Abstract
Purpose
In this prospective trial, we evaluated the influence of chemotherapy for breast cancer on women’s health-related quality of life (HR-QoL), sexual function, and mental status.
Methods
The patients completed validated questionnaires on HR-QoL, sexual function, and depression before, during, and at the end and finally 6 months after chemotherapy. Special attention was paid to possible differences between pre- and postmenopausal patients.
Results
Between 2008 and 2012, 79 patients were enrolled in the trial (mean age 47.46 years). Premenopausal participants were 63.3 %. Sexual activity dropped from 71.9 % before chemotherapy to a minimum of 47 % at the end of chemotherapy. A similar effect was seen for pleasure and discomfort. Depression values were the highest at the beginning of chemotherapy, with spontaneous improvement in many patients during the course of time. HR-QoL and global health status both increased 6 months after therapy. For almost all parameters, changes were more obvious in pre- than in postmenopausal patients.
Conclusions
In a close monitoring, we observed significant changes in HR-QoL, depression, and sexual function in breast cancer patients. Special attention needs to be paid to premenopausal patients. The knowledge of effective recovery and spontaneous improvement of HR-QoL in spite of still impaired sexuality are important information in counseling both pre- and postmenopausal patients with diagnosis of breast cancer prior to upcoming therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of breast cancer is approximately 12 %, and the prevalence rises with increasing age. Independent of age, the diagnosis is a life-threatening event for every woman. Especially in young patients being diagnosed with breast cancer, life planning and long-term perspectives concerning family or professional life can be substantially destroyed. Whereas at the time of the diagnosis, the fear of death is the central point, other concerns come into focus during therapy, including quality of life, physical changes, and sexuality.
The impact of breast cancer on body image may not be underestimated. Surgery has become increasingly less invasive and breast-conserving therapy combined with radiotherapy is an option for most of the patients [1]. Still, scars remain, and both adjuvant irradiation and chemotherapy influence the long-term appearance and well-being [2]. The physical changes due to oncological therapy are an important issue in long-term breast cancer survivors [3]. Furthermore, the incidence of sexual dysfunction is reported with an incidence of up to 77 % in these patients [2]. Problems are being attributed to several factors like weight gain after therapy or mastectomy [2]. Apart from this, antihormonal treatment may deeply affect sexual function and is considered a neglected problem [4]. For women of younger age, infertility and the sudden onset of premature menopause due to chemotherapy or ovarian ablation are threatening circumstances [5]. Particularly, women between 30 and 40 years are often still in a family planning process and can be affected due to the increased incidence of breast cancer at an early age [6].
Another important issue is the mental well-being of breast cancer patients. As recently reported by Vodermaier et al., depression is strongly associated with mortality in younger breast cancer patients with early stage disease [7]. Worries about recurrence are frequent in breast cancer survivors, lasting for a long time, and the patients should be encouraged to discuss this with their treating physicians [8].
In this trial, we prospectively evaluated the influence of chemotherapy for breast cancer on women’s health-related quality of life (HR-QoL), sexual function, and depression. It is widely accepted that menopause is a major change in women’s life, for example, regarding sexual life. Therefore, we hypothesized that the changes in sexual function and quality of life would differ between pre- and postmenopausal patients. Starting at the time of diagnosis, follow-up was performed over the period of therapy until 6 months after the end of chemotherapy.
Materials and methods
All patients planned for adjuvant or neoadjuvant chemotherapeutic treatment with a primary diagnosis of breast cancer at Freiburg University Medical Center were eligible for the trial and were screened for participation by the nursing staff. Exclusion criteria were age <18 or >70 years, pre-existing psychiatric diagnosis, or sexually inactive patients. To define sexual activity, the treating physician asked the patient at each visit whether she considered herself sexually active. It was not restricted to patients living in a relationship. Agreement of the local ethics committee of Freiburg University was obtained (EK 80/08). After informed consent, the patients completed the paper-based questionnaires for evaluation of the baseline scores regarding the last 4 weeks before administration of the first cycle of chemotherapy (“T1”). The evaluation was repeated after the first half of chemotherapy (“T2”), at the end of chemotherapy (“T3”), and 6 months after completion of the chemotherapy (“T4”).
Three different validated questionnaires were chosen to cover the following domains: health-related quality of life, sexual function, and depression. Regarding the possible influence of age and menopausal status, premenopausal patients were compared with postmenopausal patients in all items.
Health-related quality of life: EORTC-QLQ-C30
For evaluation of health-related quality of life, the EORTC-QLQ-C30 (version 3.0) was used. This internationally acknowledged questionnaire has been established for assessing the HR-QoL of cancer patients participating in clinical trials and has an internal consistency of >0.70 [9]. It incorporates two questions on global health and quality of life, each ranging from 0 to 100, with the maximum implying perfect health. Furthermore, it contains multi-item scales functional (physical, role, cognitive, emotional, and social) and symptom scales (fatigue, pain, and nausea and vomiting). Here again, the range is from 0 to 100, with a score of 100 representing a perfect function in the functional scales, and a score of 100 indicating very strong symptoms in the symptom scales [10, 11].
Sexual function-SAQ
The second questionnaire was the Sexual Activity Questionnaire (SAQ), originally developed to investigate the long-term impact of tamoxifen on sexual functioning of women at high risk of developing breast cancer [12]. Three aspects of sexual functioning are highlighted in this questionnaire: pleasure from sexual intercourse, discomfort during sexual intercourse, and habit. Pleasure and discomfort have scores ranging from 0 to 18 and 0 to 6, with a high value representing high pleasure and a low discomfort score meaning high discomfort. Habit ranges from 0 to 3, with 3 corresponding to less frequent intercourse. The SAQ was used to define the number of patients being sexually active, to determine the reasons for sexual inactivity and to evaluate sexual functioning in those patients who had intercourse. For the evaluation of orgasm function, three items from the Female Sexual Function Index questionnaire (FSFI) were taken [13]. Each item ranged from 0 to 4, resulting in a maximum of 12, meaning very good orgasm function. The SAQ was found to have an internal consistency between 0.74 and 0.82 [14].
Depression-ADS
The “Allgemeine Depressionsskala” is the German-validated equivalent of the CES-D-Scale (Center for Epidemiological Studies Depression Scale), a questionnaire representing mental problems and their consequences on daily life [15, 16]. We used the short form with 15 items, including questions on exhaustion, hopelessness, loneliness, and fear. A value of >17 points implicated a depressive disorder (range 0 to 45). The internal consistency of the ADS-K is between 0.88 and 0.95 [15].
Statistical analysis
The Statistical Package for the Social Sciences, IBM SPSS® Statistics version 22, was used for statistical analyses. All data are shown as means (± SD). For continuous variables, between-group differences were assessed by the non-parametric Mann-Whitney U-test for unpaired samples. Correlations were estimated with Spearman’s correlation. Continuous variables within each group were assessed for differences using Wilcoxon test for paired samples. The p values ≤0.05 were considered statistically significant.
Results
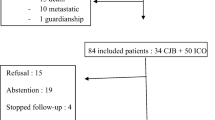
Between 2008 and 2012, 79 women met the inclusion criteria and gave their informed consent for the study. The mean age of the women participating in the study was 47.46 years (min 23, max 70 years, SD 9.75). The majority (n = 48, 60.8 %) received adjuvant chemotherapy, the remaining 31 (39 %) women neoadjuvant treatment. The mean body mass index (BMI) was 24.55 ± 4.58 kg/m2 (range 17.8–36.3 kg/m2) with a statistically higher value in postmenopausal compared to premenopausal patients (p = 0.035). Of the participating women, 63.3 % (n = 50) were premenopausal at the time of therapy. A detailed description of the demographic data can be found in Table 1.
Sexual function
At the beginning of the trial, 57 women lived in a relationship (89.1 %, missing n = 15) and 46 of them (71.9 %, missing n = 15) were sexually active. Out of the 79 patients recruited, 15 (19 %) women were active during the whole period of the chemotherapy. After the beginning of chemotherapy (T2), a growing number of patients refrained from sexual intercourse (active n = 35, 55.6 %, missing n = 16). This was mainly due to loss of libido and weariness. For example, one statement was “the illness and the treatment dominate the everyday life, making it impossible to have a relaxed time”. Furthermore, women reported pain due to the operation, vaginal dryness, or problems with arousal, making it difficult to enjoy sexual intercourse. At T3, the percentage of sexually active women significantly dropped to the lowest value of 47 (n = 31, missing n = 13, p = 0.003). At this time point, even more women reported loss of libido and weariness, and changes in the body image became more important. Also, chemotherapy-induced menopausal symptoms including hot flashes and sleeplessness were mentioned among the sexuality-impairing factors. Six months after chemotherapy, a higher percentage was sexually active again, reaching almost the value before chemotherapy (65.7 %, n = 44, missing n = 12). The difference in sexual activity between T1 and T4 did not reach significance (p = 0.13). Still at this point, patients reported arthralgia, hot flashes, and sleeplessness due to antihormonal medication. For a detailed description, see Table 2.
Pleasure, discomfort, and habit
At the beginning of chemotherapy, the mean pleasure score was 11.21 ± 3.4 (n = 47). The further values were all significantly lower compared to T1. Only in postmenopausal patients, the difference between T1 and T4 was not significant (p = 0.071). Six months after therapy, in both patient groups, the baseline scores were almost achieved. The lowest value was observed at the end of chemotherapy (mean 8.00 ± 3.77, n = 29) (see Fig. 2a).
Discomfort also increased under therapy and the score dropped: mean value at T1 was 5.17 (± 1.39, n = 47) and then declined to 4.3 ± 1.94 at T2 (n = 33), 3.90 ± 1.86 at T3 (n = 29), and finally remained at 4.04 ± 2.03 (n = 46) at T4. Change from T1 to all other examinations was significant in the whole patient cohort and in the subgroup of premenopausal patients. In postmenopausal patients, only the difference between T1 and T3 was significant (p = 0.038).
Among the sexually active patients, the percentage of women who were less frequently active also increased significantly: at T1 10.6 %, T2 72.7 %, T3 75 %, and finally at T4 43.2 %. Regarding habit, we did not observe a significant difference between pre- and postmenopausal participants.
Orgasm
The baseline score of the orgasm function was 10.04 ± 2.35 (n = 47). It did not differ significantly between pre- and postmenopausal women. During chemotherapy, the score in all patients dropped significantly compared to T1 (p < 0.005). Other than in the premenopausal group, in postmenopausal women, the value at T4 was almost back to the baseline score and did not differ significantly from T1 (p = 0.68) (see Fig. 1c).
EORTC
Both the global health status (GH) and quality of life (QoL) did not show a significant difference between pre- and postmenopausal patients before therapy. Both GH and QoL had similar baseline values (55.77 ± 21.10 and 59.51 ± 22.57). There was not much change from T1 until T3, but after chemotherapy, we could observe a significant increase for both parameters: GH increased to 71.31 ± 14.79 and QoL to 73.81 ± 18.00 (p = 0.000) (see Fig. 2). Regarding the functional and symptom scales, we also saw a negative impact due to therapy. Interestingly, the mean values at T4 were better than baseline values, however, only significantly for the symptom scales (p = 0.005).
Depression
For the depression score, the course was a different one: the highest levels were seen at the beginning of chemotherapy, with a continuous decline from this point on. This could be observed both in the mean value and in the number of patients with an ADS value of >17 as a cut-off point for depression. At the beginning of chemotherapy, 23.19 % (16/69) women showed an ADS value pointing towards a depressive disorder, whereas at T2 only 15.71 % (11/70) women had elevated scores. There was a further decline to 10.45 % (7/67) at the end of chemotherapy and to 9.38 % (6/64) at T4. Baseline scores of pre- and postmenopausal women did not differ significantly, but in premenopausal women the depression score dropped significantly after chemotherapy (T1 13.21 ± 7.84 vs. T4 6.87 ± 6.14; p = 0.000) (Fig. 3).
Correlation ADS and EORTC
In the analysis of the relationship between the ADS and the EORTC-QLQ-C30 questionnaire, we saw a correlation between depression scores and both global health status and quality of life (p = 0.000), meaning a higher depression score was associated with lower global health status and quality of life.
Discussion
The aim of this study was to analyze the effect of chemotherapy on health-related quality of life and sexual function in breast cancer patients during and shortly after treatment. In this time period, significant changes in quality of life, sexual functioning, and depression were observed. Importantly, this effect was more distinctive in premenopausal compared to postmenopausal patients. In premenopausal women, the diagnosis of cancer represents possibly an even greater change in daily life compared to older patients. Concerns regarding the professional career, children, or fertility may be experienced more important than in older patients. Whereas premenopausal women in our study suffered more from side effects of treatment than postmenopausal patients, they seemed to recover faster and resulting in a higher score of “global health” and “quality of life” 6 months after the chemotherapy compared to baseline values. After treatment, the physical recovery is more promising in younger patients and the positive feeling after defeating cancer may contribute to better health awareness and therefore improved quality of life. This is in line with other published results and may be due to the fact that the diagnosis of cancer is especially difficult for younger women [17]. In our study, premenopausal women also showed less depressive disorders at T4 compared to the older patient group.
Regarding depression, we saw a relevant number of patients with high depression scores at the beginning of therapy. Vodermaier et al. showed a higher mortality rate in younger breast cancer patients due to depression [7]. This could imply that for most patients, there is the need for counseling especially in the early course of treatment, and patients should be checked for depression. Interestingly, there was no intervention in this trial, but still there was spontaneous change for the better. This is in line with a recent report showing that women are most vulnerable for depression and anxiety at the time of diagnosis, with improvement over time [18]. Gradually, patients may get used to the new circumstances and fears may be reduced. Still unclear is the correct treatment option for women with depression under breast cancer therapy, including either psychotherapy or pharmacotherapy [19].
The reasons for sexual inactivity were variable, including both physical and psychological factors. Whereas the number of women without a partner remained stable, we observed a growing number of patients with no interest in sex. Furthermore, physical reasons and tiredness were addressed to an increasing degree. Segrin et al. showed that the level of distress in breast cancer patients is significantly interdependent with their partners’ mental health [20]. The patients emphasized the overriding importance of nearness and affection compared to sexual intercourse. Women felt less attractive than before therapy, inter alia due to scars or alopecia, representing a huge psychological burden.
Regarding sexual function, we observed a statistically significant change for the worse in all categories (pleasure, discomfort, habit, and orgasm). In postmenopausal patients, all scores returned to baseline values indicating possibly the temporary nature of sexual problems during the oncological treatment. In premenopausal women, only the pleasure score returned to baseline. Thus in younger patients, the impaired sexuality persists longer after completion of chemotherapy compared to postmenopausal patients. The sexuality is an important topic for patients and should be addressed by the consulting physician. Nevertheless, the quality of life, as mentioned before, was not affected by reduced sexuality. The observed differences between pre- and postmenopausal woman should be considered in advising patients prior to, during, and after oncological treatment.
One shortcoming of the trial is the relatively small number of patients and no consistently answered questionnaires at all investigation time points. Patients over 70 years of age were excluded although also in this patient group, changes in sexual function and QoL are possible. Furthermore, the investigation was finished 6 months after therapy. To improve the results of our study, the evaluation should be continued to examine whether there are further changes during long-term follow up both regarding sexual function and quality of life.
In this trial, we closely monitored the quality of life and sexual activity, reporting short-term changes of all categories before, during, and 6 months after completion of chemotherapy. Continuous monitoring and psychological counseling are necessary for all breast cancer patients. In line with other studies, special attention needs to be paid to premenopausal patients. They show a higher bandwidth of changes in sexual function and quality of life with early onset shortly after the start of treatment. The knowledge of effective recovery and improved quality of life after chemotherapy in spite of still impaired sexuality presents important information in counseling both pre- and postmenopausal patients. Great progress has been made in breast cancer therapy regarding the evaluation of side effects of the disease and its treatment, and this study emphasizes the need for further work in this field.
Abbreviations
- HR-QoL:
-
Health-related quality of life
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- SAQ:
-
Sexual Activity Questionnaire
- FSFI:
-
Female Sexual Function Index questionnaire
- ADS:
-
Allgemeine Depressionsskala
References
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1271
Munshi A, Kakkar S, Bhutani R, Jalali R, Budrukkar A, Dinshaw KA (2009) Factors influencing cosmetic outcome in breast conservation. Clin Oncol (R Coll Radiol) 21(4):285–293. doi:10.1016/j.clon.2009.02.001
Raggio GA, Butryn ML, Arigo D, Mikorski R, Palmer SC (2014) Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol Health 29(6):632–650. doi:10.1080/08870446.2013.879136
Baumgart J, Nilsson K, Evers AS, Kallak TK, Poromaa IS (2013) Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause 20(2):162–168. doi:10.1097/gme.0b013e31826560da
Perz J, Ussher J, Gilbert E (2013) Loss, uncertainty, or acceptance: subjective experience of changes to fertility after breast cancer. Eur J Cancer Care (Engl). doi:10.1111/ecc.12165
Kasum M, Beketić-Orešković L, Peddi PF, Orešković S, Johnson RH (2014) Fertility after breast cancer treatment. Eur J Obstet Gynecol Reprod Biol 173:13–18. doi:10.1016/j.ejogrb.2013.11.009
Vodermaier A, Linden W, Rnic K, Young SN, Ng A, Ditsch N, Olson R (2014) Prospective associations of depression with survival: a population-based cohort study in patients with newly diagnosed breast cancer. Breast Cancer Res Treat 143(2):373–384. doi:10.1007/s10549-013-2795-4
Janz NK, Friese CR, Li Y, Graff JJ, Hamilton AS, Hawley ST (2014) Emotional well-being years post-treatment for breast cancer: prospective, multi-ethnic, and population-based analysis. J Cancer Surviv 8(1):131–142. doi:10.1007/s11764-013-0309-3
Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S (1998) Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: the QLQ=C30 (+ 3). J Clin Oncol 16(3):1188–1196
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al. (1993) The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 3;85(5):365–376.
Fayers PM, Aaronson NK, Bjordal K, et al., on behalf of the EORTC Quality of Life Group (2001) The EORTC QLQ-C30 Scoring Manual (3rd edition). European Organisation for Research and Treatment of Cancer, Brussels.
Thirlaway K, Fallowfield L, Cuzick J (1996) The Sexual Activity Questionnaire: a measure of women’s sexual functioning. Qual Life Res 5(1):81–90 Erratum in: Qual Life Res 1997 Aug;6(6):606
Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D'Agostino Jr R (2000) The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 26(2):191–208
Wenzel L, DeAlba I, Habbal R, Kluhsman BC, Fairclough D, Krebs LU, Anton-Culver H, Berkowitz R, Aziz N (2005) Quality of life in long-term cervical cancer survivors. Gynecol Oncol 97(2):310–317
Hautzinger M, Bailer M (1993) Allgemeine Depressions Skala. Manual. Göttingen, Beltz Test GmbH
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1:385–401
Jankowska M (2013) Sexual functioning in young women in the context of breast cancer treatment. Rep Pract Oncol Radiother 18(4):193–200 eCollection 2013
Stafford L, Judd F, Gibson P, Komiti A, Mann GB, Quinn M (2015) Anxiety and depression symptoms in the 2 years following diagnosis of breast or gynaecologic cancer: prevalence, course and determinants of outcome. Support Care Cancer 223(8):2215–2224. doi:10.1007/s00520-014-2571-y
Callari A, Mauri M, Miniati M, Mancino M, Bracci G, Dell'osso L, Greco C (2013) Treatment of depression in patients with breast cancer: a critical review. Tumori 99(5):623–633. doi:10.1700/1377.15313
Segrin C, Badger TA (2014) Psychological and physical distress are interdependent in breast cancer survivors and their partners. Psychol Health Med 19(6):716–723. doi:10.1080/13548506.2013.871304
Acknowledgments
We thank Mr. A. Allignol, Clinical Trials Unit, for the statistical analysis. We thank Mrs. A. Warnke-Kockrow, for data acquisition.
Author information
Authors and Affiliations
Corresponding author
Additional information
Juliane Farthmann and A. Hanjalic-Beck contributed equally to this work.
Rights and permissions
About this article
Cite this article
Farthmann, J., Hanjalic-Beck, A., Veit, J. et al. The impact of chemotherapy for breast cancer on sexual function and health-related quality of life. Support Care Cancer 24, 2603–2609 (2016). https://doi.org/10.1007/s00520-015-3073-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-3073-2