Abstract
Background
Aspirin (ASA) causes gastrotoxicity by hampering the epithelial defense against luminal contents through cyclooxygenase inhibition. Since cell survival in tough conditions may depend on rescue mechanisms like autophagy, we analyzed whether epithelial cells rely on this process to defend themselves from aspirin’s damaging action.
Methods
Rats received a single dose of ASA (150 mg/kg, p.o.) with or without pretreatment with the autophagy inhibitor 3-methyladenine, and gastric injury and epithelial autophagy were evaluated 3 h later. The effects of ASA on cell viability and autophagy were also evaluated in gastric epithelial AGS cells.
Results
Basal autophagy in the gastric mucosa was inhibited by ASA as demonstrated by increased levels of p62 and ubiquitinated proteins and total LC3 and a reduced LC3-II/LC3-I ratio. Similarly, ASA increased p62 and decreased LC3-II accumulation and the number of EmGFP/LC3B puncta in AGS cells. ASA activated the PI3K/Akt-GSK3-mTOR pathway, which phosphorylates ULK1 to prevent autophagy initiation, changes that were inhibited by the PI3K-inhibitor wortmannin. Autophagy inhibition seems to enhance the vulnerability of gastric epithelial cells as a combination of ASA with 3-methyladenine exacerbated rat gastric damage and AGS cell apoptosis.
Conclusions
Our data highlight the importance of autophagy in the gastric mucosa as a protective mechanism when the epithelium is injured. In the stomach, aspirin induces mucosal damage and reduces autophagy, thus, eliminating a protective mechanism that epithelial cells could use to escape death. We hypothesize that the combination of aspirin with drugs that activate autophagy could protect against gastric damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspirin is one of the most used NSAIDs due to its particular anti-platelet action, which is very valuable in the prevention of atherothrombotic disease. The main shortcoming of aspirin regimes is their gastrointestinal toxicity, which ranges from mild upper gastrointestinal problems to peptic ulcer disease and its complications, and whose consequences may be worsened by an enhanced tendency to bleeding. Although several risk factors for NSAID-induced upper gastrointestinal complications have been identified, the specific patient conditions determining low-dose aspirin gastrotoxicity are less well defined [1–4].
Gastrotoxicity induced by NSAIDs, including low-dose aspirin, is clearly related with their common ability to block cyclooxygenase activity and inhibit the endogenous synthesis of prostaglandins. These autacoids mediate several mechanisms that conform to what is called the mucosal barrier and which are aimed to defend gastric epithelial cells from their hostile environment. Prostanoids are also involved in the process of epithelial restitution that repairs erosions and maintains the epithelial barrier integrity [5]. NSAIDs, by deregulating this physiological safeguard, render the gastric mucosa more susceptible to the damaging actions of the gastric juice components. However, this mechanism does not entirely explain the gastrointestinal deleterious effects of NSAIDs, which are a chemically heterogeneous group of drugs that can act on specific sites of action other than cyclooxygenase enzymes [6]. In particular, aspirin directly affects several intracellular signaling pathways, like NF-κB or MAP kinases, with relevance for cell survival or death, and causes profound effects on mitochondrial function where, by uncoupling oxidative phosphorylation in the respiratory chain, alters the cellular energetic and redox homeostasis and favors cytochrome C release, which may lead to apoptosis [6, 7].
In cells exposed to challenging circumstances, the occurrence or avoidance of apoptosis often depends on the activation of rescue mechanisms. One of the resources is the catabolic process called macroautophagy (hereafter referred to as autophagy) [8, 9], which aims to degrade superfluous and damaged organelles, cytosolic proteins, and invasive microbes. To do so, the cell forms a double-membrane sequestering compartment termed the phagophore, which matures into an autophagosome. Following delivery to the lysosome, the cargo is degraded and the resulting macromolecules are released back into the cytosol [10]. The breakdown products are recycled and used as macromolecular constituents and energy sources in order to maintain cell viability under the unfavorable conditions that have triggered this response [8, 9]. Although this is the most common situation, autophagy can also promote cellular death, by either catabolizing indispensable portions of cells or facilitating the activation of apoptotic or necrotic programs [9].
The autophagic process depends on a complex machinery that involves multiple autophagy-related gene products (Atg) hierarchically recruited at the phagophore assembly site, the main players being: (a) the ULK1 complex, that regulates the induction of autophagosome formation; (b) the PtdIns 3-kinase (PtdIns3K) complex, that drives the nucleation of the isolation membrane; and (c) two ubiquitin-like (Ubl) conjugation systems (ATG12 and protein light chain 3/LC3), that play roles in vesicle expansion. This process is under the influence of many signaling pathways that converge on the regulation of the ULK1 complex activity by the mTOR complex 1 (mTORC1), which inhibits the process in nutrient-rich conditions [11], and by AMPK, which activates autophagy under nutrient depletion. In the first case, mTOR is activated in response to growth factors and insulin acting through the PI3K/Akt signaling pathway, or by amino acids through a still not fully understood mechanism. In the second situation, low cellular energy levels activate AMPK and GSK3 and both energy sensors inhibit mTOR activity. AMPK can also activate autophagy through a direct effect on ULK1 [12, 13].
In the present study we aim to determine whether the sensitivity of gastric epithelial cells to the damaging action of aspirin depends on their autophagic activity. We observed that aspirin inhibits autophagy in the rat gastric mucosa and in human gastric epithelial cells through the mTOR-mediated ULK1 phosphorylation, and that a further inhibition of autophagy exacerbates aspirin-induced gastric damage and epithelia apoptosis, which suggests that an insufficient autophagic flux is part of the ethiopathogenesis of aspirin gastrotoxicity.
Methods
Aspirin-induced gastric damage
All the animals were housed under appropriate conditions and all procedures followed the Spanish laws and were approved by the faculty ethic committee. Male Sprague–Dawley rats (220–250 g, Charles River) received a single dose of 3-MA (30 mg/kg, i.p., Calbiochem) or saline 45 min before aspirin (150 mg/kg, p.o., Sigma) or vehicle (1 % carboxymethylcellulose, Sigma). Another set of animals received either normal drinking water or drinking water containing the autophagy activator trehalose (30 mg/l) during 3 weeks before aspirin administration. After 3 h they were sacrificed by cervical dislocation. Their stomachs were removed, opened along the greater curvature, and photographed using a digital camera (Nikon). Total and damaged areas were measured in a blinded manner by using the Image J software (USNIH).
Male and female BALB/c mice (20–25 g, Charles River) received either normal drinking water or drinking water containing 50 mg/l aspirin for 7 days. With an average intake of 2–3 ml of water per day, this treatment corresponds to 5–7.5 mg/kg. This dose tries to mimic the human antiplatelet regimes (100–300 mg/day) and is intermediate between the dose obtained using the body weight or the body surface area as the reference (1.7–5 mg/kg or 20–60 mg/kg, respectively) [14].
Immunohistochemistry
The gastric corpus was fixed, embedded in paraffin, and cut in 5 µm sections as previously described [15]. Primary antibodies against ubiquitin, LC3, p62, and phosphorylated mTOR were used (Supplementary Table 1). Samples were counterstained with hematoxylin, and the specificity of the immunostaining was confirmed by the absence of signal when primary or secondary antibodies were omitted.
Plasmid constructs
The sequence encoding the EmGFP protein from the pcDNA 6.2-GW/miRNA (Life Technologies) was cloned into the expression vector p3xFLAG/MYC (Sigma) by PCR, using primers designed to contain restriction sites for HindIII and EcoRI. The sense primer was 5′-GAGAAAGCTTGTGAGCAAGGGCGAGGAG-3′ and the antisense primer was 5′-GAGAGAATTCCGCTTGTACAGCTCGTCCATGC-3′. The cDNA encoding the complete coding region of human LC3B (378 bp, GenBank® Accession No. NM_022818) was amplified by PCR from human cDNA and cloned into the p3xFLAG/EmGFP/MYC construct to obtain a fusion protein with EmGFP at the N-terminal end of LC3B (p3xFLAG/EmGFP/LC3B). The sense primer was 5′-GAGATCTAGAATGCCGTCGGAGAAGACCTTC-3′ and the antisense primer was 5′-GAGAGGATCCTTACACTGACAATTTCATCCCG-3′.
Cell culture and transfection
AGS cells (ATCC) were cultured in F12K medium (Life Technologies) supplemented with 10 % inactivated FBS, with 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 mM sodium pyruvate. For treatment purposes, cells were incubated in culture medium with reduced serum (0.5 % inactivated FBS).
AGS cells were transfected with the p3xFLAG/EmGFP/LC3B or the p3xFLAG/EmGFP/MYC plasmids by using Lipofectamine-2000 (Life Technologies) as transfection reagent, and were used 24 h post-transfection.
Fluorescence microscopy
The p3xFLAG/EmGFP/LC3B-transfected AGS cells were observed through an inverted microscope (Olympus IX81). Nine photographs (20×) per well were taken, and the number of LC3-puncta per cell were counted by an observer unaware of the treatment.
Protein extraction and Western blot analysis
Cells and tissues were homogenized in lysis buffer containing proteases (Complete Mini tablets, Roche) and phosphatases inhibitors. Equal amounts of protein were loaded onto SDS/PAGE gels and analyzed by Western blot (antibodies in Supplementary Table 1). Protein bands were detected with Supersignal chemiluminescent substrate (Thermo Scientific) in a LAS-3000 (Fujifilm). Protein densitometry was performed using the Image Gauge software (Fujifilm).
Flow cytometry
Viability of AGS cells was evaluated by flow cytometry (FacsCalibur, BD Biosciences) after incubation with AnnexinV-fluorescein and propidium iodide (Apoptosis Detection Kit, Abcam).
Statistical analysis
Data were expressed as mean ± S.E.M. and were compared by analysis of variance (one way-ANOVA) with a Newman-Keuls post hoc correction for multiple comparisons or a t test when appropriate. A p value <0.05 was considered to be statistically significant.
Results
Autophagy is impaired in the mucosa of aspirin-treated rats
First, we analyzed whether autophagic degradation is affected during induction of gastric damage. We have previously reported that treatment of rats with 150 mg/kg acetylsalicylic acid (aspirin, ASA) produced a significant level of mucosal damage, which reached a maximum 3–6 h later, and it was almost completely recovered 24 h after administration [16]. In order to study the level of autophagy in gastric mucosa during induction of damage, we orally administered ASA or vehicle (1 % carboxymethylcellulose) and sacrificed the rats 3 h later. Stomachs of aspirin-treated rats presented multiple haemorragic wounds across the gastric mucosa (Fig. 1a).
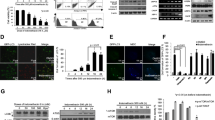
Defective autophagy in the damaged gastric mucosa of aspirin-treated rats. Rats were treated p.o. with vehicle (n = 4) or 150 mg/kg ASA (n = 6) for 3 h. a Representative photographs of stomachs, showing wounds along the gastric corpus. b Western blot of stomach lysates for ubiquitinated proteins (smear), LC3 and actin. Graphs showed relative densitometric quantification of total ubiquitinated proteins, total LC3 (LC3-I + LC3-II) and the ratio LC3-II/LC3-I. Protein densitometry was performed using the Image Gauge software (Fujifilm). c Representative ×10 images and ×100 amplifications of immunostaining with antibodies against ubiquitin, LC3 and p62 in gastric sections. d Western blot of stomach lysates for p62 and actin in mice treated with vehicle (n = 4) or ASA (50 mg/l, n = 5) for 7 days. e Representative ×10 images and ×40 amplifications of immunostaining with anti-p62 antibody in gastric sections
Accumulation of ubiquitinated protein aggregates in the brain and the liver has been described in knockout mouse models lacking key autophagy genes [17–19]. Western blot analysis of ubiquitinated protein level was performed in a piece of stomach from vehicle- and aspirin-treated rats and data showed a significant increase in the amount of ubiquitinated proteins (Fig. 1b), suggesting that their degradation is impaired. Immunostaining of paraffin-embedded slides from control rats with an anti-ubiquitin antibody showed a slight signal of ubiquitinated proteins in epithelial cells of the basal area of the mucosa (Fig. 1c). In the mucosa of aspirin-treated rats, the intensity of staining increased and was extended to the cells in the apical section.
We next analyzed the level of the autophagy marker LC3, which is transformed from the LC3-I to the LC3-II form when the autophagy process is activated, by immunohistochemistry and Western blot. Western blot analysis of stomach lysates from control and ASA-treated rats indicated that ASA significantly increases both LC3-I and LC3-II (total LC3) protein levels, whereas it reduces the LC3-II/LC3-I ratio (Fig. 1b). Immunostaining of stomach sections from control rats with the anti-LC3 antibody (which recognizes both forms of LC3) showed a similar pattern of protein expression for total LC3 than that observed for ubiquitinated proteins. In the gastric mucosa from vehicle-treated rats, LC3 was detected in the basal epithelial cells. The intensity was stronger in the mucosa of aspirin-treated rats, which also showed immunostaining for LC3 in apical areas (Fig. 1c).
Additionally, we measured the level of p62, which interacts with LC3-II at the inner autophagosome membrane, binds different autophagic substrates and is degraded by autophagy. Therefore, it serves as an indicator of autophagy completion. Notably, the intensity of immunostaining for p62 was stronger in the mucosa of aspirin-treated rats (Fig. 1c). Subchronic treatment with low dose ASA also produces an increase of p62 protein levels in the gastric mucosa of BALB/C mice, as assessed by Western blot and immunohistochemistry (Fig. 1d, e). Taken together, these data suggest that basal autophagy in the gastric mucosa is inhibited by aspirin.
Aspirin inhibits the autophagic flux in gastric epithelial cells
Inhibition of autophagy in gastric epithelial cells was confirmed by using the human gastric cell line of AGS cells as a cellular model. To evaluate autophagic flux, cells were treated with increasing doses of aspirin in the absence and presence of the lysosomal inhibitors chloroquine and ammonium chloride. Treatment of AGS cells with 10 µM chloroquine and 10 mM ammonium chloride for 24 h resulted in LC3-II accumulation, corresponding to the amount of LC3-I transformed into LC3-II during the treatment period (Fig. 2a). Aspirin decreased LC3-II accumulation in a dose dependent manner (Fig. 2a), indicating that a lower amount of LC3-I is lipidated to give LC3-II in the presence of aspirin. Interestingly, protein levels of p62 were higher in AGS cells treated with aspirin than in control cells and degradation of p62 induced by the autophagy activator rapamycin was prevented by treatment with aspirin (Fig. 2b), suggesting that this drug inhibits both basal and induced autophagy.
Inhibition of autophagy flux by aspirin in AGS cells. a AGS cells were treated with increasing doses of aspirin (ASA) in the absence and presence of 10 µM chloroquine (CQ) or 10 mM ammonium chloride. Representative Western blot for LC3 and actin (n = 4). b Representative Western blot for p62 and actin of AGS cells treated as indicated (n = 4). c AGS cells were transiently transfected with 3xFLAG/EmGFP/LC3B plasmid and treated with aspirin in the absence and in presence of CQ. Graph showed the number of LC3 puncta/cell (n = 4)
In order to confirm the inhibition of autophagy flux by aspirin, we made a plasmid construct to overexpress LC3B protein tagged with 3xFLAG and EmGFP. AGS cells were transiently transfected with this plasmid and treated with aspirin in the absence and in the presence of chloroquine (Fig. 2c). While aspirin treatment did not modify the number of EmGFP/LC3B puncta per cell, it significantly reduced the accumulation of these structures after treatment with chloroquine, which strongly suggests that autophagosome formation is impaired in the presence of aspirin.
In order to assess whether the effect of aspirin on autophagy is depending on cyclooxygenase inhibition, we studied autophagy flux in AGS cells after treatment with other NSAIDs. Sodium salicylate reproduced the effect observed with aspirin (Supplementary Fig. 1a), suggesting that the salicylate moiety is responsible for autophagy inhibition. However, two other gastrolesive NSAIDs, piroxicam and ketorolac and the cyclooxygenase-2 selective inhibitor celecoxib, failed to inhibit autophagy in AGS cells (Supplementary Fig. 1b–d) at doses that completely inhibit cyclooxygenase enzymes. These results indicate that the effect of aspirin or salicylate is unrelated to cyclooxygenase inhibition.
Activation of mTOR-mediated ULK1 phosphorylation by aspirin during the induction of gastric damage
Since the mTOR pathway is an essential regulator of autophagy, we aimed to analyze the state of mTOR phosphorylation in stomach samples from vehicle and aspirin-treated rats by means of a Western blot. Stomachs of aspirin-treated rats presented a significantly increased level of mTOR phosphorylation at Ser 2448 and Ser 2481 (Fig. 3a) compared with control rats. Immunohistochemistry of stomach slides with anti-phosphorylated mTOR at Ser 2448 also revealed that aspirin treatment increased phosphorylation of mTOR in epithelial cells of the rat gastric mucosa (Fig. 3b). Aspirin also induced a dose-dependent increase in the percentage of mTOR protein phosphorylated at both residues in AGS cells (Fig. 3c).
Induction of mTOR phosphorylation by aspirin. Rats were treated as described in Fig. 1. a Western blot of stomach lysates for Ser2448-, Ser2481-phosphorylated, and total mTOR. Graphs showed relative densitometric quantification of the ratio phosphorylated/total mTOR. b Representative ×10 images and ×40 amplifications of immunostaining with anti-phosphorylated (Ser2448) mTOR antibody in gastric sections. c Representative Western blot for Ser2448-, Ser2481-phosphorylated, and total mTOR of AGS cells treated with aspirin (n = 4)
The mTOR pathway impinges on the autophagy process by inhibiting ULK proteins, the mammalian homologs of yeast Atg1. Phosphorylation of ULK1 at Ser 757 by activated mTOR prevents its phosphorylation and activation by AMPK. Levels of phosphorylated ULK1 at Ser 757 were higher in the stomachs of aspirin-treated rats than in control rats (Fig. 4a). Furthermore, aspirin produced a dose-dependent increase in mTOR-mediated ULK1 phosphorylation in AGS cells (Fig. 4b). These data suggest that inhibition of autophagy by aspirin is due to the activation of mTOR-mediated phosphorylation of ULK1 protein.
Induction of mTOR-mediated phosphorylation of ULK1 by aspirin in a PI3K/Akt-dependent manner. Rats were treated as described in Fig. 1 and 3. a Western blot of stomach lysates for Ser757-phosphorylated and total ULK1. b Representative Western blot for Ser757-phosphorylated and total ULK1, Ser21-phosphorylated and total GSK3α, and Ser9-phosphorylated and total GSK3β of AGS cells treated with aspirin (n = 3). Western blot for tubulin was carried out as loading control. c Western blot of pooled samples (n = 4–6) from stomach lysates for Ser21-phosphorylated and total GSK3α and Ser9-phosphorylated and total GSK3β. d Representative Western blot for indicated proteins of AGS cells treated with 5 mM aspirin and increasing doses of wortmannin (n = 3)
GSK-3 kinases negatively regulate mTOR in response to fasting and nutrient deprivation [13]. Akt-mediated phosphorylation of GSK-3α at Ser 21 and GSK-3β at Ser 9 inhibits their activities and releases the negative regulation on mTOR by GSK3 proteins [20]. Aspirin treatment increased phosphorylation of both GSK-3α and GSK-3β in pooled samples from stomach lysates (Fig. 4c) and in AGS cells (Fig. 4b). Interestingly, the PI3K inhibitor wortmannin abolished aspirin-induced GSK3α and GSK-3β phosphorylation in these cells (Fig. 4d). Wortmannin also inhibited the mTOR-mediated phosphorylation of ULK1 induced by aspirin (Fig. 4d). Taken together, our results suggest that aspirin is activating the PI3K/Akt-GSK3-mTOR pathway, which phosphorylates ULK1 to block the initiation of the autophagy process. On the other hand, the effect of aspirin on ULK1 phosphorylation was mimicked when cells were treated with equimolar doses of sodium salicylate (Supplementary Fig. 2a) and was prevented by treatment with wortmannin (Supplementary Fig. 2b).
Pharmacological inhibition of autophagy exacerbates aspirin-induced gastric damage and epithelia apoptosis
Autophagy has been described as a protective mechanism against several kinds of cellular stresses, although it has been also involved in cell death. In order to investigate whether disruption of basal autophagy can aggravate gastric damage induced by aspirin, we treated rats with 3-methyladenine (3-MA), an autophagy inhibitor which selectively acts on the PI3K type III complex involved in the autophagosome membrane nucleation. Administration of 3-MA or its vehicle was followed 45 min later by treatment with aspirin or its vehicle for 3 additional hours. As shown in Fig. 5a, rats treated with 3-MA alone did not present any macroscopical damage in the gastric mucosa. However, after aspirin administration, rats previously treated with 3-MA showed a slight but significant increase in wounded area (Fig. 5a). Taken together, our data suggest that although defective autophagy is not sufficient to induce gastric damage, impairment of basal autophagy leads to a higher degree of injury when damage is induced by aspirin.
Exacerbation of gastric damage and apoptosis by inhibition of autophagy in aspirin-treated rats and AGS cells. a Rats were treated with 3-MA (30 mg/kg, i.p.) and aspirin (150 mg/kg, p.o.) as described in “Methods” (n > 4). Pictures show representative photographs of stomachs and the graph represents the percentage of the damaged area. b Western blot of stomach lysates for caspase 3 (full caspase 3, 35 kDa; cleaved caspase 3, 17/19 kDa). c AGS cells were treated with increasing doses of aspirin in the absence and the presence of 2 mM 3-MA. Graphs show the percentage of apoptotic and necrotic cells as measured by means of the annexin V and propidium iodide assay, respectively. d Rats received trehalose (30 mg/l, in the drinking water) and aspirin (150 mg/kg, p.o.) as described in “Methods” (n = 4). Pictures show representative photographs of stomachs and graph represents the percentage of the damaged area
In order to assess if 3-MA increases the sensitivity of gastric epithelium to apoptosis, we measured the level of caspase 3 activation by Western blot. Rats treated with 3-MA before aspirin administration presented a lower protein level of full caspase 3 and a higher level of active cleaved caspase 3 (Fig. 5b), suggesting that apoptosis is elevated when autophagy is inhibited. Finally we examined the effect of autophagy inhibition on apoptosis and necrosis by measuring annexin and propidium iodide fluorescence in a flow cytometer. AGS cells were treated with increasing doses of aspirin and pre-treated with 2 mM 3-MA. Dose dependent effect of 3-MA on apoptosis was tested (data not shown) in order to choose a dose of 3-MA which is not producing apoptosis by itself. Aspirin treatment did not induce apoptosis and necrosis until a concentration of 10 mM (Fig. 5c). However, in the presence of 3-MA, the dose of 5 mM aspirin is enough to induce both apoptosis and necrosis. Interestingly, use of 3-MA does not increase the percentage of cells undergoing apoptosis and necrosis with 10 mM aspirin.
Pharmacological activation of autophagy reduces aspirin-induced gastric damage
We also examined if induction of autophagy can reverse the gastric damage produced by aspirin by treating rats with trehalose. Whereas the treatment with trehalose alone has no effect on the gastric mucosa, rats receiving trehalose presented a significant reduction of wounded area after aspirin administration (Fig. 5d).
Discussion
We demonstrated here that autophagy is impaired in the gastric mucosa during aspirin-induced injury and that inhibition of basal autophagy exacerbated the damage produced by this drug. Inhibition of autophagy by aspirin was reproduced in a cellular model with a gastric epithelial cell line (AGS), indicating that the effect is mainly due to an action on epithelial cells in the gastric mucosa.
In order to study the autophagy process in vivo, we analyzed the appearance of ubiquitinated proteins and the level of LC3 and p62 proteins in the gastric mucosa of rats. In accordance with a defective autophagy, we observed that the level of p62 and ubiquitinated proteins in the stomach of aspirin-treated rats is higher as compared with control rats, as measured by immunohistochemistry and Western blot. Remarkably, immunostaining with an anti-LC3 antibody was restricted to the basal portion of the mucosa in control rats, which probably corresponds to gastric chief cells and correlates with the pattern of basal and starvation-induced autophagy described by Mizushima et al. in GFP-LC3 transgenic mice [21]. After treatment with aspirin, the intensity of immunostaining was increased not only in the basal but also in the luminal portion of the mucosa, the area most exposed to the damaging gastric contents. Given that LC3 antibody can bind both LC3-I and LC3-II isoforms, we can only conclude from these experiments that total LC3 protein level is increased, data that was confirmed by Western blotting. Since this protein is degraded by autophagy, it is assumed that the amount of total LC3 protein inversely correlates with the level of autophagy flux [22]. Moreover, the ratio between LC3-II and LC3-I protein levels was reduced after aspirin treatment, indicating indeed that the drug decreased the rate of autophagosome formation in the gastric mucosa of aspirin-treated rats.
These results were reproduced in AGS cells, where in a dose-dependent manner, treatment with aspirin inhibited the accumulation of LC3-II protein. We also detected a reduction in the appearance of LC3-II dots in the presence of inhibitors of lysosomal degradation, as well as an accumulation of the autophagic substrate p62. Therefore, it seems that aspirin can inhibit autophagy in the gastric mucosa through a direct action on epithelial cells.
Aspirin treatment increased the phosphorylation of Ser757 in ULK1 protein in the rat stomach and in AGS cells. This phosphorylation is carried out by mTOR and has an inhibitory effect on the kinase activity of ULK1 complex that initiates the cascade leading to LC3 lipidation and autophagosome formation and elongation [23, 24]. This result is then indicative of an increased activity of mTORC1 and agrees with the inhibition of autophagy that we observed. Aspirin treatment also induced mTOR autophosphorylation at Ser2481, which again indicates mTOR stimulation, and at Ser2448, which denotes a direct effect of PI3K/Akt kinase activity [25, 26]. Both modifications suppose an activating effect on mTOR and, therefore, explain the effect of aspirin on ULK1. Aspirin also increased phosphorylation of the kinases GSK3α (at Ser 21) and GSK3β (at Ser 9), two modifications that release their inhibitory action on the mTOR pathway [13, 26]. Interestingly, these phosphorylations on GSK3 isoenzymes and also that of ULK1 at Ser757 were inhibited by the PI3K inhibitor wortmannin. Thus, our results indicate that aspirin treatment activates the PI3K/Akt/GSK3/mTOR pathway to increase the phosphorylation of the Ser757 residue in ULK1 protein and, in turn, reduce autophagic activity.
This pathway is usually counterregulated by AMPK that would stimulate autophagy in response to low cellular energy levels. Aspirin probably stimulates AMPK due to its uncoupling effect on the mitochondrial oxidative phosphorylation; however, no stimulation of autophagy would be expected because the mTOR-mediated phosphorylation of ULK1 would impede its activation by AMPK [23, 27]. The inhibition of autophagy induced by aspirin, and also by sodium salicylate, also seems independent of cyclooxygenase inhibition, since it was not observed with other non-selective NSAIDS like piroxicam and ketorolac or with the cyclooxygenase 2-specific NSAID celecoxib.
To our knowledge, this is the first report analyzing the effects of aspirin on mTOR signaling pathways in gastric epithelial cells, and the results obtained differ from those reported by Din et al. in epithelial cells of more distal parts of the gut [28]. They observed that aspirin reduces the activity of mTOR in colorectal cancer cells and suggest that this effect could contribute to aspirin’s antineoplastic effect. This divergent effect of aspirin on mTOR activity in these two cell types may be related to their different origin or, alternatively, to changes in experimental conditions that may be determinant of basal mTOR activity (e.g., serum concentration in culture media). With regard to autophagy, the net effect of aspirin on colonic cells is not clear because the reported results indicate that an increased amount of LC3-II and total LC3 in the presence of bafilomycin A, suggestive of autophagy activation, co-exists with the reduced LC3-II/LC3-I ratio and augmented amount of total LC3 in the absence of this lysosome inhibitor, which are indicative of autophagy inhibition. The lack of a straight explanation for these results should make us consider the possible implication of other cellular processes in response to aspirin. A possibility may be the LC3-mediated/mTOR-independent non-autophagic process known as entosis, described in epithelial cells and human tumors [29, 30].
Apoptosis of epithelial cells has an important role during the production of gastric damage [31]. Although excessive autophagy could lead to cell death, the crosstalk between autophagy and apoptosis tells us about two antagonistic processes, pointing to the first as a protective mechanism against several kinds of stresses [32–35]. In our flow cytometry experiments, pharmacological inhibition of autophagy with the type III PI3K inhibitor 3-MA or the lysosomal inhibitor chloroquine increased the percentage of apoptotic AGS cells (data not shown). We used, therefore, submaximal doses of 3-MA and observed a synergic response with aspirin (5 mM) in that none of these drugs increased apoptosis and necrosis rates per se, but a significant augment was observed when combined. In vivo, pharmacological inhibition of autophagy by 3-MA did not cause any macroscopical damage in our experiments, but pre-treatment of rats with this drug exacerbated aspirin-induced lesions. This indicates that suppression of autophagy is not sufficient to produce damage but recognizes autophagy as a protective mechanism when injury occurs. Interestingly, several autophagy-deficient intestinal mouse models seems to need the presence of an additional stimuli, such as viral infection, pathogenic gut microbiota, or drug-induced damage, to show a barrier defect when autophagy is reduced, otherwise few alterations in the epithelium were observed [36–39]. In our experimental models this additional stimuli is the action of aspirin, which has been described to produce apoptosis and to promote mitochondrial dysfunction that can lead to the generation of radical oxygen species. Our data suggest that basal autophagy in the gastric epithelial cells suppress, at least in part, the deleterious effect of aspirin, probably eliminating defective mitochondria or counteracting the apoptotic pathways (for example, releasing Bcl-2-related anti-apoptotic factors from Beclin-1 protein). Further research will be needed to assess what is the exact mechanism involved in the protective role of gastric epithelial autophagy.
In summary, our data highlight the importance of autophagy in the gastric mucosa as a protective mechanism when the epithelium is injured, extending the observations that we and others made along the intestinal tract [16, 37, 38]. In the stomach, aspirin is inducing mucosal damage and reducing autophagy, thus eliminating a possible protective mechanism that epithelial cells could use to resist injury. We can speculate that the combination of aspirin with another drug, which activates autophagy, could protect against gastric damage.
Abbreviations
- 3-MA:
-
3-Methyladenine
- ASA:
-
Acetylsalicylic acid, aspirin
- CQ:
-
Chloroquine
- NSAIDs:
-
Non-steroidal anti-inflammatory agents
- Veh:
-
Vehicle
References
Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–61.
Leung Ki EL, Chan FK. Interaction of Helicobacter pylori infection and low-dose aspirin in the upper gastrointestinal tract: implications for clinical practice. Best Pract Res Clin Gastroenterol. 2012;26:163–72.
Sostres C, Lanas A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol. 2011;8:385–94.
Valkhoff VE, Sturkenboom MC, Kuipers EJ. Risk factors for gastrointestinal bleeding associated with low-dose aspirin. Best Pract Res Clin Gastroenterol. 2012;26:125–40.
Starodub OT, Demitrack ES, Baumgartner HK, et al. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–32.
Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–72.
Jana NR. NSAIDs and apoptosis. Cell Mol Life Sci. 2008;65:1295–301.
Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75.
Marino G, Niso-Santano M, Baehrecke EH, et al. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41.
Feng Y, He D, Yao Z, et al. The machinery of macroautophagy. Cell Res. 2014;24:24–41.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23.
Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–68.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61.
Hernandez C, Santamatilde E, McCreath KJ, et al. Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1: implications for gastric mucosal healing. Br J Pharmacol. 2009;156:262–72.
Ortiz-Masia D, Cosin-Roger J, Calatayud S, et al. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal Immunol. 2013;. doi:10.1038/mi.2013.108.
Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4.
Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9.
Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34.
Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9.
Mizushima N, Yamamoto A, Matsui M, et al. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11.
Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26.
Alers S, Loffler AS, Wesselborg S, et al. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11.
Alers S, Loffler AS, Wesselborg S, et al. The incredible ULKs. Cell Commun Signal. 2012;10:7.
Nave BT, Ouwens M, Withers DJ, et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–31.
Peterson RT, Beal PA, Comb MJ, et al. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–23.
Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41.
Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–15.
Overholtzer M, Mailleux AA, Mouneimne G, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79.
Florey O, Kim SE, Sandoval CP, et al. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–43.
Fiorucci S, Antonelli E, Morelli A. Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Dig Liver Dis. 2001;33(Suppl 2):S35–43.
Gordy C, He YW. The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell. 2012;3:17–27.
Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39.
Wei Y, Pattingre S, Sinha S, et al. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88.
Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2.
Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–63.
Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–8.
Cadwell K, Patel KK, Maloney NS, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–45.
Cabrera S, Fernandez AF, Marino G, et al. ATG4B/autophagin-1 regulates intestinal homeostasis and protects mice from experimental colitis. Autophagy. 2013;9:1188–200.
Acknowledgments
We thank Brian Normanly for his English language editing. This work was supported by Ministerio de Ciencia e Innovación [grant numbers SAF2010-20231, SAF2010-16030, SAF2013-43441-P and RYC-2011-09571], Ministerio de Sanidad y Consumo [Grant Number PI11/00327], CIBERehd [Grant Number CB06/04/0071] and Generalitat Valenciana [Grant Number PROMETEOII/2014/035]. Carlos Hernandez acknowledges support from the ‘Ramon y Cajal’ program from Ministerio de Ciencia e Innovación of Spain (RYC-2011-09571). Jesús Cosín-Roger is supported by FPU fellowships from Ministerio de Educación, Cultura y Deporte. The support received had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernández, C., Barrachina, M.D., Vallecillo-Hernández, J. et al. Aspirin-induced gastrointestinal damage is associated with an inhibition of epithelial cell autophagy. J Gastroenterol 51, 691–701 (2016). https://doi.org/10.1007/s00535-015-1137-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1137-1