Abstract
Background
NUDT15 R139C (rs116855232) is a recently identified genetic factor responsible for thiopurine-induced leukocytopenia and hair loss. In this study, we investigated the association of NUDT15 R139C with 6-thioguanine nucleotide (6-TGN) levels and thiopurine-induced leukocytopenia in Japanese patients with inflammatory bowel disease (IBD).
Methods
Two hundred and sixty-four subjects (103 healthy volunteers and 161 IBD patients treated with thiopurines) were enrolled. Genotyping for NUDT15 R139C was performed using Custom TaqMan® SNP genotyping assays.
Results
The NUDT15 C/C, C/T, and T/T genotypes were 80.7, 18.2, and 1.1 %, respectively. The allelic frequency was 10.2 %. Among 161 IBD patients, there was no significant difference in 6-TGN levels among the NUDT15 genotypes. Forty-five patients (27.9 %) developed leukocytopenia (WBC <3000/μl), and the C/T and T/T genotypes were significantly associated with the development of leukocytopenia (P = 1.7 × 10−5). In these patients, 6-TGN levels were not significantly different between NUDT15 genotypes. NUDT15 R139C was significantly associated with early (<8 weeks) (P = 1.03 × 10−4) and late (>8 weeks) leukocytopenia (P = 4.3 × 10−4). The decrease in WBC count at 2 and 4 weeks was significantly higher in patients with the C/T or T/T genotypes as compared to the patients with the C/C genotype. All patients with the T/T genotype (n = 2) developed early severe hair loss and severe leukocytopenia (<1000/μl). The logistic regression analysis revealed that NUDT15 R139C was the sole genetic factor responsible for the thiopurine-induced leukocytopenia (P = 0.001).
Conclusions
These results suggest that NUDT15 R139C-related thiopurine-induced leukocytopenia is mediated by a 6-TGN-independent mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thiopurine drugs azathioprine (AZA) and 6-mercaptopurine (6-MP) are widely used to maintain clinical remission of inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC) [1–3]. Despite its efficacy, thiopurine was reported to have drug-induced toxicity, such as myelotoxicity, hepatotoxicity, hair loss, pancreatitis and gastrointestinal intolerance [4, 5]. About 15–30 % of patients discontinue therapy due to side effects [6–9].
The unfortunate side-effect profile of thiopurine despite its clinical efficacy has been explained by individual thiopurine-metabolizing activity, based on the genetic polymorphism of thiopurine-metabolizing enzymes [10, 11]. AZA and 6-MP are metabolized to 6-thioguanine nucleotides (6-TGNs) [10, 11], and 6TGNs mediate the immunosuppressive properties of AZA/6-MP via the inhibition of immune cell proliferation [10]. In this process TPMT (thiopurine S-methyltransferase) metabolizes thiopurines to inactive molecules, and variant TPMT alleles of lower metabolizer are associated with thiopurine-induced leukocytopenia with an elevation of 6-TGN levels [12]. A SNP (single-nucleotide polymorphism) of TPMT A719G has been reported to be the most prevalent allele of lower thiopurine metabolize [1, 13].
Genetic polymorphism of inosine triphosphate pyrophosphatase (ITPase) was also suspected as partly responsible for thiopurine intolerance [5, 14–17]. Genetic ITPase deficiency has been reported to result in the cellular accumulation of thioinosine triphosphate (TITP), and some studies have suggested a role for ITPase variants in thiopurine-induced toxicity [16, 17].
Multidrug resistance protein 4 (MRP4) functions as a cellular efflux pump for 6-MP and 6-TGNs [18]. A SNP in human MRP4 G2269A dramatically reduces MRP4 function and results in the intracellular accumulation of 6-TGNs [18]. We have previously reported that MRP4 G2269A is associated with higher 6-TGN levels and leads to thiopurine-induced leukocytopenia in Japanese patients with IBD [19].
In addition to this genetic predisposition, a considerable number of patients who have not inherited the TPMT or MRP4 variant experience thiopurine-induced leukocytopenia [1], suggesting the contribution of unknown factors responsible for inter-individual variation in thiopurine sensitivity. In 2014, Yang et al. discovered a SNP in NUDT15 (nucleoside diphosphate–linked moiety X-type motif 15) (NUDT15 R139C) that was strongly associated with thiopurine-induced leukocytopenia [odds ratio (OR) = 35.6, P = 4.88 × 10−94] in Korean CD patients [20]. Sensitivity and specificity of NUDT15 R139C for thiopurine-induced early (developed within 8 weeks) leukocytopenia were 89.4 and 93.2 %, respectively. Following this study, Kakuta et al. confirmed the association of NUDT15 R139C with early leukocytopenia in Japanese IBD patients (OR = 28.4, P = 1.92 × 10−16) [21]. These findings suggest that NUDT15 R139C is a new genetic factor responsible for thiopurine-induced myelotoxicity in East Asian populations. However, these two studies did not evaluate 6TGN levels, which is quite important to understand the functional role of NUDT15 R139C in promoting thiopurine-induced leukocytopenia.
In this study, we investigated the association of NUDT15 R139C genotypes with thiopurine-induced leukocytopenia and 6TGN levels. Furthermore, we focused on the association between NUDT15 R139C and decrease in WBC (white blood cell) count.
Patients and methods
Patients
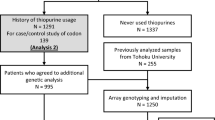
We enrolled 103 healthy volunteers (female/male 46/57) and 161 IBD patients (UC n = 89; CD n = 72) attending the gastroenterology outpatient clinic at the Hospital of Shiga University of Medical Science between May 2015 and July 2015. All subjects were Japanese, and their demographic/clinical characteristics are shown in Table 1. All patients were treated with AZA/6MP. The protocol of this study was approved by the Ethics Committee of Shiga University of Medical Science. Written informed consent was obtained from all patients.
Thiopurine treatment and adverse events
The clinical data including 6-TGN levels were reviewed from medical records. The dose of 6MP was converted to an AZA equivalent dose using a conversion factor of 2.08 [22]. Thiopurine therapy was performed according to the treatment guidelines of IBD in Japan. Thiopurines were started at a dose of 0.5–1.0 mg/kg/day (as AZA dose) and adverse events were checked at 7–14 days from the initiation. If patients have no adverse events, we increased to around 1.0 mg/kg/day. The thiopurine dose and 6-TGN levels when the WBC count reached the lowest value (nadir) were used for analysis. Leukocytopenia was graded by common toxicity criteria as follows: Grade 2, 2000–3000/μl; Grade 3, 1000–2000/μl, and Grade 4, <1000/μl [23]. Severe hair loss was defined by the need to wear wigs for long period, according to the criteria described by Kakuta et al. [21].
Genotyping of NUDT15 R139C (rs116855232), TPMT A719G (rs1142345), ITPase C94A (rs1127354) and MRP4 G2269A (rs3765534)
Mononuclear cells were isolated from heparinized blood using a Ficoll density gradient. The genomic DNA was isolated by a DNA extraction kit purchased from QIAGEN (Valencia, CA). Genotyping for NUDT15 R139C (rs116855232), TPMT A719G (rs1142345), ITPase C94A (rs1127354) and MRP4 G2269A (rs3765534) was performed using Custom TaqMan® SNP genotyping assays (ID: C_154823200_10, C_19567_20, C_27465000_10 and C_27478235_20; Applied Biosystems, Inc., Foster City, CA) in accordance with information on the Applied Biosystems website (http://www.appliedbiosystems.com).
The PCR was performed according to the manufacturer’s instructions provided by Applied Biosystems. The PCR thermal cycling was as follows: initial denaturing at 95 °C for 30 s followed by 40 cycles of 92 °C for 5 s and 60 °C for 20 s. Thermal cycling was performed using a LightCycler 480 system (Roche Diagnostics, Switzerland). Each 96-well plate contained 80 samples of an unknown genotype and 4 reaction mixtures containing the reagents, but no DNA (no-template control). The no-DNA control samples were necessary for the Sequence Detection System (SDS) 7700 signal processing, as outlined in the TaqMan Allelic Discrimination Guide. The genotypes were determined visually based on the dye component fluorescent emission data depicted in the X–Y scatter-plot of the SDS software.
6-TGN assay
The heparinized blood samples were first washed, and the red blood cells were hydrolyzed by acid, and extracted with phenylmercuric acetate/ethyl acetate. The 6-TGN levels were then measured by high performance liquid chromatography, as previously described [24].
Hardy–Weinberg equilibrium (HWE)
HWE analysis was performed on the research subjects by comparing the detected distribution of allele frequencies with the theoretical distribution estimated on the basis of the SNP allelic frequencies. P > 0.05 (Chi-squared statistics) was considered to indicate equilibrium.
Statistical analysis
The AZA dose, 6-TGN levels, and ΔWBC (ΔHb and ΔPlts) were compared using the Mann–Whitney test. The frequencies of leukocytopenia and severe hair loss for each genotype of R139C were compared by 3 × 2 Chi-square tests in univariate analyses. Logistic regression analyses were performed to identify the associations of leukocytopenia with candidate SNPs in multivariate analyses. All analyses were performed using R software version 2.14.1. P < 0.05 was regarded as statistically significant.
Results
Genotype frequency
Of the 264 subjects analyzed (161 IBD patients and 103 healthy volunteers), 213 subjects showed a wild type of NUDT15 R139C (C/C) (80.7 %) (Table 2). Forty-eight subjects showed a heterozygote of NUDT15 R139C (C/T) (18.2 %) and 3 carried a homozygote (T/T) (1.1 %) (Table 2). The allelic frequency of NUDT15 R139C was 10.2 % (54/528). No deviation from the Hardy–Weinberg equilibrium (HWE) was detected (P = 0.66).
In the same cohort, 7 subjects had a heterozygote of TPMT A719G (2.7 %) (Table 2), and the allelic frequency of TPMT A719G was 1.3 % (7/528). Sixty-three subjects showed a heterozygote of MRP4 G2269A (23.9 %) and 5 carried a homozygote (1.9 %) (Table 2). The allelic frequency of MRP4 G2269A was 13.8 % (73/528). A heterozygote of ITPase C94A was detected in 55 subjects (20.8 %) and a homozygote of ITPase C94A was detected 6 subjects (2.3 %) (Table 2) [25]. The allelic frequency of ITPase C94A was 12.7 % (67/528). These results agreed with previous reports on TPMT, MPR4 and ITPase polymorphism in the Japanese population [7, 13, 16, 19, 25].
Association of NUDT15 genotype with 6-TGN levels and leukocytopenia
The associations of the NUDT15 variant with 6-TGN levels and the white blood cell (WBC) count were analyzed in 161 IBD patients treated with AZA/6-MP (Table 3). These subjects consisted of 127 patients with the wild genotype (C/C), 32 patients with the C/T genotype, and 2 patients with the T/T genotype. In 161 IBD patients (including those with and without leukocytopenia), there was no significant difference in AZA dose and 6-TGN levels at WBC nadir among the NUDT15 genotypes (Table 3), suggesting that the NUDT15 genotype did not affect thiopurine metabolism.
Forty-five of 161 IBD patients (27.9 %) experienced leukocytopenia (Grade 2 or higher; WBC <3000/μl), and NUDT15 variants (C/T and T/T genotype) were significantly associated with the development of leukocytopenia [P = 1.70 × 10−5, odds ratio (OR) 5.82, 95 % confidence interval (CI) 2.59–13.1] (Table 3). This result was compatible with recent reports on Koreans (P = 5.49 × 10−5) [20] and Japanese (P = 1.61 × 10−5) [21].
Next, we investigated the association of NUDT15 R139C with early (developed within 8 weeks) and late (developed after 8 weeks) leukocytopenia. As shown in Table 3, NUDT15 R139C was significantly associated with the development of early leukocytopenia (P = 1.03 × 10−4, OR 9.33, 95 % CI 2.83–30.8), and a much stronger association was detected with the development of early severe leukocytopenia (Grades 3 and 4) (P = 6.8 × 10−6, OR 22.8, 95 % CI 4.25–122.6). In particular, all patients with the T/T genotype (n = 2) developed Grade 4 leukocytopenia (<1000/μl) within 8 weeks.
Recently, Kakuta et al. reported that the presence of NUDT15 R139C was not related to the development of late leukocytopenia in the Japanese population. However, we detected a significant association between the presence of NUDT15 R139C and the development of late leukocytopenia (P = 4.3 × 10−4, OR 4.08, 95 % CI 1.9–8.7) (Table 3). Interval to late leukocytopenia tended to be shorter in patients with the C/T genotype than those with the wild (C/C) genotype (Table 3), but statistical significance was not detected (P = 0.06).
ΔWBC at 2 and 4 week
To evaluate the association of NUDT15 R139C with the decrease rate of WBC, we calculated the ΔWBC at 2 and 4 weeks. The ΔWBC at 2 weeks was calculated by the following formula: (the ΔWBC at 2 weeks) = (WBC count at 2 weeks after thiopurine initiation−WBC count at thiopurine initiation). The ΔWBC at 4 weeks was also similarly calculated. As shown in Table 3, ΔWBC at 2 and 4 weeks was significantly higher in patients with the C/T or T/T genotypes as compared to patients with the wild genotype (C/C). In patients with the wild genotype (C/C), ΔWBC values at 2 and 4 weeks were 388.6 and 575, respectively, showing no decrease in these patients. WBC count seemed to decrease much more rapidly in patients with the T/T genotype as compared with patients with the C/T genotype (statistical analysis was not possible due to small sample number). A similar tendency was observed in the rate of decrease of platelets, but the findings were not significant (Supplementary Table 1).
Early severe hair loss
All patients with the NUDT15 T/T genotype (n = 2) developed early severe hair loss, but this phenomenon was not observed in patients with the wild genotype (C/C) or C/T genotype (Table 3).
AZA dose, 6-TGN levels and prednisolone dose in IBD patients with leukocytopenia
In IBD patients with leukocytopenia, AZA dose, 6-TGN levels and prednisolone dose at WBC nadir were not different between NUDT15 genotypes (Table 4). Furthermore, there were no statistically significant differences in AZA dose and 6-TGN levels among the NUDT15 genotypes in patients with early and late leukocytopenia (Table 4).We also evaluated the association of other candidate SNPs (TPMT A719G, MRP4 G2269A, and ITPA C94A) with the development of leukocytopenia, but no significant association was detected (Supplementary Table 2).
Multivariate analysis
In the logistic regression analysis with NUDT15, TPMT, MRP4, ITPase variants and 6TGNs levels, the NUDT15 variant was only genetic factor significantly contributing to the thiopurine-induced leukocytopenia (P = 0.001, OR 5.38, 95 % CI 1.91–15.2) (Table 5). In addition, interaction of NUDT15 variant with MRP4 or ITPase variants was not observed (Supplementary Table 3). Interaction between NUDT15 and TPMT variant could not be evaluated due to small number of patients with TPMT variant.
Discussion
In the current study, we demonstrated that NUDT15 R139C is common in the Japanese population (allelic frequency of 10.2 %), and this finding was compatible with other recent reports of East Asians [20, 21, 26]. The genotype frequency (wild type C/C 80.7 %, heterozygote C/T 18.2 %, homozygote 1.1 %) was similar to that found in recent reports on Koreans [20] and Japanese [21].
Thiopurine-induced leukocytopenia has been mainly explained by TPMT variants that induce the intracellular accumulation of 6-TGNs [1, 12]. However, TPMT variants can be found in some patients who experience thiopurine-associated leucopenia [19, 27]. Recently, NUDT15 R139C (rs116855232) has been identified as a novel predictor of thiopurine-induced leukocytopenia in Korean and Japanese patients with IBD [20, 21]. The GWAS (genome-wide association study) in child patients with acute lymphocytic leukemia also proved the association between NUDT15 R139C and thiopurine sensitivity and showed that NUDT15 R139C was found frequently in East Asians (9.8 %) and Hispanics (3.9 %) but rarely in Europeans (0.2 %) and Africans (0 %) [26]. However, none of these studies analyzed 6-TGN levels that might aid in explaining the molecular mechanism underlying the association of NUDT15 variants with thiopurine-induced leukocytopenia. In the present study, we confirmed that NUDT15 R139C is a strong predictor for thiopurine-induced leukocytopenia and found that NUDT15 R139C-associated thiopurine leukocytopenia was not accompanied by an elevation of 6-TGN levels, suggesting a thiopurine metabolism-independent mechanism.
Functional changes to NUDT15 by the T to A substitution of rs116855232 remain unclear. 8-xo-7,8-Dihydroguanine (8-oxo-Gua) is the most abundant oxidized purine base [28] generated by reactive oxygen species [29]. NUDT15 hydrolyzes the 8-oxo-Gua-containing DNA precursor (8-oxo-dGTP and 8-oxo-dGDP) to the monophosphate, thereby preventing the incorporation of 8-oxo-Gua-containing nucleotides into DNA [28, 30]. NUDT15 plays an important role in accurate DNA replication as well as in the prevention of abnormal protein synthesis under oxidative stress. Yang et al. explained NUDT15 variant-related thiopurine leukocytopenia from the viewpoint of thiopurine metabolism [26]. Thio-dGTP is one of the active molecules of 6-TGNs, and due to its molecular similarity to 8-oxo-dGDP, thio-dGTP may be hydrolyzed by NUDT15 to the inactive thio-dGMP or thio-dGDP. If NUDT15 R139C exerts loss-of-function, NUDT15 R139C will lead to intracellular accumulation of thio-dGTP and result in extensive DNA damage and cytotoxicity. However, this hypothesis does not align with the findings in the present study that 6-TGN levels (including thio-dGTP) were not elevated in patients with NUDT15 variants.
Rapid development of severe leukocytopenia and severe hair loss was characteristic for patients with the NUDT15 T/T genotype, and no patients with the C/T genotype had these issues. Severe leukocytopenia and hair loss are also manifestations of strong chemotherapy, and this suggests direct injury to bone marrow- and hair follicle-stem cells. Acute bone marrow suppression is the result of the induction of apoptosis in the proliferating bone marrow stem cells, and several studies have shown that the induction of oxidative stress is primarily responsible for the loss of bone marrow- and hair follicle-stem cells [31–33]. These findings suggest that if NUDT15 R139C induces loss-of-function, accumulation of oxidative stress and increased apoptosis in bone marrow progenitor cells might be a potential mechanism of NUDT15 R139C-related thiopurine leukocytopenia. The thiopurine metabolism-independent mechanism as well as the functions of NUDT15 should be extensively investigated in the future.
There have been different observations in recent studies of NUDT15 variants in East Asians. Yang et al. reported that NUDT15 R139C was associated with both early (developed within 8 weeks) and late (after 8 weeks) leukocytopenia in the Korean population [20], but Kakuta et al. showed that NUDT15 R139C was associated with only early leukocytopenia, but not with late leukopenia, in the Japanese population [21]. The current study showed an association of NUDT15 R139C with early and late leukopenia in the Japanese population, and this was compatible with the findings of Yang et al. The reason for the discrepancy in late leukocytopenia might be due to different adjustments of AZA dosage based on the development of adverse events. Based on these observations, NUDT15 R139C is considered to be a major genetic factor contributing to the heightened thiopurine sensitivity of East Asians. This is supported by the high frequency of NUDT15 R139C [26] and low frequency of the TPMT variant [13] in East Asians.
In the current study, we evaluated the effects of NUDT15 R139C not only by evaluating the frequency of leukocytopenia but also by examining the decrease in WBC count. ΔWBC indicates overall alteration of WBC count including patients whose WBC did not decrease below 3000/μl. Therefore, ΔWBC is considered to be a better marker for the evaluation of the rapid effects of NUDT15 R139C compared to the frequency of leukocytopenia development. ΔWBC was significantly enhanced in patients with the C/T and T/T genotypes as compared to those with the wild C/C genotype. WBC count decreased in patients with the C/T and T/T genotypes after 2 and 4 weeks, but such a decrease was not observed in patients with the wild genotype. These findings clearly indicate a rapid influence of NUDT15 R139C on hematopoietic activity.
In conclusion, we found that NUDT15 variant-related thiopurine-induced leukopenia was not associated with 6-TGN levels, and that a decrease in the WBC count was rapidly (within 2 weeks) induced after thiopurine initiation in patients carrying NUDT15 R139C. Our observations suggest that NUDT15 R139C is a new genetic factor related to the increased thiopurine sensitivity/toxicity of IBD patients. The utility of routine genotyping for NUDT15 variants before initiating thiopurine therapy should be considered to reduce the risk of thiopurine-related severe leukocytopenia and hair loss.
References
Amin J, Huang B, Yoon J, et al. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis. 2015;21:445–52.
Lee MN, Kang B, Choi SY, et al. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21:1054–62.
Timmer A, McDonald JW, Tsoulis DJ, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478.
Gearry RB, Barclay ML, Burt MJ, et al. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–7.
Van Dieren JM, Hansen BE, Kuipers EJ, et al. Meta-analysis: inosine triphosphate pyrophosphatase polymorphisms and thiopurine toxicity in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2007;26:643–52.
Gisbert JP, Gomollon F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–800.
Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24:1258–64.
Lees CW, Maan AK, Hansoti B, et al. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:220–7.
Winter JW, Gaffney D, Shapiro D, et al. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:1069–77.
Derijks LJ, Gilissen LP, Engels LG, et al. Pharmacokinetics of 6-thioguanine in patients with inflammatory bowel disease. Ther Drug Monit. 2006;28:45–50.
Haglund S, Taipalensuu J, Peterson C, et al. IMPDH activity in thiopurine-treated patients with inflammatory bowel disease—relation to TPMT activity and metabolite concentrations. Br J Clin Pharmacol. 2007;65(1):69–77.
Hiratsuka M, Inoue T, Omori F, et al. Genetic analysis of thiopurine methyltransferase polymorphism in a Japanese population. Mutat Res. 2000;448:91–5.
Ban H, Andoh A, Tanaka A, et al. Analysis of thiopurine S-methyltransferase genotypes in Japanese patients with inflammatory bowel disease. Intern Med. 2008;47:1645–8.
Kurzawski M, Dziewanowski K, Lener A, et al. TPMT but not ITPA gene polymorphism influences the risk of azathioprine intolerance in renal transplant recipients. Eur J Clin Pharmacol. 2009;65:533–40.
Allorge D, Hamdan R, Broly F, et al. ITPA genotyping test does not improve detection of Crohn’s disease patients at risk of azathioprine/6-mercaptopurine induced myelosuppression. Gut. 2005;54:565.
Uchiyama K, Nakamura M, Kubota T, et al. Thiopurine S-methyltransferase and inosine triphosphate pyrophosphohydrolase genes in Japanese patients with inflammatory bowel disease in whom adverse drug reactions were induced by azathioprine/6-mercaptopurine treatment. J Gastroenterol. 2009;44:197–203.
Zelinkova Z, Derijks LJ, Stokkers PC, et al. Inosine triphosphate pyrophosphatase and thiopurine s-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin Gastroenterol Hepatol. 2006;4:44–9.
Krishnamurthy P, Schwab M, Takenaka K, et al. Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Res. 2008;68:4983–9.
Ban H, Andoh A, Imaeda H, et al. The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2010;45:1014–21.
Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–20.
Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. The pharmacogenomics journal. 2015;. doi:10.1038/tpj.2015.43.
Sandborn W, Sutherland L, Pearson D, et al. Azathioprine or 6-mercaptopurine for inducing remission of Crohn’s disease. Cochrane Database Syst Rev. 2000;(2):CD000545.
NCI N. National Cancer Institute, common terminology criteria for adverse events v4. NIH publication, Bethesda, MD. 2009;# 09-7473.
Pike MG, Franklin CL, Mays DC, et al. Improved methods for determining the concentration of 6-thioguanine nucleotides and 6-methylmercaptopurine nucleotides in blood. J Chromatogr B Biomed Sci Appl. 2001;757:1–9.
Marinaki AM, Ansari A, Duley JA, et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. 2004;14:181–7.
Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33:1235–42.
Booth RA, Ansari MT, Loit E, et al. Assessment of thiopurine S-methyltransferase activity in patients prescribed thiopurines: a systematic review. Ann Intern Med. 2011;154:814–23 (W-295-818).
Takagi Y, Setoyama D, Ito R, et al. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J Biol Chem. 2012;287:21541–9.
Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
McLennan AG. The Nudix hydrolase superfamily. Cell Mol Life Sci: CMLS. 2006;63:123–43.
Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51.
Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002.
Zhao J, Li H, Zhou R, et al. Foxp1 Regulates the proliferation of hair follicle stem cells in response to oxidative stress during hair cycling. PLoS One. 2015;10:e0131674.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K08967), a grant for the Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan, a grant from the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and development, AMED, and a grant from Smoking Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors, except Akira Andoh, disclose no conflict of interest. Akira Andoh reports speaker fees from AbbVie and Eisai Pharmaceutical.
Additional information
Updates
This article was updated on December 1, 2015. Two values were corrected in Table 3 (−4.550 should be −4550 and −4.430 should be −4430).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asada, A., Nishida, A., Shioya, M. et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol 51, 22–29 (2016). https://doi.org/10.1007/s00535-015-1142-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1142-4