Abstract
Purpose
Febrile neutropenia (FN) during adjuvant chemotherapy is associated with morbidity, mortality risk, and substantial cost, and subsequent chemotherapy dose reductions may result in poorer outcomes. Patients at high risk of, or who develop FN, often receive prophylaxis with granulocyte colony-stimulating factors (G-CSF). We investigated whether different prophylaxis strategies with G-CSF offered favorable value-for-money.
Methods
We developed a decision model to estimate the short- and long-term costs and outcomes of a hypothetical cohort of women with breast cancer receiving adjuvant taxotere + cyclophosphamide (TC) chemotherapy. The short-term phase estimated upfront costs and FN risks with adjuvant TC chemotherapy without G-CSF prophylaxis (i.e., chemotherapy dose reductions) as well as with secondary and primary G-CSF prophylaxis strategies. The long-term phase estimated the expected costs and quality-adjusted life years (QALYs) for patients who completed adjuvant TC chemotherapy with or without one or more episodes of FN.
Results
Secondary G-CSF was associated with lower costs and greater QALY gains than a no G-CSF strategy. Primary G-CSF appears likely to be cost-effective relative to secondary G-CSF at FN rates greater than 28 %, assuming some loss of chemotherapy efficacy at lower dose intensities. The cost-effectiveness of primary vs. secondary G-CSF was sensitive to FN risk and mortality, and loss of chemotherapy efficacy following FN.
Conclusions
Secondary G-CSF is more effective and less costly than a no G-CSF strategy. Primary G-CSF may be justified at higher willingness-to-pay thresholds and/or higher FN risks, but this threshold FN risk appears to be higher than the 20 % rate recommended by current clinical guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women worldwide [1]. Most patients present with early-stage (I–III) disease [1] and are treated with curative-intent surgery followed by adjuvant systemic treatments, including chemotherapy, in an attempt to reduce the risk of cancer recurrence and improve survival outcomes [2]. Adjuvant chemotherapy is associated with a risk of febrile neutropenia (FN), defined as a fever (≥38.5 or >38.0 C for 1 h) associated with a low absolute neutrophil count (<0.5 × 1000/L or <1.0 × 1000/L with predicted decrease to <0.5 × 1000/L) [3]. FN is associated with significant morbidity, potential mortality, and cost [4–6] and can lead to chemotherapy delays and/or dose reductions that may compromise treatment efficacy and lead to inferior survival outcomes [7]. Patients who develop FN are often prescribed granulocyte-colony stimulating factors (G-CSF) with their subsequent chemotherapy cycles, in an attempt to reduce the risk of further episodes (secondary prophylaxis) and maintain chemotherapy relative dose intensity (RDI) [8, 9]. Guidelines also recommend G-CSF from the first cycle of chemotherapy (primary prophylaxis) for patients treated with chemotherapeutic regimens associated with a FN risk of more than 20 % and patients treated with regimens associated with a 10 to 20 % FN risk in the presence of other risk factors, such as older age (≥65 years), underlying co-morbidities, low baseline neutrophil count, anemia, and abnormal liver function tests [8]. Primary G-CSF is not recommended for regimens associated with a FN risk of less than 10 %.
In many countries, including Canada, taxane-based regimens have become standard adjuvant chemotherapy strategies [10]. Adjuvant TC, (T, taxotere; C, cyclophosphamide) chemotherapy has been shown to be associated with significant improvements in disease-free survival (DFS) and overall survival (OS) in a phase III randomized clinical trial compared with AC (A, adriamycin; C, cyclophosphamide) regimen [11]. Adjuvant TC, delivered every 3 weeks for 4 cycles, was associated with a 5 % FN risk in the clinical trial, but a substantially higher 29 % meta-analytic risk (95 % confidence interval (CI) 23.8–35.2 %) was later observed among 902 patients from 13 studies outside a clinical trial setting [12]. As such, current guidelines would recommend primary over secondary G-CSF prophylaxis for TC chemotherapy [8]. Primary G-CSF prophylaxis for all patients undergoing adjuvant TC chemotherapy, however, is an expensive strategy. The Canadian ($) acquisition cost is $1400–$2300 per cycle for 7 days of filgrastim or single dose peg-filgrastim, respectively, or $5600–$9200 per patient over the 4 cycles of TC chemotherapy.
Given this cost, and uncertainty regarding the baseline risk of TC-associated FN in the clinical setting, we developed a decision analytic model to examine the costs of secondary and primary G-CSF prophylaxis in the context of their potential benefits, as well as a strategy of dose reduction or delay without G-CSF prophylaxis. In particular, the model estimated the cost per quality-adjusted life year (QALY) gained of each strategy and examined the FN threshold at which primary G-CSF with TC regimen may be economically justified compared to a secondary G-CSF strategy.
Methods
The model
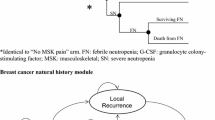
The decision model estimated short- and long-term costs and outcomes for a hypothetical cohort of women with breast cancer following curative-intent surgery. The short-term phase of the model (12 weeks) estimated expected costs and QALYs following 4 cycles of TC for each prophylactic G-CSF strategy (primary, secondary, or none). Possible outcomes, as shown in Fig. 1, included (i) completion of therapy with no FN, (ii) a FN episode with chemotherapy completion at full or reduced doses, (iii) FN-related death, and (iv) death unrelated to FN (background mortality). Each FN episode was associated with treatment-related costs and quality-of-life (QoL) penalty and could also lead to lower chemotherapy RDI reflecting potential reductions in chemotherapy dose delivery subsequent to a FN event and/or chemotherapy dose delays. We assumed that chemotherapy RDI would be reduced in a stepwise manner, to ≥85 % (dose −1) following the first FN occurrence in the primary and no G-CSF strategies or the second FN event in the secondary G-CSF strategy, and to less than 85 % (dose −2) following a subsequent episode of FN in all strategies.
The long-term phase of the model estimated disease-free survival (DFS) over a lifetime horizon, conditional on chemotherapy effectiveness at the conclusion of the 12-week short-term phase. The long-term phase of the model was based on a previously published model [13, 14] and began with all patients in a disease-free state following adjuvant therapy. Patients could experience a local or distant recurrence or die as a result of state-specific or background mortality. The data sources for the overall model are detailed below and in Table 1. Key model assumptions are outlined in Box 1.
Box 1: key assumptions
1. No cancer recurrences would occur beyond year 15. |
2. No background mortality was assumed during the 12-week chemotherapy phase of the model. |
3. Two-thirds of all incident FN cases would occur during the first chemotherapy cycle [12]. |
4. FN episodes were assumed to last for 7 days. |
5. The relative risk (RR) of FN is dependent on both G-CSF use and the chemotherapy RDI. |
6. The combined effects of G-CSF and reduced dose intensity on the RR of FN were multiplicative. Such combinations occur if a patient has an episode of FN while receiving primary or secondary G-CSF. |
7. The relative reduction in the risk of FN with primary or secondary G-CSF (HR 0.24; 95 % CI 0.14, 0.41) is constant regardless of chemotherapy dosage. |
8. The RR of FN associated at dose −2 is comparable to prophylactic G-CSF, and the RR at dose −1 is midway between that at full dose and dose −2. As the primary analysis assumed no loss of chemotherapy efficacy at dose −1 dose, it would have been counterintuitive to assume that dose −1 would be associated with a similar FN risk as secondary G-CSF. |
9. The primary analysis assumed that completion of chemotherapy at dose −1 dose (RDI ≥85 %) would not negatively impact survival outcomes, but completion at dose −2 reduces the relative effectiveness of chemotherapy and increases the risk of cancer recurrence [15]. |
Event probabilities
The baseline rate of FN with adjuvant TC in the absence of G-CSF (29.1 and 95 % CI 23.8 and 35.2 %) was derived from a recent meta-analysis of FN following TC chemotherapy outside of a clinical trial setting [12]. The relative risk (RR) of FN was based on the reduction in the risk of FN given prophylactic G-CSF and/or reduced chemotherapy dose intensity. The RR of FN with primary or secondary G-CSF and full chemotherapy dose (RR = 0.24; 95 % CI 0.14, 0.41) was derived from the same meta-analysis, with a number of assumptions (see Box 1) required to compute FN risk at dose −1 and −2 with and without G-CSF (Table 2). A breast cancer-specific estimate of FN mortality (1.4 %) was derived from the literature [12].
Non-parametric estimates of natural history recurrence rates over the first 15 years of the long-term model were derived from a large meta-analysis of adjuvant systemic therapies in breast cancer [16]. The relative DFS benefit with full-dose adjuvant TC was derived from the relevant clinical trial [11] and from Adjuvant! Online [17]. The potential impact of dose reductions on chemotherapy effectiveness and risk of cancer recurrence was derived from a study by Chirevella et al. [15], which reported that DFS among patients completing chemotherapy at RDI greater than 85 % was 1.57 times greater than that of patients treated with a RDI less than 85 %. The inverse of this ratio was used to adjust the risk of cancer recurrence at dose −2. We assumed no loss of chemotherapy efficacy at dose level −1 for the base case analysis, and this assumption was tested in a sensitivity analysis.
Costs and utilities
We adopted a third-party, direct-payer cost perspective. The drug acquisition cost per course of G-CSF was based on the 2013 Nova Scotia formulary reimbursement prices for Neupogen® and Neulasta®, accounting for differences in the dosing schedules. The baseline analysis used the lower cost alternative (Neupogen) administered for 7 days based on a 1:4 mix of higher and lower standard vial doses (20 % 480 μg vs. 80 % 300 μg dose). As per a recent Canadian study [18], we assumed that the initial G-CSF injection would be delivered by a home care nurse and that 6 % of patients would require on-going home care support. Given the large variation in G-CSF administration practices in Canada, and the potential for future changes in G-CSF acquisition and administration, we also tested the impact of higher and lower G-CSF costs in a one-way sensitivity analysis. The costs of FN hospitalization were derived from a Canadian cost study [6]. The cost of chemotherapy was based on local costs at the Queen Elizabeth II Health Sciences Centre, Halifax, Canada, and included chemotherapy drug acquisition (assuming a body surface area of 1.7), administration, supportive medications, laboratory and diagnostic testing, and human resources. Drug acquisition cost was adjusted for dose reductions (dose −1 or −2). The costs of treating cancer recurrences were derived from a Canadian model of cancer costs [19]. As in our previous work, other health state costs, including adverse events, were derived from the literature [14, 20]. All costs were converted into monthly or one-time event-driven costs and adjusted to 2014 Canadian dollars using the Statistics Canada Consumer Price Index health component [21].
Each health state in the model was also associated with a quality weight, or utility, which was derived from the Center for the Evaluation of Value and Risk in Health Cost-Effectiveness Analysis Registry [20].
Sensitivity analyses
We performed a probabilistic sensitivity analysis which allowed all the parameters in the model to vary simultaneously according to specified probability distributions (see Table 1). These probabilistic results were used to generate 95 % confidence intervals (CIs) and a cost-effectiveness acceptability frontier (CEAF) which illustrated the economically preferred strategy over a range of possible willingness-to-pay thresholds while accounting for uncertainty in multiple parameters. We also performed a series of one-way and two-way sensitivity analyses to test the impact of changes in key parameters and assumptions.
Our base case scenario used a 29 % FN rate and incorporated FN-related quality-of-life (QoL) penalties and increased mortality risk, as well as reduced chemotherapy effectiveness for patients with FN treated at dose level −2. Consistent with previous economic evaluations of G-CSF in breast cancer [22–25], we assumed that there would be no loss of chemotherapy effectiveness at −1 dose or following FN episodes without a dose reduction. We tested a number of other alternative scenarios, including a 24 % baseline FN rate to conform more closely with two previous evaluations of primary vs. secondary G-CSF [22, 25], as well as the 20 % threshold rate for recommending primary G-CSF in current clinical guidelines [8].
We also tested the sequential impact of incorporating various potential benefits of G-CSF prophylaxis. We started from a conservative perspective where the only impact of FN was in terms of cost and QoL penalties during each 7-day episode and on chemotherapy effectiveness only if FN led to chemotherapy dose adjustment to −2. We sequentially expanded this impact to include some loss of chemotherapy effectiveness at dose −1 (assuming a recurrence risk midway between full dose and dose −2, or a 50 % penalty), and some loss of effectiveness at a full dose following any FN episode (assuming a recurrence risk midway between full dose and dose −1, or a 25 % penalty) to account for the potential impact of delays in chemotherapy without any dose reductions. Finally, we tested the impact of reduced FN-related mortality with G-CSF, with and without a loss of chemotherapy effectiveness following any FN episode.
Results
The base case results for each G-CSF strategy are summarized in Table 3. With a 29 % baseline FN rate, a secondary G-CSF strategy tended to be cost-saving (Δcost = −$217; 95 % CI −$655, $51 per patient) and slightly but significantly more effective in terms of expected lifetime QALYs per patient (ΔQALYs = 0.02; 95 % CI 0.01, 0.04) than a no G-CSF strategy. Relative to secondary G-CSF, the primary G-CSF strategy was associated with an incremental cost of $4380 (95 % CI $912, $5807) and a small but significant lifetime QALY gain of 0.05 QALYs (95 % CI: 0.01, 0.11), for an incremental cost of $94,327 per QALY gained. The CEAF in Fig. 2, which accounts for simultaneous uncertainty in the parameters, suggests that primary G-CSF would only be preferred to a secondary prophylaxis strategy at a willingness-to-pay greater than the commonly cited $50,000 and $100,000 per QALY gained thresholds.
Sensitivity analyses
The results of the one-way sensitivity analyses (Fig. 3), which illustrate the impact of a proportional change in each parameter, suggest that cost-utility was most sensitive to changes in baseline FN risk, mortality, and the price of G-CSF. Chemotherapy characteristics, including price and relative effectiveness, had relatively little impact. A baseline FN risk of 24 % rather than 29 % increased the cost per QALY gained of primary relative to secondary G-CSF to $119,518, while using the 20 % risk recommended by current guidelines increased the cost per QALY to $151,968.
The scenarios considering the sequential impacts of FN (Appendix Table) demonstrated that the cost-effectiveness of primary vs. secondary G-CSF, but not secondary vs. a no G-CSF strategy, was sensitive to a reduction of chemotherapy efficacy following any FN event, as well as the impact of FN-related mortality. If the impact of FN is limited to QoL penalties and decreased chemotherapy effectiveness at dose level −2, the cost per QALY gained with a primary vs. secondary G-CSF strategy was $8.4 million. If there is also some loss of chemotherapy effectiveness at dose −1, primary G-CSF was dominated by the secondary strategy. If the impact of FN included some loss of effectiveness following any FN event (e.g., if all FN episodes were associated with significant dose delays leading to impaired chemotherapy efficacy), the cost-utility of primary G-CSF improved to $168,524 per QALY gained. Finally, the cost per QALY gained of primary vs. secondary G-CSF improved further to $61,030 when incorporating all potential FN impacts, including QoL penalties, FN-related mortality, and some loss of chemotherapy effectiveness following any FN event.
The minimum risk of FN necessary to achieve a cost per QALY gained of less than $100,000 was highly sensitive to the assumed relative effectiveness of chemotherapy following chemotherapy dose reductions or delays. Assuming a reduction in effectiveness at dose −2 (the base case), the threshold FN risk was 28 %. If there was also a 50 % loss of effectiveness at dose −1 relative to dose −2 (i.e., the negative impact of dose −1 on chemotherapy effectiveness is half that assumed for dose −2), the threshold FN risk increased to 32 %. This counterintuitive result will be discussed below and reflects a higher proportion of patients in the primary vs. secondary strategy who completed chemotherapy at a reduced −1 dose (Appendix Table). A further 25 % relative loss of effectiveness following any FN event (i.e., the negative impact of dose delays following FN event on chemotherapy effectiveness is 25 % of that assumed for −2 dose) improved this threshold to 22 %.
Discussion
Our primary analysis, based on a 29 % expected FN rate in the absence of primary G-CSF prophylaxis, suggested that a strategy of secondary G-CSF was less costly and more effective in terms of QALYs gained than a no G-CSF strategy. Primary G-CSF did not appear to be cost-effective relative to secondary G-CSF at willingness-to-pay thresholds much less than $100,000 per QALY gained. Critically, sensitivity analysis suggested that the minimum FN risk necessary to meet a $100,000 threshold was substantially higher than the 20 % currently recommended by clinical guidelines [8] if the benefits were limited to improved QoL, reduced FN-related mortality, and preservation of chemotherapy effectiveness by maintaining a treatment RDI ≥85 %.
These results appear consistent with two evaluations from other jurisdictions that have compared primary and secondary prophylactic G-CSF strategies in breast cancer. Ramsey et al. [22] reported a cost of US $116,000 per QALY gained with primary G-CSF based on a higher price and a lower baseline FN risk than we used in our base case (24 vs. 29 %), but this result was quite similar to our sensitivity analysis using a comparable FN risk. Using the same FN risk as Ramsey et al., Whyte et al. [25] reported a cost of £26,824 per QALY gained for primary G-CSF and estimated that the threshold FN risk necessary to meet a £30,000/QALY threshold was 29 %; comparable to our estimate of 28 % to meet a $100,000/QALY threshold. Together, our base case results and these previous evaluations suggest that primary G-CSF does not provide reasonable value for money relative to secondary G-CSF when judged against common cost-effective thresholds (CDN/US $50,000–100,000 or £20,000–30,000 per QALY gained) at the 20 % FN threshold currently recommended by practice guidelines.
To our knowledge, this is the first evaluation in breast cancer to explicitly test the impact of varying chemotherapy effectiveness following a FN episode, with or without subsequent chemotherapy dose reductions. Our analysis shows that the cost-effective FN threshold is influenced by chemotherapy effectiveness at lower RDIs and/or following dose delays. While a reduction in chemotherapy efficacy following significant dose reductions (dose −2 or RDI <85 %) have been previously demonstrated [15], it remains unclear if there is also loss of efficacy at the −1 dose (≥85 % RDI) or following any FN episode regardless of subsequent dose (e.g., due to treatment delays). Previous evaluations of G-CSF in breast cancer [22–25] have implicitly assumed that there is no loss of effectiveness at dose level −1, consistent with Chirevella et al. [15]. Our sensitivity analysis suggested that the minimum FN risk for primary G-CSF to be cost-effective relative to secondary G-CSF was higher than the baseline 28 % threshold noted earlier if there was a greater risk of cancer recurrence among patients treated at dose level −1. Under a primary G-CSF strategy, all patients who develop FN would receive their next chemotherapy cycle at dose level −1. Under a secondary strategy, patients who develop FN would receive G-CSF and be maintained at the planned chemotherapy dose with subsequent cycles, unless there was a second FN event. Overall, we observed a smaller proportion of patients reaching −1 dose levels, and correspondingly higher proportions maintaining full chemotherapy dose, in the secondary compared to the primary G-CSF strategy (Appendix Table). Conversely, the cost-effective FN threshold was lower than the baseline 28 % under the assumption of reduced chemotherapy effectiveness following any FN event. Our analysis showed that primary G-CSF was a cost-effective strategy compared to secondary G-CSF at a 22 % FN threshold under the assumptions of reduced FN mortality, and some reduction in chemotherapy effectiveness following any FN event, with or without subsequent dose reductions.
The primary driver of G-CSF benefit in the model, however, appeared to be FN-related mortality avoided and not the maintenance of chemotherapy RDI, as illustrated by the impact of excluding FN mortality in a sensitivity analysis. As shown in Table 3, the expected number of cancer recurrences was only slightly improved by a primary or secondary G-CSF strategy, as more than 96 % of the patients in the no G-CSF strategy still finished therapy at full dose or dose −1 and were not at increased risk of cancer recurrence under the assumptions of the base case analysis. Conversely, although the absolute number of FN-related deaths was small in all three strategies, the proportional differences in QALY due to FN mortality between strategies were substantial. In the absence of FN mortality reduction by G-CSF, primary G-CSF would only be a cost-effective strategy relative to secondary G-CSF if all FN events were associated with a loss of chemotherapy efficacy (e.g., due to dose delays) regardless of subsequent chemotherapy dosages. As such, the value for money for primary vs. secondary G-CSF must be examined within the context of these plausible but uncertain assumptions.
Our study, like others [22–25], was limited by the need for assumptions about the potential impact of FN and G-CSF on chemotherapy effectiveness, as well as the assumed reduction in FN mortality with G-CSF. Clinical research investigating the dose-response relationship over a continuous range of chemotherapy RDIs would be valuable in addressing this question. Our evaluation was also specific to the TC regimen, but sensitivity analyses suggested that cost-utility estimates were not particularly sensitive to chemotherapy price or relative effectiveness.
In summary, our evaluation suggests that secondary prophylaxis of FN with G-CSF is economically preferred to a no G-CSF strategy and that primary G-CSF may be justified over a secondary strategy at higher FN risks and higher willingness-to-pay thresholds. Most importantly, the threshold FN risk necessary to meet a cost-effective threshold of $100,000 per QALY gained, even with the lowest cost G-CSF option, appeared to be higher than the 20 % rate recommended by current clinical guidelines under most of the scenarios tested here. Critically, however, the cost-effectiveness of primary vs. secondary G-CSF appears to be dependent on a number of implicit and/or untested assumptions, such as FN mortality avoided with G-CSF, and relative chemotherapy efficacy following an episode of FN. Further clinical research is required to ascertain the true benefit of primary G-CSF and its value for money in FN prophylaxis.
References
Canadian Cancer Society, National Cancer Institute of Canada (2007) Canadian cancer statistics 2007. Canada, Toronto
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 379:432–444. doi:10.1016/S0140-6736(11)61625-5
Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group (2010) Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol 21(Suppl 5):v248–v251. doi:10.1093/annonc/mdq195
Lyman GH, Kuderer N, Greene J, Balducci L (1998) The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer 34:1857–1864. doi:10.1016/S0959-8049(98)00222-6
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167. doi:10.1200/JCO.2006.08.8823
Lathia N, Mittmann N, DeAngelis C et al (2010) Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer 116:742–748. doi:10.1002/cncr.24773
Lyman GH, Rolston KVI (2010) How we treat febrile neutropenia in patients receiving cancer chemotherapy. J Oncol Pract 6:149–152. doi:10.1200/JOP.091092
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. J Clin Oncol 47:8–32. doi:10.1016/j.ejca.2010.10.013
Cooper KL, Madan J, Whyte S et al (2011) Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404. doi:10.1186/1471-2407-11-404
National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 24 Jul 2012
Jones SE, Savin MA, Holmes FA et al (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387. doi:10.1200/JCO.2006.06.5391
Younis T, Rayson D, Thompson K (2012) Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer 20:2523–2530. doi:10.1007/s00520-011-1375-6
Younis T, Rayson D, Sellon M, Skedgel C (2007) Adjuvant chemotherapy for breast cancer: a cost-utility analysis of FEC-D vs. FEC 100. Breast Cancer Res Treat 111:261–267. doi:10.1007/s10549-007-9770-x
Younis T, Rayson D, Skedgel C (2011) The cost–utility of adjuvant chemotherapy using docetaxel and cyclophosphamide compared with doxorubicin and cyclophosphamide in breast cancer. Curr Oncol 18:e288–e296. doi:10.3747/co.v18i6.810
Chirivella I, Bermejo B, Insa A et al (2008) Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat 114:479–484. doi:10.1007/s10549-008-0018-1
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. doi:10.1016/S0140-6736(05)66544-0
Adjuvant! Online. https://www.adjuvantonline.com/index.jsp. Accessed 13 Apr 2012
Zhu X, Bouganim N, Vandermeer L et al (2012) Use and delivery of granulocyte colony–stimulating factor in breast cancer patients receiving neoadjuvant or adjuvant chemotherapy—single-centre experience. Curr Oncol 19:e239–e243. doi:10.3747/co.19.948
Will BP, Berthelot JM, Le Petit C et al (2000) Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer 36:724–735
Skedgel C, Rayson D, Younis T (2013) Is adjuvant trastuzumab a cost-effective therapy for HER-2/neu-positive T1bN0 breast cancer? Ann Oncol 24:1834–1840. doi:10.1093/annonc/mdt069
Statistics Canada (2011) Consumer price index, health and personal care, by province. http://www.statcan.gc.ca.ezproxy.library.dal.ca/tables-tableaux/sum-som/l01/cst01/econ161a-eng.htm. Accessed 13 Apr 2012
Ramsey SD, Liu Z, Boer R et al (2009) Cost-effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health 12:217–225. doi:10.1111/j.1524-4733.2008.00434.x
Lyman GH, Lalla A, Barron RL, Dubois RW (2009) Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther 31:1092–1104. doi:10.1016/j.clinthera.2009.05.003
Liu Z, Doan QV, Malin J, Leonard R (2009) The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy 7:193–205. doi:10.2165/11314740-000000000-00000
Whyte S, Cooper KL, Stevenson MD et al (2011) Cost-effectiveness of granulocyte colony–stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health 14:465–474. doi:10.1016/j.jval.2010.10.037
Center for the Evaluation of Value and Risk in Health. The Cost-Effectiveness Analysis Registry. Institute for Clinical Research and Health Policy Studies, Tufts Medical Center. www.cearegistry.org. Accessed 24 Jul 2012
Acknowledgments
Funding was provided by the Beatrice Hunter Cancer Research Institute, with funding from the Breast Cancer Society of Canada, and the Queen Elizabeth II Foundation award for breast cancer research. No support was received from a pharmaceutical company. The authors have no conflicts of interest to declare. The authors would like to acknowledge the assistance of Patrick Berrigan in conducting the sensitivity analyses, as well as Marlene Sellon for her assistance with the drug acquisition costs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17.7 kb)
Rights and permissions
About this article
Cite this article
Skedgel, C., Rayson, D. & Younis, T. Is febrile neutropenia prophylaxis with granulocyte-colony stimulating factors economically justified for adjuvant TC chemotherapy in breast cancer?. Support Care Cancer 24, 387–394 (2016). https://doi.org/10.1007/s00520-015-2805-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2805-7