Abstract
Purpose
Oral mucositis induced by radiation or chemoradiation can compromise the quality of life of oral squamous cell carcinoma (OSCC) patients. The present study was designed to evaluate the preventive effects of elemental diet (ED), Elental®, on radiotherapy- or chemoradiotherapy-induced mucositis in OSCC patients.
Patients and methods
Seventy-four patients who underwent radiation (60–70 Gy) with/without chemotherapy [S-1, cisplatin (CDDP), CDDP plus S-1] were enrolled in this retrospective study; 37 had received Elental® during treatment (Elental® group) and 37 had not (control group). Factors related to alleviation of oral mucositis were identified by multivariate logistic regression analysis. Rates of completion of chemoradiation treatments were compared between Elental® and control groups according to the treatment regimen. The comparison of the nutritional status between groups was also performed.
Results
Multivariate analysis indicated that the administration of Elental® and no combined chemotherapy (radiation alone) were significant factors associated with the degree of oral mucositis, i.e., most of the patients who consumed Elental® suffered from a lower degree of mucositis compared to the control group. Elental® was associated with a significantly improved rate of completion of chemoradiation (no interruption). There was no significant difference between Elental® group and control group in terms of mean change of body weight or total protein and albumin levels in blood serum before and after (chemo)radiation.
Conclusions
The present study indicates that Elental® is effective for ameliorating oral mucositis induced by (chemo)radiation in OSCC patients. Elental® was also associated with improved completion rates of (chemo)radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of oral squamous cell carcinoma (OSCC) is increasing gradually, and about 300,000 patients are annually estimated to get oral cancer worldwide [1–3]. Especially, incidence rates for oral cancer sites related to HPV infections, such as the oropharynx, tonsil, and base of the tongue, are increasing in young adults in the USA and in some countries in Europe [4]. OSCC is the most common malignant neoplasm of the oral cavity, which represents about 90 % of all oral malignancies [5]. The standard treatment for early stage OSCC is surgical operation. However, we select chemoradiotherapy (CRT) for patients with advanced or recurrent OSCC because CRT can contribute to improved survival rates and preservation of oral function. In addition, we can choose radiotherapy (RT) or CRT for the tumor that could not be removed completely by surgery. In spite of these advantages, RT or intensified CRT in the oral cavity or head and neck region causes severe mucositis of the oral cavity, pharynx, and larynx, which often results in acute oral pain and compromised nutritional intake. It is reported that acute mucositis occurs in 100 % of head and neck cancer patients undergoing RT or concurrent CRT, presenting significant clinical and economic problems [6]. Interruptions to RT or concurrent chemotherapy due to pain and swallowing dysfunction associated with radiation-induced mucositis can adversely influence outcomes of cancer treatment and prolong the hospitalization period [7–13]. Although numerous types of therapy have been offered for preventing or decreasing radiation-induced mucositis, the efficacy of these treatments is still limited [14–21].
Japan’s first ED, Elental® (Ajinomoto Pharmaceutical Ltd., Tokyo, Japan) has been widely used in Japan for four decades for improving nutritional status in patients [22]. It has an easily digestible nutrition formula that combines amino acids, carbohydrates, vitamins, minerals, and minimal fat [22, 23]. This ED is effective in the treatment of a number of diseases and has been reported to decrease mucosal inflammation in acute Crohn’s disease by reducing the mucosal proinflammatory cytokine production [23–26]. It is well documented that l-glutamine helps to reduce the incidence and severity of chemotherapy- and radiotherapy-induced mucositis, and Elental® is a good source of l-glutamine [23, 27, 28]. Animal studies have suggested that supplementation of an elemental diet (ED) with glutamine may protect the gut from radiation and some chemotherapeutic agents [29, 30]. Moreover, ED with glutamine (Elental®) has been found to significantly decrease the severity of chemotherapy-induced stomatitis in patients with colorectal cancer and esophageal cancer [31, 32]. Therefore, Elental® with glutamine could be useful in the treatment of mucositis in cancer patients.
The purpose of this study is to understand whether ED with glutamine is capable of recovering the cells from chemotherapy- and/or radiotherapy-induced damage. It has almost the same formula as VIVONEX® T.E.N. (Nestlé) available in many Western countries [33]. Here, we assessed the efficacy of an ED with glutamine (Elental®) in minimizing or preventing RT- or CRT-induced mucositis in patients with OSCC.
Patients and methods
The records of 74 patients with OSCC who were scheduled for 60–70 Gy of RT or concurrent chemoradiotherapy at the Yamaguchi University Hospital of Japan from January 2011 to December 2014 were retrospectively reviewed. All patients received conventional fractioned radiation (2 Gy per day and 5 days per week) to their oral cavity with or without concurrent chemotherapy. The range of total radiation dose was 60–70 Gy (mean 60.9 Gy). Concurrent chemotherapies were S-1 (65 mg/m2/day, 2-week administration and 1-week rest, twice during RT), cisplatin (CDDP, 100 mg/m2, intravenous infusion triweekly, three times during RT), S-1 plus CDDP (S-1, 60 mg/m2, days 1–14 and days 36–49, and CDDP, 20 mg/m2, intravenous infusion days 8–11 and days 43–46), or none (no chemotherapy, RT alone). We have prescribed Elental® (one bottle/day) for OSCC patients receiving RT with or without chemotherapy since January 2011 to prevent or treat mucositis during and after the RT or CRT regimen. Thus, we recorded data on a daily basis and compared the data from 37 patients who received Elental® (Elental® group) with those from 37 patients who did not receive Elental® (the control group) during the study period (6 weeks).
One bottle of Elental® (80 g, 300 kcal) powder was dissolved in 300 mL water for an eventual concentration of 1 kcal/mL, and the patients swished it around their mouths and swallowed it orally once a day during the (chemo)radiation period. In addition, patients in both groups used a similar regimen of oral brushing and gargling with 4 % azulene sodium sulfonate mixed with tap water, and patients were treated with azulene sodium sulfonate plus lidocaine gargle, NSAIDs, and/or opioids, if needed, when they experienced severe oral pain with mucositis.
We performed univariate and multivariate analyses to identify the clinicopathological and therapeutic factors involved in alleviation of oral mucositis during the (chemo)radiation period. Degree of oral mucositis was graded according to the Common Terminology Criteria for Adverse Events version 4.0 (National Cancer Institute CTCAE v4.0). The CTCAE v4.0 grades for oral mucositis are defined as follows: grade 0, no mucositis; grade 1, asymptomatic or mild symptoms and intervention not indicated; grade 2, moderate pain not interfering with oral intake and modified diet indicated; grade 3, severe pain interfering with oral intake; grade 4, life-threatening consequences and urgent intervention indicated; grade 5, death. Moreover, we evaluated incidence rates of grade 3 or 4 oral mucositis and completion rates of scheduled (chemo)radiation treatments by regimen. Treatment completion included patients who underwent all scheduled chemotherapy and >60 Gy of radiation without interruption. Furthermore, nutritional status before and after (chemo)radiation was investigated in terms of body weight and levels of total protein and albumin in blood serum.
All statistical significance was set at p < 0.05. Statistical analyses including chi-square for independence test, Fisher’s exact test, Mann-Whitney U test, Student’s t test, and multivariate logistic regression analysis were performed using StatView software (version 5.0J, SAS Institute Inc. Cary, NC, USA).
This retrospective study was approved by the ethical committee of the Yamaguchi University Hospital of Japan.
Results
The clinicopathological characteristics of all patients are summarized in Table 1. All patients in this study were advanced-stage OSCC (stage III/IV). We identified significant clinicopathological and therapeutic factors associated with differences in the CTCAE v4.0 oral mucositis severity grade by univariate analysis and found that Elental® administration (p = 0.003) and combined chemotherapy (p = 0.001) were significantly associated with the degree of oral mucositis (Table 2). The subsequent multivariate logistic regression analysis included factors with p values of <0.05 from the univariate analysis, and the results suggested that Elental® administration (p = 0.004) and none (no chemotherapy, RT only) (p = 0.004) were significant factors affecting the grade of oral mucositis during RT or CRT, i.e., patients in the Elental® group and those who received RT only mostly suffered from lower grade of mucositis (grade 1/2) than the other groups (Table 3).
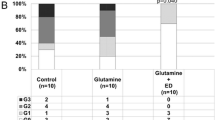
We also compared the efficacy of Elental® for oral mucositis between CRT and RT alone because the presence or absence of combined chemotherapy strongly affects the severity of side effects. Characteristics of patients with Elental® administration added to the basic supportive care for oral mucositis (Elental® group) and patients who had only supportive care (control group) are compared in Table 4. The stage and completion of regimen were significantly different between the Elental® group and the control group. Therefore, we compared the two groups based on CTCAE v4.0 oral mucositis grade and rates of completion of (chemo)radiation therapy by regimen. For RT alone, 30.8 % of patients in the control group had grade 3/4 mucositis vs 8.3 % in the Elental® group (p = 0.193). For CRT, the rates of grade 3/4 mucositis were 79.2 vs 40.0 %, respectively, in the controls and the Elental® group (p = 0.005) (Fig. 1a). Elental® showed a statistically significant difference in reducing the severity of oral mucositis in CRT cases, though the statistical significance was not observed in RT alone cases possibly because of the small number of cases we investigated by regimen. The rates of completion by group (control vs Elental®) were 76.9 and 100 %, respectively, in RT alone (p = 0.089), and 33.3 and 76.0 % in CRT (p = 0.757) (Fig. 1b). Elental® helped to reduce the interruptions of treatment regimen in RT cases in spite of the absence of statistically significant difference between Elental® and control group. Most treatment interruptions were related to the cessation of combined chemotherapy. In addition, seven patients discontinued RT, and all of these patients belong to the control group. The reasons of treatment interruptions for more than half of the treatment-interrupted patients were severe pain and bleeding by oral mucositis. Moreover, treatment interruptions were also caused by febrile neutropenia and sepsis in some cases. The addition of Elental® appears to be associated with a decreased severity of (chemo)radiation-induced oral mucositis and improved completion rates of chemo(radiation) regardless of the regimen of RT or CRT.
Effects of Elental® on oral mucositis grade according to anticancer regimen. Statistical significance was seen only in the rates of grade 3/4 mucositis of CRT (p = 0.005). However, Elental® was associated with a tendency to decreased severity of (chemo)radiation-induced oral mucositis (a) and improved completion rates of (chemo)radiation (b) in both regimens. RT radiotherapy, CRT chemoradiotherapy
Finally, we evaluated the nutritional status of patients in both groups before (chemo)radiation and after chemo(radiation) and compared the changes in pre- and post-(chemo)radiation nutrition status for each group. We retrospectively evaluated body weight, serum total protein, and serum albumin. Of these indicators, however, we could not detect any significant difference between the Elental® group and the control group (Table 5). Also, the number of cases that required nutrition management by tube feeding was seven in the Elental® group and eight in the control group. Nine cases in the control group underwent parenteral nutrition, whereas only two cases received the same in the Elental® group.
Discussion
The present study demonstrates that elemental diet (ED), Elental®, is effective for ameliorating oral mucositis induced by RT or CRT in patients with OSCC. In addition, Elental® administration was associated with improved completion rates of RT or CRT.
Mucositis, dysphagia, xerostomia, and hematological toxicities are well known as major side effects of RT and CRT. Incidence of severe oral mucositis leads to higher unplanned breaks and delays in RT, which are invariably associated with poorer outcome [34–37]. However, effective treatments for radiation-induced mucositis have not been established yet [14, 38–40]. At present, the recombinant keratinocyte growth factor-1 (rKGF1), palifermin, is thought to be a promising agent for the management of oral mucositis associated with cancer treatment. Briefly, palifermin could reduce severe oral mucositis in patients treated with postoperative or definitive CRT for head and neck cancer in two randomized trials, though neither trial showed an improvement in chemoradiation completion rates [17, 41]. Regardless of its usefulness in decreasing oral mucositis, the safety of this growth factor in cancer patients remains unclear and it is expensive compared to other type of therapies for oral mucositis [42].
Conversely, Elental® has been approved and covered by public insurance as a prescription medication specified for the treatment of malnutrition in Japan, and its safety has been well established [22]. Elental® contains nutrients in a close to dissolved state, and it is easily digested and absorbed through the digestive tract without digestive juice secretion [22, 26]. Elental® is also a good source of l-glutamine (2415 mg l-glutamine/100 g Elental®). This nonessential amino acid, glutamine, has recently become the subject of extensive scientific interest for the treatment of mucositis and stomatitis. Studies with laboratory animal models have demonstrated that glutamine supplement is safe and serves to reduce the incidence and severity of cytotoxicity-induced mucositis [27, 28]. Elental® is inexpensive, costing <US$4.00 per day and the estimated cost for a 7-week course of Elental® is about $112. Several authors have reported the benefit of Elental® against oral mucositis during chemotherapy in patients with esophageal or colorectal cancer [31, 32]. However, there is no published report on the efficacy of Elental® for the treatment of (chemo)radiation-induced oral mucositis.
The multivariate analysis performed in this retrospective study has demonstrated that Elental® was significantly associated with prevention and amelioration of (chemo)radiation-induced mucositis in patients with OSCC. Moreover, our findings suggested that Elental® as an adjunct to conventional anti-mucositis therapies helped to improve the completion rate of RT or CRT for OSCC. In this study, no adverse effects related to the clinical use of Elental® were recorded.
Elental® nutrition therapy was reported to have supportive effects in patients with Crohn’s disease, including active or quiescent disease remission, wound healing, pain relief, and nutritional status improvement, and also useful in the treatment of chronic pancreatitis [23–25]. However, contrary to our expectations, Elental® was not associated with prevention of deterioration of nutrition status in terms of the value of nutrition indicators in our study, but it did manage to minimize mucositis (Table 5).
It is also possible that consumption of Elental® only might not be able to prevent or ameliorate the severe oral mucositis induced by the more intensive (chemo)radiation regimens than those administered in our current study. Also, the records indicated that tube feeding as a nutrition therapy was not different between two groups, though central venous alimentation were more aggressively applied in the control group compared with the Elental® group, which may have been required to compensate for marked decreases of oral intake that were seen in control group patients. Further randomized study must be needed to clarify the effect of Elental® on the maintenance of nutritional status. We are preparing for prospective randomized clinical trials to confirm the benefits indicated from this retrospective study. Investigations are also necessary to clarify the mechanism of action of elemental diet against (chemo)radiation-induced mucositis in OSCC.
Conclusion
This retrospective study demonstrated that ED prevented and alleviated severe oral mucositis induced by RT or CRT and improved the completion rates of RT or CRT with chemotherapeutic agents for head and neck cancer patients. Elental® is effective, safe, and inexpensive and is therefore a promising agent for the treatment of RT- or CRT-induced oral mucositis. A higher level of evidence is required via further prospective randomized study with a large group of patients in order to confirm the efficacy of Elental® for RT- or CRT-induced oral mucositis.
References
Rautava J, Luukkaa M, Heikinheimo K, Alin J, Grenman R, Happonen RP (2007) Squamous cell carcinomas arising from different types of oral epithelia differ in their tumor and patient characteristics and survival. Oral Oncol 43:911–919
Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT (2002) Presentation, treatment, and outcome of oral cavity cancer: a national cancer data base report. Head Neck 24:165–180
Mehrotra R, Singh MK, Pandya S, Singh M (2008) The use of an oral brush biopsy without computer-assisted analysis in the oral lesions: a study of 94 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:246–253
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Lawoyin JO, Lawoyin DO, Aderinokun G (1997) Intra-oral squamous cell carcinoma in Ibadan: a review of 90 cases. Afr J Med Med Sci 26:187–188
Sonis ST (2011) Oral mucositis. Anti-Cancer Drugs 22:607–612
Robertson C, Robertson AG, Hendry JH, Roberts SA, Slevin NJ, Duncan WB, MacDougall RH, Kerr GR, O’Sullivan B, Keane TJ (1998) Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int J Radiat Oncol Biol Phys 40:319–329
Bese NS, Hendry J, Jeremic B (2007) Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 68:654–666
Withers HR, Taylor JM, Maciejewski B (1998) The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27:131–146
Herrmann T, Jakubek A, Trott KR (1994) The importance of the timing of a gap in radiotherapy of squamous cell carcinomas of the head and neck. Strahlenther Onkol 170:545–549
Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R and Maciejewski B (2003) Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys 56:399–412, 2003.
Russo G, Haddad R, Posner M, Machtay M (2008) Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 13:886–898
Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, Hayden V, Eilers J, Epstein JB, LeVeque FG, Miller C, Peterson DE, Schubert MM, Spijkervet FK, Horowitz M (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19:2201–2205
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, DB MG, Hutchins RD, Peterson DE, Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Peterson DE, Bensadoun RJ, Roila F, ESMO Guidelines Working Group (2009) Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol 20(Suppl 4):174–177
Quinn B, Potting CM, Stone R, Blijlevens NM, Fliedner M, Margulies A, Sharp L (2008) Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer 44:61–72
Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG, Berger D (2011) Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol 29:2815–2820
Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, Elting L, Mittal BB, Schattner MA, Spielberger R, Treister NS, Trotti 3rd AM (2008) NCCN Task Force Report. Prevention and management of mucositis in cancer care. J Natl Compr Canc Netw 6(Suppl 1):S1–21 quiz S22–24
Svanberg A, Ohrn K, Birgegard G (2010) Oral cryotherapy reduces mucositis and improves nutrition—a randomised controlled trial. J Clin Nurs 19:2146–2151
Scully C, Epstein J, Sonis S (2004) Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck 26:77–84
Cowen D, Tardieu C, Schubert M, Peterson D, Resbeut M, Faucher C, Franquin JC (1997) Low energy helium-neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. Int J Radiat Oncol Biol Phys 38:697–703
Online Ajinomoto Products Information, Elental®. http://www.ajinomoto.com/en/aboutus/history/chronicle_2014/09.html
Ikeura T, Takaoka M, Uchida K, Miyoshi H, Okazaki K (2014) Beneficial effect of low-fat elemental diet therapy on pain in chronic pancreatitis. Int J Chronic Dis:1–5, Article ID 862091.
Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K (2006) Acute duodenal Crohn’s disease successfully managed with low-speed elemental diet infusion via nasogastric tube: a case report. World J Gastroenterol 12:649–651
Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K (2005) Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis 11:580–588
Nakayama G, Morioka D, Murakami T, Takakura H, Miura Y, Togo S (2012) Chylous ascites occurring after low anterior resection of the rectum successfully treated with an oral fat-free elemental diet (Elental(®)). Clin J Gastroenterol 5:216–219
Choi K, Lee SS, Oh SJ, Lim SY, Lim SY, Jeon WK, Oh TY, Kim JW (2007) The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr 26:57–62
Savarese DM, Savy G, Vahdat L, Wischmeyer PE, Corey B (2003) Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev 29:501–513
Shou J, Lieberman MD, Hofmann K, Leon P, Redmond HP, Davies H, Daly JM (1991) Dietary manipulation of methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr 15:307–312
Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortos K, Bye R, Morrow FD, Jacobs DO, Smith RJ, Antin JH, Wilmore DW (1992) Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med 116:821–828
Ogata Y, Takeuchi M, Ishibashi N, Kibe S, Takahashi K, Uchida S, Murakami N, Yahara T, Shirouzu K (2012) Efficacy of Elental on prevention for chemotherapy-induced oral mucositis in colorectal cancer patients. Gan To Kagaku Ryoho 39:583–587 Japanese
Fukui T, Itoh Y, Orihara M, Yoshizawa K, Takeda H, Kawada S, Yoshioka T (2011) Elental prevented and reduced oral mucositis during chemotherapy in patients esophageal cancer. Gan To Kagaku Ryoho 38:2597–2601 Japanese
Online Nestle Products Information, VIVONEX® T.E.N. http://www.nestle-nutrition.com/products/Product.aspx?ProductId=a3bc4b10-3701-4020-9742-d947a30ed612
Bieri S, Bentzen SM, Huguenin P, Allal AS, Cozzi L, Landmann C, Monney M, Bernier J (2003) Early morbidity after radiotherapy with or without chemotherapy in advanced head and neck cancer. Experience from four non-randomized studies. Strahlenther Onkol 179:390–395
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, Van Glabbeke M, European Organization for Research and Treatment of Cancer Trial 22931 (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350:1945–1952
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK, Radiation Therapy Oncology Group 9501/Intergroup (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937–1944
Lin A, Jabbari S, Worden FP, Bradford CR, Chepeha DB, Teknos TN, Liao JJ, Nyquist GG, Tsien C, Schipper MJ, Urba S, Wolf GT, Eisbruch A (2005) Metabolic abnormalities associated with weight loss during chemoirradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 63:1413–1418
Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijlstra M, King EE, Stringer AM, Van der Velden WJ, Yazbeck R, Elad S, Bowen JM, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) (2013) Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 21:313–326
Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti 3rd A, Von Hoff D, Schuchter LM (2009) American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 27:127–145
Peterson DE, Bensadoun RJ, Roila F, ESMO Guidelines Working Group (2011) Management of oral and gastrointestinal mucositis: ESMO clinical practice guidelines. Ann Oncol 22:vi78–vi84
Le QT, Kim HE, Schneider CJ, Muraközy G, Skladowski K, Reinisch S, Chen Y, Hickey M, Mo M, Chen MG, Berger D, Lizambri R, Henke M (2011) Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol 29:2808–2814
Bossi P, Locati LD, Licitra L (2012) Palifermin in prevention of head and neck cancer radiation-induced mucositis: not yet a definitive word on safety and efficacy profile. J Clin Oncol 30:565–567
Conflict of interest
There is no financial support for the present study, and the authors declare that there are no competing interests. The authors are fully responsible for the content of this paper. All authors reviewed and approved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harada, K., Ferdous, T., Horinaga, D. et al. Efficacy of elemental diet on prevention for chemoradiotherapy-induced oral mucositis in patients with oral squamous cell carcinoma. Support Care Cancer 24, 953–959 (2016). https://doi.org/10.1007/s00520-015-2866-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2866-7