Abstract
Purpose
In chemotherapy-induced nausea and vomiting (CINV), the superiority of the second-generation 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) over the first-generation 5-HT3RA is shown in the delayed emesis in cycle 1. We evaluate the antiemetic efficacy in real-world clinical practice that has not been sufficiently investigated in clinical trials.
Methods
We included patients who were diagnosed with gastric cancer between April 2012 and June 2017 from the medical claims databases and were treated with cisplatin (≥ 50 mg/m2) and standard antiemetic therapy (5-HT3RA + neurokinin-1 receptor antagonist [NK1RA] + dexamethasone). We compared the second-generation 5-HT3RA (2nd group) and the first-generation 5-HT3RA (1st group) groups to evaluate the additional antiemetic drug as the CINV event.
Results
In total, 3798 patients were extracted; 1440 and 2358 patients were included in the 1st and 2nd groups, respectively. The clinical and demographic characteristics did not differ between the groups. In the overall (days 1–6) in cycle 1, 51.7% and 44.3% of patients in the 1st and 2nd groups, respectively, had a CINV event. In the acute phase (days 1–2), 38.7% and 30.2% and in the delayed phase (days 3–6), 35.8% and 32.1% of patients in the 1st and 2nd groups, respectively, had a CINV event. Furthermore, the CINV event trend was the same as in cycles 1 to 5.
Conclusion
The proportion of CINV events in the 2nd group was smaller than that in the 1st group at any cycle. These findings may suggest consistent antiemetic efficacy of second-generation 5-HT3RA throughout the cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) induces poor chemotherapy compliance and poor patient quality of life [1, 2]. New antiemetics have significantly improved CINV, but CINV remains a critical adverse event associated with chemotherapy [3].
Patients receiving highly emetogenic chemotherapy (HEC) will have more than 90% of the risk of vomiting without antiemetics [4]. HEC includes anticancer drugs such as cisplatin, dacarbazine, and anthracycline, as well as cyclophosphamide regimens. CINV is classified into three types according to the time of CINV expression. Acute emesis is observed within 24 h after the initiation of chemotherapy, delayed emesis is observed after 24 h following chemotherapy, and anticipatory emesis is observed before chemotherapy in patients who have experienced significant CINV during previous chemotherapy. Delayed emesis often occurs after the patient is discharged from the hospital, which causes underestimation of CINV for the healthcare providers and inadequate control of CINV for the patient [5].
The purpose of antiemetic therapy is complete prevention of CINV. For HEC, the triplet combination of 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) + neurokinin-1 receptor antagonist (NK1RA) + dexamethasone (DEX) is the standard antiemetic therapy specified in the guidelines [6,7,8]. If CINV occurs even after administration of these antiemetics, additional antiemetic with different mechanisms, dopamine receptor antagonists, benzodiazepine anxiolytics, and multi-receptor antipsychotics will be administered.
Among the antiemetic agents, 5-HT3RAs consist of first-generation 5-HT3RA (azasetron, granisetron, indisetron, ondansetron, dolasetron, ramosetron, and tropisetron) and second-generation 5-HT3RA (palonosetron). In the phase III trial that investigated the differences between 5-HT3RA generations, the second-generation 5-HT3RA showed a better antiemetic effect in the delayed emesis for patients receiving HEC for the first time [9]. However, in almost all clinical trials, the antiemetic effect was evaluated only during the first cycle, but the continuity of HEC was not evaluated. In real-world clinical practice, HEC is repeatedly administered to the patient until the therapeutic goal is obtained. Additionally, for the second and subsequent HECs, the suppression of CINV would become more difficult due to anticipatory emesis [10]. Therefore, the effect of the second-generation 5-HT3RA on the first-generation 5-HT3RA for HEC repeatedly administered under clinical practice has not been sufficiently investigated.
In Japan, patients with advanced or recurrent gastric cancer are treated with cisplatin-based regimens as first-line chemotherapy [11]. The most common regimen is the fluoropyrimidine anticancer drug (tegafur, gimeracil, oteracil potassium (S-1), or capecitabine) + cisplatin; if the human EGFR–related 2 (HER2) is positive, trastuzumab is also used. Although cisplatin is effective for the treatment of gastric cancer, control of CINV caused by cisplatin is important for maintaining compliance.
The aim of this study was to evaluate the real-world effectiveness of second-generation 5-HT3RA compared with first-generation 5-HT3RA for CINV caused by the repeatedly administered cisplatin-based regimen in clinical practice in patients with gastric cancer. Efficacy was assessed using the medical claims database with additional antiemetics as the frequency of CINV events. In order to evaluate the continuity of HEC, we also evaluated the cisplatin administration status by cycle.
Method
Data source
This was a retrospective nationwide cohort study in Japan in which the medical claims database provided by the Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan) was used. This database was derived from acute care hospitals under the Japanese Diagnosis and Procedure Combination (DPC) system. As of June 2017, it contained approximately 20 million patients, which included 162 designated cancer care hospitals (approximately 40% of all such hospitals in Japan) and included 307 acute care hospitals (approximately 18%). This database contained information on sex, age, diagnosis (International Classification of Diseases [ICD]-10 code), prescription information (date, dose, and frequency), height, weight, and smoking status. Several studies using this database in oncology have been reported [12, 13].
Study cohort
The study cohort was identified based on the diagnosis and prescription of drugs. The initial administration of cisplatin defined the index date (day 1). Eligibility criteria included patients with gastric cancer (ICD-10 code C16) from April 2012 to June 2017, who received anticancer drugs (fluoropyrimidine (S-1 or capecitabine) (day − 59–day 60) and cisplatin (≥ 50 mg/m2) (day 1)), in addition to the triplet combination antiemetics (5-HT3RA (1st-generation 5-HT3RA or 2nd-generation 5-HT3RA), NK1RA, and DEX) (day 1).
The exclusion criteria were as follows: less than 20 years of age; the combined use of radiation therapy; a medical history of nausea, vomiting, dehydration, or mental illness from day − 29 to day 0; the use of antiemetic drugs; cases with death from days 1 to 5; and cisplatin over use.
Based on the generation of 5-HT3RA used at the first cisplatin administration (cycle 1), the groups were divided into first-generation (1st group) and second-generation (2nd group) 5-HT3RA groups.
Cisplatin administration was excluded from the analysis if it was administered more than 1 year after the first dose or if the interval between doses of cisplatin exceeded 90 days in order to evaluate a series of treatments. Moreover, the cisplatin was administered up to five times in order to evaluate the CINV events.
With the exception of dolasetron, all first-generation 5-HT3RAs were approved in Japan before 2004. The approvals for aprepitant (NK1RA), fosaprepitant (NK1RA), and palonosetron (second-generation 5-HT3RA) were in 2009, 2011, and 2010, respectively. Olanzapine’s insurance coverage for CINV was after June 2017.
Outcome measures
The outcome of CINV events was defined as the additional antiemetic drug administered from day 1 to day 6 in each cycle. The proportion of CINV events was evaluated by cycle (cisplatin administered every time) for up to five cycles and it was also evaluated daily. Patients who discontinued cisplatin were excluded from the analysis of the cycle. The use of additional antiemetic drugs was evaluated according to the antiemetic guidelines [7]. The acute, delayed, and overall phase events were defined as days 1–2, days 3–6, and days 1–6, respectively.
Statistical analysis
Mean and standard deviation (SD) were calculated for continuous variables, and numbers and proportions were calculated for categorical data. Fisher’s exact test was used for comparisons between proportions, and the Wilcoxon rank sum test was used for comparisons between continuous variables.
Primary analysis was performed by calculating the risk difference in the CINV events. The proportion of CINV events was determined for each cycle based on grouping. An unadjusted risk difference and a Cochran-Mantel-Haenszel (CMH) adjusted risk difference by patient risk factors (sex, smoking history, and age [≥ 60 years or < 60 years]) with 95% confidence intervals (CI) were calculated for the acute, delayed, and overall phases. Subgroup analyses (patient risk factors, cisplatin dose [≥ 70 mg/m2 or < 70 mg/m2], and NK1RA [aprepitant or fosaprepitant]) were conducted to investigate the consistency of the effect by calculating the unadjusted risk difference. The period until the first CINV event in cycle 1 was described using the Kaplan-Meier method and estimated the median and 95% CI. The log-rank test was used to compare the groups. The time course of the CINV events by the day period in cycle 1 was analyzed.
Three sensitivity analyses were conducted to evaluate the robustness of the proportion of CINV events by cycle. First, a comparison was conducted for the generation of 5-HT3RA actually used for each cycle as that may have changed between cycles in this study. Therefore, we evaluated the efficacy of 5-HT3RA in each cycle as a sensitivity analysis. Second, we also analyzed the group using propensity score (PS) matching based on patient risk factors and cisplatin dose per body surface area (BSA) using 1:1 matching and a caliper width of 0.2 SD with the greedy nearest neighbor matching method. Third, we conducted inverse probability of treatment weighting (IPTW) with average treatment effect (ATE) weighting using PS.
Two-sided p values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics
This study was approved by the Ethics Committee of the Graduate School and Faculty of Medicine, Kyoto University (approval number: R1893, February 20, 2019). The study was performed according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Health, Labor and Welfare. The need for informed consent was exempted since anonymized data were used.
Results
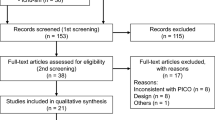
Overall, 5417 patients were extracted from the database according to the eligibility criteria; 1619 patients were excluded according to the exclusion criteria; thus, 3798 patients were included in the analysis (Fig. 1). The primary reasons for exclusion were prior antiemetic drug use (n = 1075) and the patient’s medical history (n = 899). Among the eligible patients, 1440 and 2358 patients were included in the 1st and 2nd groups, respectively. Patient demographics are shown in Table 1. The patient risk factors (sex, smoking history, and age) and the dose of cisplatin per BSA in cycle one did not significantly differ between the groups.
The use of antiemetic drugs, concomitant anticancer agents, and opioids is shown in Supplementary Table 1. In the 1st group, 1311 (91.0%) patients received granisetron, followed by 96 (6.7%) and 58 (4.0%) of patients who received ramosetron and azasetron, respectively. In the 2nd group, all patients received palonosetron. In the 1st group, 1242 (86.3%) and 199 (13.8%) patients received aprepitant and fosaprepitant, respectively. In the 2nd group, 1760 (74.6%) and 602 (25.5%) patients received aprepitant and fosaprepitant, respectively. In the 1st group, 100%, 79.3%, 72.6%, 35.0%, and 9.3% patients received DEX on days 1, 2, 3, 4, and 5, respectively. In the 2nd group, 100%, 77.7%, 70.4%, 35.2%, and 6.1% patients received DEX on days 1, 2, 3, 4, and 5, respectively. Among other concomitant anticancer drugs, 238 (16.5%) and 35 (2.4%) patients received trastuzumab and docetaxel in the 1st group, respectively. Additionally, 375 (15.9%) and 101 (4.3%) patients received trastuzumab and docetaxel in the 2nd group, respectively.
Outcome
Table 2 shows a summary of the CINV events. The risk difference in cycle 1 was − 7.39% (95% CI − 10.66 to − 4.12), − 8.49% (− 11.61 to − 5.36), and − 3.66% (− 6.77 to − 0.55) for the overall, acute, and delayed phases. There were significantly fewer events in the 2nd group for cycle 1. There was a similar trend for the overall phase and acute phase events from cycles 2–5. However, the delayed phase events did not significantly differ between the groups, with the exception of cycle 1. The CMH-adjusted risk difference showed the same tendency. In the sensitivity analyses, the same tendency was observed in the PS-matched group (Supplementary Tables 2 and 3) and the IPTW analyses (Table 2). The analysis of the actually administered 5-HT3RA taking into consideration the switch of generation was shown in Supplementary Table 4. In the subgroup analysis in cycle 1, the 2nd group showed consistently good trends in the most baseline characteristics (Table 3). The additional antiemetic drugs used in cycle 1 are shown in Supplementary Table 5.
The time-to-first-CINV event was significantly longer in the 2nd group than in the 1st group (p < 0.0001) (Fig. 2). The median time was 6 days (95% CI 5 to not to be estimated) in the 1st group and more than 6 days (95% CI could not be estimated) in the 2nd group. The proportion of patients with CINV events was higher in the 1st group than in the 2nd group at day 1 and slightly higher at days 3–4 (Fig. 3).
The treatment status of cisplatin and the proportion of patients who had their 5-HT3RA switched for each cycle are shown in Supplementary Table 6. The median number of cycles in each group was three; 514 (35.7%) and 857 (36.3%) patients in the 1st and 2nd groups were able to receive cisplatin for 5 cycles. There was no significant difference in the treatment status of cisplatin between the groups. After cycle 2, more than 5% of the patients in the 1st group were switched to the 2nd generation 5-HT3RA.
Discussion
In this study, we investigated the intergenerational comparison of 5-HT3RA with NK1RA and DEX using an administrative database. We found that cisplatin-induced CINV events in the acute and overall phases were significantly lower in the 2nd group than in the 1st group from cycles 1 to 5 in real-world clinical practice. The consistent efficacy in the sensitivity analyses indicated the robustness of the efficacy in the 2nd group over the 1st group. Furthermore, 2nd group also showed effectiveness in the time-to-first-CINV event. Conversely, the administration status of cisplatin did not differ between the groups, and the treatment intensity in the next cycle did not change even if the frequency of CINV events decreased.
We observed more substantial differences in the acute phase, with an 8.49% improvement compared to the delayed phase, with a 3.66% improvement in cycle 1. These findings differed from those used in clinical trials, one of which evaluated the three-drug combination (5-HT3RA + NK1RA + DEX) in HEC. That study found a complete response (CR) proportion for the acute and delayed phase improvement between the second-generation 5-HT3RA and first-generation 5-HT3RA which were reported to be approximately 0% and 8%, respectively [9]. The following differences may have caused this discrepancy. First, we could not obtain an accurate prescription time from the database. The definitions for the acute (0–24 h) and delayed (24–120 h) phases in clinical trials could not be accurately distinguished in this study; we used days 1–2 and 3–6 for the acute and delayed phases. Second, a CINV event in this study was defined as the use of only an additional antiemetic drug, which differs from that used in clinical trials. In clinical trials, the CR criteria have been defined as no use of rescue therapy (additional antiemetic drugs) and no vomiting. The validity of the CINV definition in this study has not been examined.
Additionally, unlike clinical trials, the actual administration of an additional antiemetic drug was not clear in real-world clinical practice. The use of rescue medication on days 1 and 5 has been reported to be approximately 8% and 20%, respectively, in clinical trials; however, they were approximately 20% and 10% in this study [14]. This difference was probably due to prophylactic administration, which might reduce the difference in the delayed phase and increase the difference in the acute phase in this study. However, the effectiveness of the 2nd group was observed at days 3–4 in the time course of CINV events. This may be due to the suppressing the peak of delayed emesis by the 2nd group.
The proportion of CINV events up to 5 cycles tended to decrease in both groups, but the differences between groups were consistent. This trend was also consistent with the results previously reported in clinical trials [15]. Patients who received cisplatin multiple times are considered to be in a relatively good condition in which cancer has not progressed, and no serious adverse events have occurred. Therefore, the CINV events decreased with each cycle.
The 5-HT3RA switch was often observed in the 1st group. In cycle 5, 8.2% of patients in the 1st group were using the second-generation 5-HT3RA, and 2.0% of patients in the 2nd group were using the first-generation 5-HT3RA, respectively. Insufficient antiemetic effect would cause this switching of the generation of 5-HT3RA in the 1st group more frequently. The frequency of CINV events that evaluated the switching of patients increased slightly in the 2nd group, but the overall trend did not change. HEC after cycle 2 would be more difficult to suppress CINV due to anticipatory emesis. Not causing the first CINV is important to avoid anticipatory emesis. It might have been better to use second-generation 5-HT3RA from the beginning instead of switching after CINV occurred.
Olanzapine has recently been reported to affect CINV positively [14, 16]. Therefore, the four-drug combination is a new option for the prevention of CINV in HEC. However, the use of olanzapine was only 0.1% in this study. In almost all the study periods, olanzapine was not covered by insurance for nausea and vomiting in Japan [7]. Moreover, the guidelines in Japan have not been recommended the use of olanzapine yet. Therefore, we could adequately compare the CINV effect in the three-drug combination during this study period.
In this study, there was little variation in subgroups of patient risk factors between the groups. Based on the subgroup analysis, the 2nd group showed consistently good trends in most subgroups. Although the number of patients was small, the difference between groups might be small for the cisplatin dose (≥ 70 mg/m2) subgroup at all phases. For this subgroup, more powerful antiemetic therapy, like adding olanzapine, may be useful.
Additionally, the percentage of NK1RA (aprepitant or fosaprepitant) usage was slightly different between the groups. But the efficacy of aprepitant and fosaprepitant for prevention of CINV have been reported to be the same [17]. And the subgroup analysis of NK1RA and other subgroups tended to be about the same trend. Therefore, this difference may have little impact on the comparison of CINV efficacy.
Gastric cancer patients were extracted from the database using the ICD-10 code and prescribed drugs in this study. The validity of the diagnosis of solid metastatic tumors in the DPC was previously reported as having a sensitivity and specificity of 58.5% and 98.5%, respectively [18]. Although the diagnosis of gastric cancer with the ICD-10 code has not been validated. The proportion of HER2-positive advanced or recurrent gastric cancer in Japan was reported to be approximately 10–20%, and the proportion of concomitant use of trastuzumab was approximately 16.1% in this study [19,20,21]. Other concomitant drugs (docetaxel and irinotecan) are also commonly used drugs for gastric cancer. Therefore, we believe that the patients included in this study were appropriately extracted as advanced gastric cancer.
Comparability between groups is often a problem in non-randomized studies. However, there were no significant differences in the clinical and demographic characteristics including sex, age, smoking history, and initial dose of cisplatin, which were patient risk factors for CINV without any adjustment in this study. We cannot eliminate the impact of unmeasured confounding factors, but we expected the comparability between the groups to be high in this study.
The two sensitivity analyses conducted using PS reduced the effect of observed confounding factors. Almost all the results were the same for the PS-adjusted and PS-unadjusted analyses. This might also support the robustness of the findings regarding CINV in this study.
The administration status of cisplatin did not change between the groups. Nausea and vomiting are unpleasant adverse events for patients and are known to reduce quality of life. However, in clinical practice, the cisplatin dose reduction or modification, which is solely due to nausea and vomiting, may not have been actively conducted. The median number of treatment cycles in clinical trials was reported to be three to six; however, in this study, the median number of treatment cycles was three in both groups [22, 23]. The early discontinuation of treatment before dose adjustment may be one reason for the lack of difference between the groups. The cycle of cisplatin in this study may have been too short to evaluate the continuity of HEC.
Limitations
We were not able to obtain the patient-reported outcomes of nausea and vomiting that are acquired in clinical trials from this database. Therefore, the sensitivity of the CINV event in this study was expected to be low. In clinical trials, the most commonly reported patient-reported outcomes were nausea, followed by the administration of rescue medication, and finally, vomiting [14]. Perhaps nausea first occurred, and rescue medication was administered before vomiting. Therefore, we could evaluate severe nausea, which required additional antiemetics and vomiting in this study. Due to this property, the definition of CINV event in this study was considered to have a certain level of sensitivity.
It was also difficult to distinguish the additional antiemetics needed for prophylactic administration or the treatment of nausea and vomiting. Therefore, the CINV event in this study may overestimate nausea and vomiting events. Similarly, because the prescription time could not be obtained, it was difficult to clearly distinguish the CINV events which occurred between the acute and delayed phases. However, in cycle 1, there were significant differences in the proportion of CINV events both in the acute and delayed phases, and even after cycle 2, the 2nd group had fewer CINV events than the 1st group. Therefore, second-generation 5-HT3RA antiemetics would not be inferior to the first-generation 5-HT3RA.
Finally, this study only evaluates the CINV event in gastric cancer patients receiving cisplatin-based chemotherapy. Therefore, different results may be obtained with other cancers and other HEC. Especially, the median number of treatment cycles of cisplatin was short in this study, so other HEC may have been suitable for assessing the continuity of HEC.
Conclusions
We investigated the effect of the second-generation 5-HT3RA on the first-generation 5-HT3RA for HEC in the triplet combination antiemetics repeatedly administered under clinical practice for the first time. Although we could not observe the improvement in the continuity of cisplatin administration, the observed consistent reduction of additional antiemetics would mean the prevention of CINV in the second-generation 5-HT3RA and this benefit was not observed in the clinical trials. The antiemetic effect against cisplatin-based chemotherapy in gastric cancer patients would be more effective in the second-generation 5-HT3RA than in the first-generation 5-HT3RA throughout the entire cycle.
Data availability
Not applicable.
References
Morita S, Kobayashi K, Eguchi K, Matsumoto T, Shibuya M, Yamaji Y, Sakamoto J, Ohashi Y (2003) Influence of clinical parameters on quality of life during chemotherapy in patients with advanced non-small cell lung cancer: application of a general linear model Jpn J Clin Oncol 33: 470–476
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Gilmore J, D’Amato S, Griffith N, Schwartzberg L (2018). Recent advances in antiemetics: new formulations of 5HT3-receptor antagonists. Cancer management and research 10: 1827-1857
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
Majem M, Moreno ME, Calvo N, Feliu A, Perez J, Mangues MA, Barnadas A (2011). Perception of healthcare providers versus patient reported incidence of chemotherapy-induced nausea and vomiting after the addition of NK-1 receptor antagonists. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 19: 1983-1990
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M (2016). 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27: v119-v133
Takeuchi H, Saeki T, Aiba K, Tamura K, Aogi K, Eguchi K, Okita K, Kagami Y, Tanaka R, Nakagawa K, Fujii H, Boku N, Wada M, Akechi T, Udagawa Y, Okawa Y, Onozawa Y, Sasaki H, Shima Y, Shimoyama N, Takeda M, Nishidate T, Yamamoto A, Ikeda T, Hirata K (2016) Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. International journal of clinical oncology 21: 1-12
Network NCC (2020) Anitiemesis (Version .2020 - February 19,2020). https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
Suzuki K, Yamanaka T, Hashimoto H, Shimada Y, Arata K, Matsui R, Goto K, Takiguchi T, Ohyanagi F, Kogure Y, Nogami N, Nakao M, Takeda K, Azuma K, Nagase S, Hayashi T, Fujiwara K, Shimada T, Seki N, Yamamoto N (2016). Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 27: 1601-1606
Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ (1998) Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 6:244–247
Japanese Gastric Cancer A (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer 14: 113–123
Nakashima M, Takeuchi M, Kawakami K (2020) Effectiveness and safety of regorafenib vs. trifluridine/tipiracil in unresectable colorectal cancer: a retrospective cohort study. Clin Colorectal Cancer
Mizuno K, Takeuchi M, Kanazawa Y, Kitamura M, Ide K, Omori K, Kawakami K (2019) Recurrent laryngeal nerve paralysis after thyroid cancer surgery and intraoperative nerve monitoring. Laryngoscope 129: 1954–1960
Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, Hoshina Y, Sakata Y, Takahashi TY, Nakashima K, Nakao M, Takei D, Zenda S, Mizukami K, Iwasa S, Sakurai M, Yamamoto N, Ohe Y (2020) Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21: 242-249
Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, Borroni ME, Jordan K (2014) A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 25: 1333-1339
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, Dietrich L, Biggs D, Lafky JM, Loprinzi CL (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375: 134–142
Grunberg S, Chua D, Maru A, Dinis J, DeVandry S, Boice JA, Hardwick JS, Beckford E, Taylor A, Carides A, Roila F, Herrstedt J (2011) Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol--EASE J Clin Oncol 29: 1495-1501
Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H (2017) Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 27:476–482
Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, Miyata Y, Takiuchi H, Yamaguchi K, Sasaki Y, Nishina T, Satoh A, Baba E, Tamura T, Abe T, Hatake K, Ohtsu A (2012) Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study Gastric Cancer 15: 313-322
Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A (2006) Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer Oncol Rep 15: 65-71
Matsusaka S, Nashimoto A, Nishikawa K, Miki A, Miwa H, Yamaguchi K, Yoshikawa T, Ochiai A, Morita S, Sano T, Kodera Y, Kakeji Y, Sakamoto J, Saji S, Yoshida K (2016) Clinicopathological factors associated with HER2 status in gastric cancer: results from a prospective multicenter observational cohort study in a Japanese population (JFMC44-1101) Gastric Cancer 19: 839-851
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial Lancet 376: 687-697
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial Lancet Oncol 9: 215-221
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. K is an company employee of Taiho Pharmaceutical Co., Ltd., Japan. K. K received advisory fees from Shin Nippon Biomedical Laboratories, Ltd., Japan, JMDC Inc., Japan, and LEBER Inc., Japan; holds stocks of Real World Data, Co., Ltd., Japan; and research funds from Sumitomo Dainippon Pharma Co., Ltd., Japan, Pfizer Inc., Japan, Stella Pharma Corporation., Japan, Cmic Co., Ltd., Japan, Suntory Beverage & Food Limited, Japan, Medical Platform Co., Ltd., Japan, Eisai Co., Ltd., Japan, Kyowa Hakko Kirin Co., Ltd., Japan, Mitsubishi Corporation, Japan, and Real World Data, Co, Ltd., Japan. The other authors have no direct or indirect conflicts of interest.
Ethics approval
This study was approved by the Ethics Committee of the Graduate School and Faculty of Medicine, Kyoto University (approval number: R1893, February 20, 2019). The study was performed according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Health, Labor, and Welfare.
Consent to participate
The need for informed consent was exempted because anonymized data were used.
Consent to publish
All authors provided consent to publish this work.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Kunitomi, Y., Nakashima, M., Seki, T. et al. Intergenerational comparison of 5-HT3RA in the prevention of chemotherapy-induced nausea and vomiting in gastric cancer patients receiving cisplatin-based chemotherapy: an observational study using a Japanese administrative claims database. Support Care Cancer 29, 3951–3959 (2021). https://doi.org/10.1007/s00520-020-05958-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05958-0