly, ISX expression in adjacent tissues was strongly correlated with its in advanced gastric cancer tissues (R = 0.746, p < 0.001, Fig. 5c). In addition, ISX immunohistochemical staining was highly correlated with high grade of pathological differentiation (Fig. 5d). These results indicated that high ISX expression in gastric cancer may be associated with gastric cancer progression.
ISX protein expression in human gastric cancer. a Representative immunohistochemical staining of ISX in early gastric cancer resected by ESD (original magnification ×100). b Representative immunohistochemical staining of ISX in adjacent gastric tissue and advanced gastric cancer (GC) as indicated (original magnification ×100). c Correlation of ISX expression in advanced gastric cancer with ISX expression in adjacent gastric tissue. Correlation coefficient was R = 0.746, p < 0.001. d Correlation of tumor grade with ISX expression in advanced gastric cancer. Correlation coefficient was R = 0.702, p < 0.001
ISX expression accelerates tumorigenesis in gastric cancer cells
Since cells stably expressing ISX were CD44-positive (Fig. 3b), we assessed the association between ISX expression and tumorigenesis using a spheroid colony formation assay in serum-free medium [25]. Stable-ISX MKN45 cells formed a greater number of spheroid colonies compared with parent cells (colony number per well; 86.5 vs. 3.8, ave., p ≤ 0.001, Fig. 6a, b), suggesting that ISX expression in gastric cancer cells accelerates tumorigenesis, resulting in growth and invasion of tumor cells.
Spheroid colony formation assay and xenograft model of Stable-ISX MKN45 gastric cancer cells. a Representative spheroid colony under light microscopy, (left) mock, and (right) Stable-ISX MKN45 (original magnification ×200). b Area of spheroid colonies after 2 weeks. Values are mean ± SEM. Stable-ISX MKN45 showed more spheroid colony formation compared with control (mock vs. Stable-ISX MKN45 **p < 0.001). c, d Tumor volume in nude mice injected with mock or Stable-ISX MKN45 cells. Tumor sizes were measured every week, and the average of volumes were plotted (n = 8 each). Values are mean ± SEM. Stable-ISX MKN45 showed more proliferation compared to mock, by volume (mock vs. stable, volume: 761 vs. 1278 mm3 at 4 weeks, 1003 vs. 1645 mm3 at 5 weeks, *p < 0.05) and weight (at 5 weeks, 535 ± 63 vs. 333 ± 59 mg, *p < 0.05)
We next validated the tumorigenicity of Stable-ISX MKN45 cells in xenograft model. Stable-ISX MKN45 cells showed significantly increased in tumor size compared with that of parent MKN45 cells (mock vs. stable, volume: 761 vs. 1278 mm3 at 4 weeks, 1003 vs. 1645 mm3 at 5 weeks, p ≤ 0.05 and weight: 333 vs. 535 mg, p ≤ 0.05) at 5 weeks post inoculation (Fig. 6c, d). All these data suggested that ISX played a key role in tumorigenicity and tumor progression of human gastric cancer.
Discussion
This is the first report focusing on ISX expression and intestinal metaplasia in the setting of Helicobacter-associated gastric carcinogenesis. Based on our previous observation regarding mRNA expression profile of organoids, we isolated the novel gene ISX that may play key roles in intestinal metaplasia and cancer (unpublished observation). ISX is normally expressed only in the intestine but is also expressed in Helicobacter-infected stomach, suggesting that ISX may be a novel transcription factor for Helicobacter-induced intestinal metaplasia [10]. Apart from the expression pattern of CDX2, an intestinal homeobox gene and regulator of intestinal homeostasis [6, 37], ISX was distinctly expressed in Helicobacter-infected gastric epithelial cells, suggesting that ISX may have a distinct biological role in regulating intestinal metaplasia in the stomach.
We demonstrated that ISX expression was induced, at least in part, by activating the NF-κB pathway, which was not contradicted by a previous study [14]. A variety of intracellular signaling pathways are activated in H. pylori-infected gastric epithelium, and the NF-κB and mitogen-activated protein kinase (MAPK) pathways are key in the development of inflammation and carcinogenesis [38, 39]. As ISX expression depended on the presence of the Helicobacter virulence factor cagPAI, we hypothesized that the ISX expression mechanism may be shared with inflammation amplifiers, such as TNF-α, IL-1β, or IL-6, which induce chemokine release via an enhanced positive-feedback loop in the NF-κB pathway, and are maintained in gastric cancer and gastritis [40, 41]. It has been reported that ISX mRNA expression may be activated through increased NF-κB (p65) binding to ISX promoter in HCC cell [14]. Promoter analysis of ISX gene is required to determine whether ISX contains NF-κB and/or MAPK binding sites and whether ISX is truly regulated by these signaling pathways.
Cancer stem cell theory has been broadly discussed, and some stomach markers, such as CD44 and Sox-2, have been reported as putative stem or cancer stem cell markers [25, 26, 42]. Our previous observation in organoids from H. felis mice model revealed that ISX was expressed in Helicobacter-infected primary gastric cells. As forced expression of ISX induced the expression of CD44, ISX may trigger the conversion of matured epithelial cells into undifferentiated stem cells by activating Wnt/β-catenin signaling [43]. We also noticed that CDX1 and CDX2 were upregulated after forced expression of ISX. Since CDX1 confers an intestinal phenotype on gastric epithelial cells by inducing a stemness-associated reprogramming factor [44], ISX may function as a master reprogramming regulator in mature cells.
Some limitations of our study should be mentioned. Although we confirmed that ISX enhanced transcriptional activity of CDX2, and overexpression of ISX induced increase of CDX2 expression, we have no direct evidence that ISX protein binds directly to the promoter region of CDX2. As for cyclinD1, it has already been confirmed to have a putative region to which ISX binds and then functions as a transcriptional regulator [14]. The putative sequence of the ISX binding site was detected in the upstream regions of CDX2 after an in silico database search, suggesting that ISX may regulate transcription of CDX2 by binding directly to the promoter.
Few studies have reported the biological functions of ISX [12, 14]. We demonstrated that overexpression of ISX enhanced cell proliferation and tumorigenic activity in gastric cancer cells, at least in part, through upregulation of cyclinD1 expression. CyclinD1 is induced by gastrin or H. pylori infection in the gastric milieu and is associated with gastric carcinogenesis [45, 46]. Overexpression of cyclinD1 in breast cancer plays a key role in cancer development [47]. All of these data suggest that ISX exerts its effect on carcinogenesis through cyclinD1 expression. Further investigation is required to determine whether ISX per se has a potential to initiate cancer using an ISX-knockout mouse cancer model in vivo [12].
We showed that Stable-ISX MKN45 cells developed spheroid colonies. Liu et al. found that spheroid colony-forming cells from MKN-45 cells contain gastric cancer stem cell characteristics of sustained self-renewal, high proliferation, resistance to drugs, and high CD44 expression [25, 48]. Thus we think that ISX expression may be a novel therapeutic target for cancer cells that have resistance to chemotherapy. We hypothesized that chronic inflammation by H. pylori activates several signaling pathways, including NF-κB and signal transducer and activator of transcription 3 (STAT3), which induces release of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6. ISX expression by these cytokines, in turn, induces CD44 expression possibly through the WNT/β-catenin pathway in mature gastric stem cells, resulting in generation of gastric cancer stem cells.
Patients with poorly differentiated adenocarcinoma have shorter survival than those with well-differentiated stomach carcinoma [49]. We found a positive correlation between ISX expression and poorly differentiated cancer using a microarray, suggesting that ISX expression could become a biomarker of intestinal metaplasia useful for prediction of gastric cancer prognosis [50]. One study proposed three subtypes of H. pylori-associated gastric cancers; genomically stable tumors are enriched for a diffuse histological variant (69 %), whereas tumors with chromosomal instability and microsatellite unstable tumors are a poorly diffuse type (12 and 11 %, respectively) [51]. Further investigation is required to determine which cancer subtype fits with ISX-positive cancer, as with CDX2 [52].
In conclusion, we proposes here that H. pylori-induced ISX expression is a key factor in intestinal metaplasia and gastric carcinogenesis (acquisition of cancer stem cell characteristics and promoting cancer cell survival and proliferation). Therefore, ISX-targeted therapy may inhibit “field cancerization” and resistance to cancer chemotherapy.
References
Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133(2):659–72.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Sue S, Shibata W, Maeda S. Induced signaling pathways contribute to intestinal metaplasia and gastric carcinogenesis. BioMed Res Int. 2015;2015:737621.
Barros R, Freund JN, David L, Almeida R. Gastric intestinal metaplasia revisited: function and regulation of CDX2. Trends Mol Med. 2012;18(9):555–63.
Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99(5):836–42.
Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, et al. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64(21):7740–7.
Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104(6):488–92.
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–7.
Ushijima T. Epigenetic field for cancerization: its cause and clinical implications. BMC Proc. 2013;7(Suppl 2):K22.
Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, et al. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133(20):4119–29.
Gehring WJ, Affolter M, Burglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487–526.
Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, et al. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283(8):4905–11.
Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem. 2013;288(13):9017–27.
Hsu SH, Wang LT, Lee KT, Chen YL, Liu KY, Suen JL, et al. Proinflammatory homeobox gene, ISX, regulates tumor growth and survival in hepatocellular carcinoma. Cancer Res. 2013;73(2):508–18.
Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488(7412):527–30.
Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–6.
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006;66(19):9339–44.
Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci USA. 2008;105(11):4358–63.
Ferrand J, Lehours P, Schmid-Alliana A, Megraud F, Varon C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-kappaB-dependent pathway. PLoS One. 2011;6(12):e29007.
Pilpilidis I, Kountouras J, Zavos C, Katsinelos P. Upper gastrointestinal carcinogenesis: H. pylori and stem cell cross-talk. J Surg Res. 2011;166(2):255–64.
Noto JM, Khizanishvili T, Chaturvedi R, Piazuelo MB, Romero-Gallo J, Delgado AG, et al. Helicobacter pylori promotes the expression of Kruppel-like factor 5, a mediator of carcinogenesis, in vitro and in vivo. PLoS One. 2013;8(1):e54344.
Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36.
Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306(5701):1568–71.
Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288(22):16085–97.
Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27(5):1006–20.
Wada T, Ishimoto T, Seishima R, Tsuchihashi K, Yoshikawa M, Oshima H, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104(10):1323–9.
Shibata W, Takaishi S, Muthupalani S, Pritchard DM, Whary MT, Rogers AB, et al. Conditional deletion of IkappaB-kinase-beta accelerates helicobacter-dependent gastric apoptosis, proliferation, and preneoplasia. Gastroenterology. 2010;138(3):1022–34 (e1–10).
May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289(5484):1550–4.
Saegusa M, Hashimura M, Kuwata T, Hamano M, Wani Y, Okayasu I. A functional role of Cdx2 in beta-catenin signaling during transdifferentiation in endometrial carcinomas. Carcinogenesis. 2007;28(9):1885–92.
Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115(3):642–8.
Akanuma M, Maeda S, Ogura K, Mitsuno Y, Hirata Y, Ikenoue T, et al. The evaluation of putative virulence factors of Helicobacter pylori for gastroduodenal disease by use of a short-term Mongolian gerbil infection model. J Infect Dis. 2002;185(3):341–7.
Mesquita P, Jonckheere N, Almeida R, Ducourouble MP, Serpa J, Silva E, et al. Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J Biol Chem. 2003;278(51):51549–56.
Taylor JK, Levy T, Suh ER, Traber PG. Activation of enhancer elements by the homeobox gene Cdx2 is cell line specific. Nucleic Acids Res. 1997;25(12):2293–300.
Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13(4):470–6.
Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–7.
Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300(4):813–8.
Mutoh H, Hayakawa H, Sashikawa M, Sakamoto H, Sugano K. Direct repression of Sonic Hedgehog expression in the stomach by Cdx2 leads to intestinal transformation. Biochem J. 2010;427(3):423–34.
Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, et al. H. pylori activates NF-κB through a signaling pathway involving IκB kinases, NF-κB—inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119(1):97–108.
Hirata Y, Maeda S, Mitsuno Y, Tateishi K, Yanai A, Akanuma M, et al. Helicobacter pylori CagA protein activates serum response element-driven transcription independently of tyrosine phosphorylation. Gastroenterology. 2002;123(6):1962–71.
Atsumi T, Singh R, Sabharwal L, Bando H, Meng J, Arima Y, et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014;74(1):8–14.
Lee J, Nakagiri T, Oto T, Harada M, Morii E, Shintani Y, et al. IL-6 amplifier, NF-kappaB-triggered positive feedback for IL-6 signaling, in grafts is involved in allogeneic rejection responses. J Immunol. 2012;189(4):1928–36.
Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9(4):317–29.
Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14(3):342–56.
Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, et al. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci USA. 2012;109(50):20584–9.
Song DH, Rana B, Wolfe JR, Crimmins G, Choi C, Albanese C, et al. Gastrin-induced gastric adenocarcinoma growth is mediated through cyclin D1. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G217–22.
Hirata Y, Maeda S, Mitsuno Y, Akanuma M, Yamaji Y, Ogura K, et al. Helicobacter pylori activates the cyclin D1 gene through mitogen-activated protein kinase pathway in gastric cancer cells. Infect Immun. 2001;69(6):3965–71.
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369(6482):669–71.
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J, et al. Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol. 2013;42(2):453–9.
Kong X, Wang JL, Chen HM, Fang JY. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol. 2012;106(3):346–52.
Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104(4):643–52.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Seno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T, et al. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: prognostic implications. Int J Oncol. 2002;21(4):769–74.
Acknowledgments
We thank Mrs. Yuki Yamashita for excellent technical assistance. This study was funded by Grants-in Aid for Scientific Research (S.M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sue, S., Shibata, W., Kameta, E. et al. Intestine-specific homeobox (ISX) induces intestinal metaplasia and cell proliferation to contribute to gastric carcinogenesis. J Gastroenterol 51, 949–960 (2016). https://doi.org/10.1007/s00535-016-1176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1176-2