Abstract
Background
Breast cancer remains the number 1 lethal malignancy in women. With rising incidence and decreased mortality, the number of breast cancer survivors has increased. Consequently, sequelae, such as pain, are becoming more important.
Purpose
The purpose of this study was to identify risk factors for the development of pain in breast cancer survivors.
Methods
PubMed and Web of Science were systematically screened for studies encompassing risk factors for the development of pain in breast cancer survivors. Meta-analyses were carried out for risk factors described in more than one article. Moderator analysis was performed in case of high heterogeneity (I 2 > 50%) across studies.
Results
Seventeen studies were found eligible. Meta-analyses were performed for 17 factors. Significant differences for the odds of developing chronic pain were found for BMI (overall OR: 1.34, 95%CI 1.08–1.67, p = 0.008), education (overall OR: 1.23, 95%CI 1.07–1.42, p = 0.005), lymphedema (overall OR: 2.58, 95%CI 1.93–3.46, p < 0.00001), smoking status (overall OR: 0.75, 95%CI 0.62–0.92, p = 0.005), axillary lymph node dissection (overall OR: 1.25, 95%CI 1.04–1.52, p = 0.02), chemotherapy (overall OR: 1.44, 95%CI 1.24–1.68, p < 0.00001), and radiotherapy (overall OR: 1.32, 95%CI 1.17–1.48, p < 0.00001). After performing moderator analyses for age, comorbidities, hormone therapy, and breast surgery, hormone therapy became a significant risk factor as well (overall OR: 1.33, 95%CI 1.15–1.54, p = 0.0001).
Conclusion
BMI > 30, education < 12–13 years, lymphedema, not smoking, axillary lymph node dissection, chemotherapy, hormone therapy, and radiotherapy were significantly associated with higher odds for the development of chronic pain, with lymphedema being the biggest risk factor. Lack of uniformity across the studies in defining pain, follow-up, measurement tools, and cut-off values for the diagnosis of pain was noted, resulting in greater inter-study variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer remains the most lethal malignancy among women worldwide [1]. Over the last decades, the incidence of breast cancer has increased [1, 2]. According to estimates made by the International Agency for Research on Cancer (IARC), 1.67 million new cancer cases were diagnosed in 2012 worldwide [1]. The increasing incidence can partially be explained by improved detection, population growth, and aging of the population. Furthermore, a decline in mortality has been observed as a result of improved screening strategies and more effective treatment strategies [3]. Due to the combination of the declined mortality rate and the increased incidence, the number of breast cancer survivors has increased [4].
However, an important portion of breast cancer survivors has to deal with complications and sequelae of physical (lymphedema, neuropathy/pain, fatigue, menopausal symptoms, weight gain, etc.) and psychological nature (fear of recurrence, fear of death, change in body image, change in relationship, financial stress, etc.). These complications can arise during the treatment or can persist long after treatment cessation [5]. The development of chronic pain is one of the most frequently seen sequelae in the cancer survivor population [6]. Forsythe et al. reported that about 30% of the breast cancer survivors are confronted with above-average pain 10 years after ending the treatment [7].
The International Association for the Study of Pain (IASP) defined pain as “An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [8]. As the lesion recovers or the threat disappears, the pain sensation should normally decrease. However, when the pain remains persistent after the normal tissue healing process, it can be considered as chronic. To differentiate acute from chronic pain, the cut-off point of less than 1 month can be used, but 6 months is favored for research purposes [8].
So far, chronic pain in breast cancer survivors has been poorly defined in the literature.
In previous studies, there is a lack of uniformity in the applied definitions for chronic pain. Yet, postmastectomy pain syndrome has been defined by the IASP as “Chronic pain commencing immediately or soon after mastectomy or removal of a lump, affecting the anterior thorax, axilla, and/or medial upper arm.” [9]. However, this definition may be too limiting, as it only focuses on the operated region and rules out other natures of chronic pain like central sensitization.
The exact etiology underlying the development of pain in cancer survivors remains an enigma. Several researchers attempted to identify risk factors for the development of pain in breast cancer survivors [10, 11], which are not only of a crucial matter for the improvement of the prevention, but also for the implementation of treatment strategies.
To our knowledge, two systematic reviews were previously conducted regarding risk factors for the development of chronic pain in cancer survivors, but neither of them performed a meta-analysis or focused on the post-cancer treatment phase exclusively [10, 11]. In addition to reporting several risk factors for the development of chronic pain after breast surgery, Chang et al. [10] mainly focused reviewing the literature on the use of analgesic techniques for breast cancer surgery. However, eligibility criteria were not presented, making it difficult to assess whether the identified risk factors are applicable to breast cancer survivors specifically.
Andersen et al. identified several risk factors for the development of persistent pain after breast cancer [11], but also included studies on patients with recurrence and/or metastasis. A final reason warranting the need for a systematic review is the lack of reviews on risk factors of pain in cancer survivors since 2011. Given the increasing survival rate and associated interest of researchers, an increase in the number of studies can be expected. Therefore, a systematic review with strict eligibility criteria and exclusion of patients with recurrent cancer or metastasis is emerging.
Objective
The objectives of this review are: (1) to identify risk factors of pain in breast cancer survivors in a systematic, transparent, and reproducible way with strict eligibility criteria and (2) to conduct a meta-analysis.
We expect to find a combination of underlying factors such as cancer-related (tumor size, staging, etc.), treatment-related (chemotherapy, radiotherapy, surgery, etc.), and patient-related (age, body mass index (BMI), comorbidities, etc.) factors that might put breast cancer patients at an increased risk for the development of chronic pain.
Method
A systematic literature review was performed following the PRISMA guidelines [12]. To identify relevant studies regarding pain in cancer survivors, a systematic search of literature was conducted in databases PubMed and Web of Science up to March 2017. Authors were contacted if the full texts of studies could not be retrieved.
Eligibility criteria
In order to be included, studies needed to meet the following criteria:
-
(1)
subjects needed to fulfill our definition of a cancer survivor. According to the definition of the National Cancer Institute’s Office of Cancer Survivorship, a cancer survivor is “A patient with a history of cancer that is beyond the acute diagnosis and treatment phase” [13]. We used a dissimilar definition, as the cancer survivors had to be at least 6 months post-treatment (with exception of hormone therapy) and without recurrence or metastasis.
-
(2)
subjects needed to be diagnosed with breast cancer in the past
-
(3)
data to determine risk factors of pain had to be available.
The following exclusion criteria were applied:
-
(1)
study design: case reports, reviews, protocol, commentary, and letters
-
(2)
subjects not fulfilling the cancer survivor definition due to recurrence of cancer or diagnosed metastasis
-
(3)
time since completion of radiotherapy or chemotherapy was less than 6 months
-
(4)
time since surgery was less than 6 months
-
(5)
time since diagnosis was less than 1 year
-
(6)
subjects being diagnosed with other cancers besides breast cancer
-
(7)
pain was not presented as an outcome.
All articles were restricted to recent publications between 1990 and 2017 with a primary emphasis on English abstracts concerning humans.
Search
The primary search was performed in PubMed using MeSH terms and free key words. The search was based on the PECO method in which the population was represented as the cancer survivors, the exposure as risk factors, and the outcome as pain. A similar search was conducted in Web of Science, using free key words.
Study selection
The study selection encompassed two phases. In the first phase, duplicates were removed. Subsequently, all titles and abstracts were screened for eligibility in a blinded standardized manner by three independent researchers (S.V., T.B., and L.L.), using the Rayyan software [14]. Any disagreement between the three reviewers was resolved by consensus. In the second phase, the remaining articles were screened for full textual review by two researchers. In both phases, reasons for exclusion were registered.
Data collection process
A self-created extraction form was used to collect following data: year of publication, study design, sample size of participants, age (year ± SD), follow-up (year ± SD), type of pain, risk factor variable, number of patients with pain exposed to risk factor, number of patients without pain exposed to risk factor, number of patients with pain unexposed to risk factor, number of patients without pain unexposed to risk factor, odds ratio or relative risk, adjusted odds ratio or relative risk, significance, standard error (SE), pain outcome measures, cut-off values, and opioid analgesic use. Three authors extracted the data from included studies. Two authors double-checked the extracted data afterwards.
Four authors were contacted to obtain supplementary data concerning the risk factors BMI, age, and tumor size [7, 15,16,17]. Both number of patients with pain and the number of patients without pain of each subgroup were requested to make dichotomization possible.
Quality assessment
Three authors assessed the methodological characteristics of the included studies using the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) checklist.
This checklist contains 22 items, related to the different sections of the articles. Four items are specific for cohort, case-control, or cross-sectional studies; the remaining 18 items are common to all three study designs [18]. When an item was discussed in an article, it received one point. When nothing was mentioned about the item, a score of 0 was given. Disagreements were resolved by consensus.
Summary measures
Odds ratio (OR) with 95% confidence intervals (CIs) was the primary outcome measure. When an article did not mention the needed odds ratio, raw data (number of patients with and without pain of both the exposed and unexposed groups) were used to calculate the odds ratio.
These data were used afterwards to perform a meta-analysis by applying the random effect model [19].
Planned methods of analysis
Heterogeneity was assessed by the I 2 statistics using the method proposed by Higgens et al. [19]. The I 2 statistic represents an estimation of the inter-study variability. The significance of the heterogeneity was determined on the basis of the p - value obtained by the Chi-squared (χ2) test. Given the limited number of included studies for several risk factors, a p - value of 0.10 was used as cut-off for statistical significance instead of the more conventional level of 0.05 [20] (Deeks JJ et al. 2008). An I 2 value >50% was classified as an important presence of heterogeneity [19]. In this case, a moderator analysis was carried out, investigating possible underlying true systematic differences that may explain heterogeneity.
Not only the total score on the STROBE checklist, but also the presence of the most important items were taken into account. The study with the respectively highest scores on the items ‘data measurement’, ‘bias’, ‘outcome data’, ‘limitations’, ‘generalizability’ of the STROBE checklist were considered as best evidence. Studies that did not attain the average score of the STROBE checklist and scored a minimum of 2 out of 5 on the best evidence items were excluded from the meta-analyses.
The data acquired from the studies needed to be comparable to perform a meta-analysis. Therefore, several transformations were conducted: age was dichotomized as >50–55/<50–55 years [15, 21,22,23], alcohol use as yes/no [21], BMI as >30/<30 [7, 21, 22, 24, 25], chemotherapy as yes/no [21], radiotherapy as yes/no [26], hormone therapy as yes/no [21], education as >12–13/<12–13 years [21, 22, 25], tumor size as >20 mm/<20 mm [21, 23], cancer stage as stage 1/>stage 1 [21, 23, 25], smoking status as ex-or no-smoker/smoker [25], and cohabitation status as single/cohabiting [15, 22, 25].
Instead of using the given adjusted OR (<46 years) of the risk factor age by Bredal et al., which was not based on similar groups (>50–55/<50–55) as with other authors, the age groups with and without pain in this study were dichotomized and afterwards used for calculation of a comparable OR [15].
In the study of Johannsen et al., the 7–9-year post-surgery OR was preferred over the 15 months equivalent for the meta-analysis, as the 7–9-year post-surgery OR is a better approximation of the follow-up of the other studies [21].
All meta-analyses were conducted with the RevMan software (Review manager 5.3).
Results
Study selection
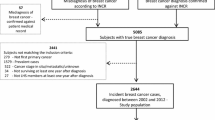
The initial search of data yielded a total of 934 articles on PubMed and 864 on Web of Science. After removing duplicates, 1431 articles remained. These studies were screened on title and abstract. Based on the prespecified exclusion criteria, 1357 articles were excluded.
The remaining 74 articles were screened for eligibility by full textual review. Fifty-seven of the 74 articles were excluded due to not fulfilling the cancer survivor definition (n = 32) or providing insufficient data (n = 25). Therefore, 17 articles were included in our synthesis. Figure 1 presents the flowchart of the study selection process.
Study characteristics
The 17 included studies consisted of five cohort [7, 16, 21, 23, 27] and 12 cross-sectional studies [15, 17, 22, 24,25,26, 28,29,30,31,32,33]. In all studies, the breast cancer survivors were at least 6 months post-treatment. The sample size of the studies ranged from 85 to 2160. Four studies were performed in the USA [7, 30, 32, 34], three in Denmark [21, 22, 24], two in Sweden [26, 33], two in Turkey [25, 29], two in Australia [16, 35], one in the Netherlands [27], one in Belgium [17], one in Spain [23], and one in Norway [15].
Across those studies, several pain measurement tools were used with varying cut-off values. For the assessment of pain, 11 articles used a study specific questionnaire [17, 21,22,23,24,25,26, 30, 32, 33, 35], and four other studies relied on a valid measurement tool [7, 15, 16, 34]. Two studies did not provide a proper description of the measurement tools used to diagnose pain [27, 29]. The studies and their characteristics are listed in Tables 1, 2, and 3.
Quality assessment
The results of the individual studies on the STROBE checklist are presented in Fig. 2, with scores ranging from 12 to 20 out of 22 points. The mean score on the STROBE checklist was 16.18 ± 2.33, indicative for a moderate quality. The main weaknesses were lack of correct information on ‘title and abstract’, ‘objectives’, ‘study design’, ‘statistical methods’, ‘funding’, and scarce efforts to address potential sources of bias.
Risk factors for the development of chronic pain in cancer survivors
Looking at the included studies, over 70 risk factors were examined for the development of chronic pain in cancer survivors.
The risk factors presented in two or more studies are as follows: age [15, 16, 21,22,23,24,25, 27], alcohol use [21, 25], BMI [7, 16, 21, 22, 24,25,26, 33], children [21, 25], comorbidities [15, 21, 25], education [15, 21, 22, 25, 35], lymphedema [15, 29, 35], relationship status [15, 21, 22, 25, 35], smoking status [21, 25], axillary surgery [15, 16, 21, 23, 24, 29], breast surgery [15, 16, 21,22,23, 25, 29, 35], chemotherapy [15, 17, 21,22,23,24, 29, 35], hormone therapy [15, 21, 29, 32, 35], radiotherapy [15, 17, 21,22,23,24,25,26, 29, 33, 35], cancer stage [21, 23, 25, 28], hormone receptor status [21, 35], and tumor size [17, 21, 23, 24, 35].
Meta-analyses were carried out for the risk factors described in more than one article (Appendix A).
Patient-related risk factors
Age
Steyaert et al. [17] did not provide sufficient data and was subsequently removed. The meta-analysis demonstrated a significant difference for the chance of developing chronic pain in breast cancer survivors between the >50–55-year group and the <50–55-year group. Data from eight studies [15, 16, 21,22,23,24,25, 27] (n = 7048) were combined, showing that the odds for developing chronic pain in subjects with an age >50–55 were lower than those with an age <50–55 (overall OR: 0.76, 95% CI 0.57–1.01, p = 0.06); however, these results were found to be not significant.
A moderator analysis was carried out since the heterogeneity across the studies was high (I 2 = 76%, p = 0.0001). After withdrawing the weakest methodological studies [17, 22, 23, 25], the heterogeneity increased a bit (I 2 = 82%, p = 0.001) and the overall odds ratio for the development of chronic pain for breast cancer survivors older than 50–55 years, in comparison with younger breast cancer survivors, lowered to 0.65 (overall OR: 0.65, 95% CI 0.42–1.01, p = 0.05). The results of all moderator analyses can be found in Table 4.
Alcohol use
Alcohol use was studied in two articles (n = 2519) [21, 25]. A significant lower chance to develop chronic pain was found in patients consuming alcohol compared to those not consuming alcohol (overall OR: 0.94, 95% CI 0.47–1.89, p = 0.86). A high heterogeneity was found (I 2 = 67%, p = 0.08) but given the fact that this risk factor was only discussed in two articles, no moderator analysis could be performed.
BMI
Bredal et al. [15] and Forsythe et al. [7] did not provide the required data. The groups, on which the given OR in Bredal et al. was based, were not clearly presented. Consequently, Bredal et al. was excluded from the meta-analysis [15]. The original OR of Forsythe et al., based on the obese and normal weighted cancer survivors without taking overweight participants into account, was used in the meta-analysis instead of a newly formed OR based on all participants [7]. This limitation was taken into account for possible moderator analysis.
A significant dissimilarity of the odds for the development of chronic pain in breast cancer survivors is presented in the meta-analysis between the two BMI groups (>30 and <30).
The odds for the development of chronic pain are 1.33 times higher in people with a BMI > 30 compared to those with a BMI < 30, according to the data from the 6 combined studies (n = 5573) (overall OR: 1.34, 95% CI 1.08–1.67, p = 0.008) [7, 16, 21, 22, 24, 25]. No significant heterogeneity (I 2) was found (I 2 = 33%, p = 0.19).
Children
The risk factor ‘children’ was discussed in two studies (n = 2519) [21, 25]. The meta-analysis delivered no difference between the risk for developing chronic pain after having children or not (overall OR 0.92, 95% CI 0.69–1.23, p = 0.56). A low heterogeneity was found (I 2 = 0%, p = 0.74).
Comorbidities
Comorbidities were approached in three articles (n = 3353) [15, 21, 25]. No significant intergroup difference was observed (overall OR: 1.11, 95% CI 0.50–2.44, p = 0.80). Furthermore, a significant heterogeneity was found (I 2 = 93%, p < 0.00001), which could possibly be explained by the discrepancy in the applied definitions for comorbidities. Bredal et al. defined comorbidities as previous pain, whereas Johannsen et al. and Alkan et al. did not provide a proper definition for comorbidities, possibly resulting in the inclusion of non-pain-related disorders [15, 21, 25].
A moderator analysis was performed with the removal of Alkan et al. [25]. No improvement in heterogeneity was found (I 2 = 95%, p < 0.00001), nor did the analysis have an influence on the odds ratio (overall OR: 1.23, 95% CI 0.33–4.56, p = 0.76).
Education
Five articles reported on education (n = 5209) [15, 21, 22, 25, 35]. A significant higher chance to develop chronic pain was found in subjects with a lower education (<12 or 13 years) (overall OR: 1.23, 95% CI 1.07–1.42, p = 0.005). A low heterogeneity was found (I 2 = 10%, p = 0.35).
Lymphedema
Lymphedema was studied in three articles (n = 1459) [15, 29, 35]. The meta-analysis found that the odds of developing chronic pain were 2.58 times higher in the group with lymphedema (overall OR: 2.58, 95% CI 1.93–3.46, p < 0.00001). No significant heterogeneity was observed (I 2 = 0%, p = 0.40).
Relationship status
Five articles (n = 5209) declared the relationship status as risk factor [15, 21, 22, 25, 35]. The meta-analysis did not lead to a significant difference between groups (overall OR: 1.05, 95% CI 0.88–1.26, p = 0.56). A modest heterogeneity was observed (I 2 = 31%, p = 0.21).
Smoking status
A significant dissimilarity of the odds for the development of chronic pain in breast cancer survivors is presented in the meta-analysis between the smoking and ex-/no-smoking group. Smokers have a smaller chance to develop chronic pain in comparison to the ex- or no-smoking group (overall OR: 0.75, 95% CI 0.62–0.92, p = 0.005). No heterogeneity was observed (I 2 = 0%, p = 0.80).
Others
Several other patient-related risk factors were only studied once, such as the following: monthly income [25], posttraumatic stress disorder [25], psychiatric support [25], regular use of analgetics [25], social support [25], severity of initial pain [35], quality of original symptoms [35], anxiety [15], depression [15], race [30], menopausal status [34], physical activity [7], television time [7], occupational status [21], personal income [21], household net wealth per person [21], ethnicity [21], Physical Activity Scale for the Elderly (PASE) components [21], SF-36 Physical Function, physical function [16], insomnia [16], baseline pain [16], emotional function [16], arm symptoms [16], breast symptoms [16], time since surgery [22], medication use [22], Charlson index [23], height [17], weight [17], and recall of preoperative pain [17]. The data regarding these risk factors are further specified in Table 1.
Treatment-related risk factors
Axillary surgery: axillary lymph node dissection versus sentinel lymph node biopsy
This risk factor was examined in six articles (n = 4263) [15, 16, 21, 23, 24, 29]. In this meta-analysis, a significant difference between axillary dissection and sentinel dissection was found. Patients who underwent axillary dissection had a 1.25 greater chance to develop chronic pain (overall OR: 1.25, 95% CI 1.04–1.52, p = 0.02) compared to those who underwent a sentinel dissection. I 2 was 27% (p = 0.23), indicative for moderate heterogeneity.
Breast surgery: mastectomy versus breast conserving surgery
The risk factor ‘breast surgery’ was discussed in eight articles (n = 6472) [15, 16, 21,22,23, 25, 29, 35]. The meta-analysis delivered no significant difference between the risk for developing chronic pain after mastectomy compared to breast conserving surgery (overall OR 0.92, 95% CI 0.75–1.14, p = 0.47). An important heterogeneity was found (I 2 = 59%, p = 0.02). After performing a moderator analysis with withdrawal of the methodological weakest studies [22, 23, 25, 28], the heterogeneity decreased (I 2 = 44%, p = 0.15) and the odds ratio increased (overall OR: 1.03, 95% CI 0.78–1.36, p = 0.83).
Chemotherapy
Seven studies (n = 4810) encompassed this category [15, 17, 21, 23, 24, 29, 35]. Overall, a significant difference in odds for the development of chronic pain was found between subjects treated with and without chemotherapy. The odds in subjects who received chemotherapy were 1.44 times higher compared to those who did not receive chemotherapy (overall OR: 1.44, 95% CI 1.23–1.69, p < 0.00001). A slight heterogeneity was observed (I 2 = 33%, p = 0.18).
Hormone therapy
Hormone therapy was studied in eight articles (n = 5823) [15, 17, 21, 23, 24, 29, 32, 35].
The meta-analysis demonstrated a non-significant difference between participants subjected to and not subjected to hormone therapy (overall OR: 1.16, 95% CI 0.99–1.37, p = 0.07).
Since a significant heterogeneity was detected (I 2 = 47%, p = 0.07), a moderator analysis was performed. After removal of the weakest methodological studies [17, 23, 28], the results became homogenous (I 2 = 0%, p = 0.54). Survivors exposed to hormone therapy were 1.33 times more likely to develop chronic pain (overall OR: 1.33, 95% CI 1.15–1.54, p = 0.0001).
Radiotherapy
Data from 11 articles (n = 7806) were combined for the meta-analysis of radiotherapy as risk factor to develop chronic pain [15, 17, 21,22,23,24,25,26, 29, 33, 35]. A significant increased chance of developing chronic pain was found in patients exposed to radiotherapy compared to the unexposed patients (overall OR: 1.32, 95% CI 1.17–1.48, p < 0.00001). A slight heterogeneity was found (I 2 = 22%, p = 0.24).
Others
The other treatment-related risk factors that were only mentioned by one study are as follows: interval after surgery [25], taxane in adjuvant therapy [25], reoperation [25], dominant hand as side of surgery [24], boost dosage radiotherapy [27], number of lymph nodes removed [35], endocrine therapy at FQ5 [35], any surgery from FQ1-FQ5 [35], chemotherapy and locoregional radiotherapy [15], breast reconstruction [29], complications [29], Danish Breast Cancer Cooperative Group (DBCG) protocol [21], irradiated volume with ≥40 Gy (cm3) [33], highest dose (Gy) [26], detection method [23], neoadjuvant treatment [36], lymph node involvement [17], duration of surgery [17], and perioperative anesthesthetics and analgetics [17].
Specific details concerning these risk factors are outlined in Table 2.
Cancer-related risk factors
Cancer stage
The cancer stages were discussed in four articles (n = 4116) [21, 23, 25, 28].
No significant intergroup difference was observed for patient diagnosed with a stage 1 or stage >1 cancer (overall OR: 1.07, 95% CI 0.80–1.44, p = 0.64).
A significant heterogeneity was found (I 2 = 56%, p < 0.08). However, no moderator analysis could be performed since 3 out of the 4 included studies had to be removed [23, 25, 28], based on the prespecified criteria, leaving us with only 1 study in the meta-analysis.
Hormone receptor status
Two studies reported on hormone receptor status (n = 2445) [21, 35]. The meta-analysis of these results demonstrated no significant difference in the risk for development of chronic pain (overall OR: 1.10, 95% CI 0.81–1.50, p = 0.53) and no heterogeneity could be observed (I 2 = 0%, p = 0.72).
Tumor size >20 mm
Steyaert et al. [17] did not provide sufficient data and was subsequently removed. The tumor size was examined in four articles (n = 3891) [21, 23, 24, 35]. The meta-analysis revealed no significant difference (overall OR: 1.12, 95% CI 0.94–1.35, p = 0.20). Furthermore, no heterogeneity was observed (I 2 = 0%, p = 0.65).
Others
The remaining cancer-related risk factors, only discussed in one study, encompass the following: location of the tumor [24], tumor side [24], lymph node status [21], histological type [23], and phenotype [36].
A detailed overview of these risk factors can be found in Table 3.
Discussion
The purpose of this systematic review and meta-analysis was to identify factors that contribute to the development of chronic pain in breast cancer survivors. Seventeen different studies were included which together provided over 70 different risk factors. For 17 risk factors, it was possible to carry out a meta-analysis. Seven out of the 17 examined factors (BMI > 30, education <12–13 years, lymphedema, no- or ex-smoker, axillary lymph node dissection, chemotherapy, and radiotherapy) demonstrated to be significantly associated with an elevated chance for the development of chronic pain in breast cancer survivors, with lymphedema being the strongest risk factor. The remaining ten risk factors (age < 50-55, alcohol use, children, comorbidities, relation status, breast surgery, hormone therapy, cancer stage, hormone receptor status, and tumor size) are not related to the development of chronic pain in breast cancer survivors. After applying moderator analyses for the meta-analyses with a high grade of heterogeneity (age, comorbidities, hormone therapy, breast surgery), hormone therapy became a significant risk factor for the development of chronic pain in breast cancer survivors as well.
In the past, two systematic reviews concerning risk factors for the development of chronic pain were conducted [10, 11]. Chang et al. reported age younger than 65 years, type of surgery (breast-conserving surgery, breast reconstruction, axillary dissection), higher post-operative pain scores, and radiotherapy to be risk factors for the development of chronic pain following breast surgery [10]. The findings for age and radiotherapy were comparable to the results of this review. However, it should be taken into consideration that Chang et al. primarily focused on patients after breast surgery, making a true comparison with the present findings difficult [10]. The second review failed in the detection of significant risk factors due to the unclear definitions of pain, treatment, and outcome measures and methodological weakness of the found articles [11]. This study proclaimed that data collection needs to be performed in a more systematical way [11].
Whether lymphedema leads to the development of chronic pain in breast cancer survivors or not has been a point of discussion. However, the present study demonstrated that lymphedema is the strongest risk factor for the development of chronic pain in breast cancer survivors. Results from previous studies deliver indirect evidence for the relation between lymphedema and pain in gynecological cancer survivors, in which a reduction of the lymphedema was correlated with a decrease in pain after applying complex decongestive physiotherapy [37]. Furthermore, one should be aware of the fact that breast cancer-related lymphedema might cause many inconveniences in the upper extremity, such as a poor range of motion, stiffness, weakness, numbness, a general poor upper body function, and pain [38]. Jeong et al. stated that the rotator cuff tendinitis is a frequently seen complication in patients with lymphedema. A total of 53.3% of the patients with lymphedema were diagnosed with a supraspinatus tear (75% showed a partial thickness tear and 25% a full thickness tear), 53.3% with an adhesive capsulitis, 13.3% with a tenosynovitis, 13.3% with an acromioclavicular arthritis, and 13.3% with a subdeltoid bursitis [38]. All these definitive structural abnormalities in patients with lymphedema might in turn lead to the development of pain [38].
The presence of obesity has been postulated as another associated factor in the development of chronic pain, which was demonstrated to be significant in this study [39, 40]. Taylor R. Jr. et al. suggested that obesity could possibly lead to the development of pain due to mechanical stress and metabolic disruptions [39]. Furthermore, they stated that emotional factors such as stress, anxiety, and depression could arise from the significant obesity and pain burdens on the individual, the healthcare system, and society as a whole, which in turn lead to further healthcare utilization and burden [39]. According to Okifuji A. et al., there are several potential contributors linking obesity to pain such as the following: mechanical and structural changes due to the increased loading (e.g., altered body mechanics and postures), chemical mediators (e.g., proinflammatory cytokines causing a low-grade chronic inflammatory state), depression, disturbed sleep, and an inactive lifestyle [40]. The presence of both pain and obesity often lead to a vicious cycle of pain–inactivity–obesity.
After axillary dissection, axillary web syndrome (AWS) might occur. AWS is characterized by the formation of multiple chords that span from the axilla to the medial arm in a web-like matter. AWS can trigger a painful sensation when performing movements in which abduction of the shoulder is involved [41, 42]. Postoperative pain is commonly seen in breast cancer survivors and is mostly caused by changes in the peripheral and central nervous system (CNS). Due to the local tissue injury of the surgery, an increased sensitivity of the nociceptors to stimuli (primary hyperalgesia) and a spontaneous firing of these nociceptors will be observed. Secondary hyperalgesia may occur after a disproportional pain experience due to the central neural plasticity. Secondary hyperalgesia is thought to be the basis for chronic post-surgical pain [43, 44].
Chemotherapy and/or adjuvant radiotherapy are frequently administered after breast cancer surgery. It is well known that both have the ability to cause neurotoxity and neuropathic pain [45, 46]. Chemotherapy can cause damage to the nerves and induce peripheral neuropathy; however, the exact pathophysiologic mechanism underlying the nerve injury in chemotherapy-induced peripheral neuropathy (CIPN) is still not completely understood. CIPN appears to be agent-specific and is thought to be caused by drug-induced damage to components of the peripheral nervous system (PNS). As a consequence of the structural damage to the PNS, the somatosensory processing in the central and peripheral nervous systems is abnormal and results in allodynia, hyperalgesia, and pain. Pain processing abnormalities seem to play an important role in the development of chronic painful conditions [47, 48].
Despite the benefits of adjuvant radiotherapy in reducing tumor burden, it might induce late effects. As it causes a high toxicity to the skin and vital organs, it might subsequently lead to the development of significant chronic pain. Delayed painful brachial and lumbosacral plexopathies, osteoradionecrosis and fractures, pelvic pain, and in some cases secondary malignancies have been reported after radiotherapy [49,50,51].
About 75% of the postmenopausal patients are diagnosed with a hormone receptor-positive breast cancer for which endocrine treatment is prescribed [53]. Despite the fact that they improve the disease-free survival by 10–40%, about 46% will develop aromatase inhibitor-induced arthralgia (AIA), an adverse event not only leading to a decrease in health-related quality of life but also in treatment compliance [54,54,55,56,57,58,60]. The exact mechanism underlying the development of the AIA remains an enigma. Hershman et al. suggests that this phenomenon is the result of estrogen deprivation and shares components with auto-immune diseases [61]. Another study suggests that aromatase inhibitors might selectively target the transient receptor potential ankyrin 1 (TRPA1) channel [62]. The stimulation of TRPA1 through the aromatase inhibitors is associated with the release of pro-inflammatory neuropeptides from sensory nerve endings, which mediate neurogenic inflammatory responses in the innervated peripheral tissue [62].
Overall, there is not only a lack of uniformity in the definition of pain, but also in the use of measurement tools and applied cut-off values for the diagnosis of pain. These dissimilarities might possibly explain the observed differences between the studies.
Furthermore, it should be noted that the current literature regarding risk factors for the development of chronic pain in breast cancer survivors has a primary focus set on treatment-related, cancer-related, and demographic factors. There is a need to shift the research focus away from the biomedical point of view onto the broader biopsychosocial dimension, as it is demonstrated that psychosocial factors play an important role in other chronic pain conditions [63,63,64]. Turk et al., for example, described that avoiding activities due to fear of pain plays a relevant part in the persistency and aggravation of pain [63]. According to Boersma et al., fear acts as a risk factor for the development or persistence of chronic pain [64]. Additionally, psychological factors like anxiety, depression, stress, and catastrophizing are proven to strongly correlate with chronic post-surgical pain [64].
The present study has several strengths, including the compliance with the PRISMA guidelines [12] for rigorous performance and reporting of systematic literature reviews and meta-analyses, the use of multiple, blinded researchers to perform the literature searches as well as data processing and quality assessments. Also, this is the first meta-analysis of all available studies exploring possible risk factors for the development of chronic pain in breast cancer survivors. Of course, some limitations to our study should be acknowledged.
First, in order to perform the meta-analyses, the ORs of the different studies needed to be comparable with each other. Therefore, dichotomization of data was needed, which is inextricably linked to a loss of information. For instance, Johannsen et al. compared the normal weight group with four other BMI groups. The results showed that the ‘obese group’ had a higher chance for the development of chronic pain, which could not be observed for the ‘severely obese group’. This kind of information could not be retrieved from our meta-analyses.
Second, using the STROBE involves some restrictions since it is constructed to provide guidance on how to report observational studies properly. It is not meant as a methodological guideline or assessment tool, in which all items can be assigned with equal weights, as some items are more meaningful than others. Therefore, the use of overall scores is, from an objective point of view, inappropriate and makes the interpretation of the results harder. However, this limitation was countered by taking into account the five most important STROBE-items for the moderator analysis.
As this review encompasses only a limited number of studies and the strength of those studies is merely mediocre, the strength of the evidence is not sufficient to draw firm conclusions regarding the risk factors for the development of chronic pain. Future research should focus on providing a proper definition for pain in breast cancer survivors. Furthermore, a consensus should be drawn about globally accepted measurement tools with clear cut-off values for the diagnosis of chronic pain in this population. This would bring more clarity and uniformity across studies examining pain. Proper knowledge about risk factors is of imperative need to not only screen patients that are at a higher risk for the development of pain but also to preventively target those factors in order to avoid acute pain from becoming chronic.
References
Ferlay J et al (2015) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Ferlay J et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Katanoda K, Matsuda T (2014) Five-year relative survival rate of breast cancer in the USA, Europe and Japan. Jpn J Clin Oncol 44(6):611
Organization, W.H., World cancer report 2014. 2014, Lyonn, France: International Agency for Research on Cancer.
Befort CA, Klemp J (2011) Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Women's Health 20(9):1307–1313
Glare PA et al (2014) Pain in cancer survivors. J Clin Oncol 32(16):1739–1747
Forsythe LP et al (2013) Pain in long-term breast cancer survivors: The role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat 137(2):617–630
Pain, I.A.f.t.S.o. Pain, IASP Taxonomy. 2014 2014–10-06 [cited 2016 2016–03-26]; Available from: http://www.iasp-pain.org/Taxonomy - Pain.
Merskey, H. and N. Bogduk, Eds. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Second edition ed. 1994, IASP Press: Seattle
Chang SH, Metha V, Langford R (2009) Acute and chronic pain following breast surgery. Acute Pain 11(1):1–14
Andersen KG, Kehlet H (2011) Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain 12(7):725–746
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 62(10):e1–34
NCI, O.o.C.S., About Cancer Survivorship Research: Survivorship Definitions.. 2012, Washington, DC
Elmagarmid, A., et al., Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews., in Evidence-Informed Public Health: Opportunities and Challenges. Abstracts of the 22nd Cochrane Colloquium. 2014, John Wiley & sons: Hyderabad, India
Bredal IS et al (2014) Chronic pain in breast cancer survivors: Comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manag 48(5):852–862
Moloney N et al (2016) Prevalence and risk factors associated with pain 21 months following surgery for breast cancer. Support Care Cancer 24(11):4533–4539
Steyaert A et al (2016) Does the perioperative analgesic/anesthetic regimen influence the prevalence of long-term chronic pain after mastectomy? J Clin Anesth 33:20–25
von Elm E et al (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg (London, England) 12(12):1495–1499
Higgins JP et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Higgins, J.P.T. and S. Green, Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. Chapter 9: Analysing data and undertaking meta-analyses., ed. J.J. Deeks, J.P.T. Higgins, and D.G. Altman. 2008: The Cochrane Collaboration.
Johannsen M et al (2015) Socio-demographic, treatment-related, and health behavioral predictors of persistent pain 15 months and 7-9 years after surgery: A nationwide prospective study of women treated for primary breast cancer. Breast Cancer Res Treat 152(3):645–658
Peuckmann V et al (2009) Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain 13(5):478–485
Romero A et al (2016) Prevalence of persistent pain after breast cancer treatment by detection mode among participants in population-based screening programs. BMC Cancer 16
Andersen Juhl A, Christiansen P, Damsgaard TE (2016) Persistent pain after breast cancer treatment: A questionnaire-based study on the prevalence, associated treatment variables, and pain type. J Breast Cancer 19(4):447–454
Alkan A et al (2016) Breast cancer survivors suffer from persistent postmastectomy pain syndrome and posttraumatic stress disorder (ORTHUS study): A study of the palliative care working committee of the Turkish oncology group (TOG). Support Care Cancer 24(9):3747–3755
Lundstedt D et al (2015) Radiation therapy to the plexus brachialis in breast cancer patients: Analysis of paresthesia in relation to dose and volume. Int J Radiat Oncol Biol Phys 92(2):277–283
Bantema-Joppe EJ et al (2012) Simultaneous integrated boost irradiation after breast-conserving surgery: Physician-rated toxicity and cosmetic outcome at 30 months' follow-up. Int J Radiat Oncol Biol Phys 83(4):e471–e477
Bell RJ et al (2014) Persistent breast pain 5 years after treatment of invasive breast cancer is largely unexplained by factors associated with treatment. J Cancer Surviv 8(1):1–8
Gulluoglu BM et al (2006) Factors related to post-treatment chronic pain in breast cancer survivors: The interference of pain with life functions. Int J Fertil Womens Med 51(2):75–82
Calhoun C, Helzlsouer KJ, Gallicchio L (2015) Racial differences in depressive symptoms and self-rated health among breast cancer survivors on aromatase inhibitor therapy. J Psychosoc Oncol 33(3):263–277
Crandall C et al (2004) Association of breast cancer and its therapy with menopause-related symptoms. Menopause- J North Am Menopause Soc 11(5):519–530
van Londen GJ et al (2014) Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support Care Cancer 22(4):937–945
Lundstedt D et al (2010) Symptoms 10-17 years after breast cancer radiotherapy data from the randomised SWEBCG91-RT trial. Radiother Oncol 97(2):281–287
Crandall C et al (2004) Association of breast cancer and its therapy with menopause-related symptoms. Menopause 11(5):519–530
Bell RJ et al (2014) Persistent breast pain 5 years after treatment of invasive breast cancer is largely unexplained by factors associated with treatment. Journal of Cancer Survivorship 8(1):1–8
Berrios-Rivera R, Rivero-Vergine A, Romero I (2008) The pediatric cancer hospitalization experience: Reality co-constructed. J Pediatr Oncol Nurs 25(6):340–353
Kim SJ, Park YD (2008) Effects of complex decongestive physiotherapy on the oedema and the quality of life of lower unilateral lymphoedema following treatment for gynecological cancer. Eur J Cancer Care (Engl) 17(5):463–468
Jeong HJ et al (2011) Causes of shoulder pain in women with breast cancer-related lymphedema: A pilot study. Yonsei Med J 52(4):661–667
Taylor RJ et al (2014) Pain and obesity in the older adult. Curr Pharm Des 20(38):6037–6041
Okifuji A, Hare BD (2015) The association between chronic pain and obesity. J Pain Res 14(8):399–408
Piper, M., et al (2016), Axillary Web Syndrome: Current Understanding and New Directions for Treatment. Ann Plast Surg
Yeung WM, McPhail SM, Kuys SS (2015) A systematic review of axillary web syndrome (AWS). J Cancer Surviv 9(4):576–598
Cregg R, Anwar S, Farquhar-Smith P (2013) Persistent postsurgical pain. Curr Opin Support Palliat Care 7(2):144–152
Shipton EA (2011) The transition from acute to chronic post surgical pain. Anaesth Intensive Care 39(5):824–836
Delanian S, Lefaix JL, Pradat PF (2012) Radiation-induced neuropathy in cancer survivors. Radiother Oncol 105(3):273–282
Grisold W, Cavaletti G, Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro-Oncology 14:45–54
Bhagra A, Rao RD (2007) Chemotherapy-induced neuropathy. Curr Oncol Rep 9(4):290–299
Boland EG et al (2014) Central pain processing in chronic chemotherapy-induced peripheral neuropathy: A functional magnetic resonance imaging study. PLoS One 9(5):e96474
Lee E et al (2016) Characterization of risk factors for adjuvant radiotherapy-associated pain in a tri-racial/ethnic breast cancer population. Pain 157(5):1122–1131
Burton AW et al (2007) Chronic pain in the cancer survivor: A new frontier. Pain Med 8(2):189–198
Levy MH, Chwistek M, Mehta RS (2008) Management of chronic pain in cancer survivors. Cancer J 14(6):401–9
Altundag K, Ibrahim NK (2006) Aromatase inhibitors in breast cancer: An overview. Oncologist 11(6):553–562
Mao JJ et al (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115(16):3631–3639
Beckwee, D., et al. (2017) Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Support Care Cancer
Howell A et al (2005) Results of the ATAC (Arimidex, tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365(9453):60–62
Goss PE et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349(19):1793–1802
Jakesz R et al (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366(9484):455–462
Burstein HJ (2007) Aromatase inhibitor-associated arthralgia syndrome. Breast 16(3):223–234
Peppone LJ et al (2015) The effect of YOCAS(c)((R)) yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Cancer Res Treat 150(3):597–604
Hershman DL, Loprinzi C, Schneider BP (2015) Symptoms: Aromatase inhibitor induced Arthralgias. Adv Exp Med Biol 862:89–100
Fusi C et al (2014) Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun 5:5736
Turk DC, Wilson HD (2010) Fear of pain as a prognostic factor in chronic pain: Conceptual models, assessment, and treatment implications. Curr Pain Headache Rep 14(2):88–95
Boersma K, Linton SJ (2005) Screening to identify patients at risk: Profiles of psychological risk factors for early intervention. Clin J Pain 21(1):38–43
Rashiq S, Dick BD (2014) Post-surgical pain syndromes: A review for the non-pain specialist. Can J Anesth 61(2):123–130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Jo Nijs is holder of a Chair funded by the Berekuyl Academy, the Netherlands. Roselien Pas is a PhD student funded by the Berekuyl Acadely Chair.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 522 kb)
Rights and permissions
About this article
Cite this article
Leysen, L., Beckwée, D., Nijs, J. et al. Risk factors of pain in breast cancer survivors: a systematic review and meta-analysis. Support Care Cancer 25, 3607–3643 (2017). https://doi.org/10.1007/s00520-017-3824-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3824-3