Abstract
Background
Inflammatory markers are strong prognostic factors for survival in a variety of cancers. This study aimed to investigate the relationships between known inflammatory markers and their ability to predict overall survival (OS) in patients receiving docetaxel therapy.
Methods
Sixty-eight patients with advanced cancer were enrolled in a clinical trial of single agent docetaxel from 2000 to 2002. Inflammation was measured using baseline cytokine concentrations, acute phase reactant proteins and white blood cell counts. The neutrophil/lymphocyte ratio (NLR) and Glasgow Prognostic Score (GPS) were calculated. Associations between inflammatory markers and their predictive value for OS were tested.
Results
The majority of patients had elevated inflammatory markers (50–70%). Strong inter-relationships were observed between the different inflammatory indices. Only NLR and GPS were independently predictive of OS. A combined NLR and GPS score demonstrated 11 month differences in overall OS between patients with normal and elevated inflammatory status. Normalisation of NLR after three doses of chemotherapy was associated with significant improvement in survival.
Conclusion
This study found that NLR predicts the clinical outcomes for patients with advanced cancer treated with docetaxel. The clinical utilisation of NLR should be validated in a larger patient population to confirm its utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic inflammation plays an essential role in cancer development and progression [1, 2]. Current evidence strongly points to central inflammatory mediators with pro-inflammatory cytokines including tumour necrosis factor (TNF-alpha), interleukin-1 (IL-1) and interleukin-6 (IL-6) [1, 2] as the key protagonists. In addition to the role these cytokines provide in the local tumour microenvironment, TNF-alpha, IL-1 and IL-6 are elevated systemically in patients with advanced cancer [3–6] and significantly contribute to the formation of the acute phase response characterised by increased circulating C-reactive protein (CRP) concentrations and homeostasis of the innate and adaptive immune system. Together, these cells and mediators produce “smouldering” chronic systemic inflammation in patients with advanced cancer and may reflect the overall patient’s ability to tolerate and respond to chemotherapy treatments.
Several studies have reported the use of serum cytokine levels in differentiating cancer versus normal patients, stage of disease, presence of metastases and survival [7–10]. The cost of cytokine measurements and lack of standardisation with commercially available ELISA kits is prohibitive for routine use therefore limiting serum cytokine measurements to research studies [11]. Laboratory markers of systemic inflammation have been investigated as both prognostic and predictive biomarkers in several cancer populations. Examples of these include CRP [12–14], Glasgow Prognostic Score (GPS) [15–17], (a combination of CRP and albumin), neutrophil/lymphocyte ratio (NLR) [18–23] and platelet/lymphocyte ratio (PLR) [24] in predicting outcomes for patients after surgical resection but also in those with inoperable cancers. Recently we investigated the use of NLR as a predictor of chemotherapy response and survival in two sets of patients with advanced colorectal cancer (CRC) receiving first-line chemotherapy [25]. These results suggest the use of NLR as an independent marker of clinical benefit and survival but also that normalisation of NLR early during the course of treatment predicts for improved clinical outcomes.
Simple and easily assessable markers of systemic inflammation such as GPS and NLR may be more reliable clinical tools in clinical practice as predictors of chemotherapy outcomes and as a dynamic marker of the interaction between tumour, host and inflammatory responses. The aim of this study, therefore, was to (1) determine the utility of GPS and NLR in patients with advanced cancer receiving docetaxel chemotherapy, (2) assess associations between GPS and NLR with pro-inflammatory cytokines (3) develop a clinical score based on inflammatory indices to aid prognostication and (4) assess the effect of normalisation of NLR after three doses of docetaxel chemotherapy.
Methods
Study population and methods
Between July 2000 and June 2002, patients with histologically confirmed locally advanced or metastatic malignancy suitable for palliative chemotherapy at the Sydney Cancer Centre were enrolled in a trial examining the pharmacokinetics of weekly docetaxel on patients with advanced cancer. This study also examined the role of patient covariates, including acute phase reactants, nutritional status and pro-inflammatory cytokines on the clearance of docetaxel, adverse events and survival. Details of the study including eligibility and treatment protocols have been described in previously published papers [26–28]. No patients in this trial received granulocyte stimulating growth factor as part of their treatment. Informed consent was obtained from all participants in this trial and approved by the ethics committee of the Central Sydney Area Health Service. Patients enrolled in this trial with values for all relevant covariates, e.g., docetaxel clearance, adverse events, adequate survival follow-up and baseline inflammatory markers (CRP, albumin, white cell components and cytokines) plus nutritional status information were included in this analysis.
Statistical analysis
Statistical analysis was performed using SPSS Graduate Version 17.0. Overall survival (OS) was calculated from the date of starting the chemotherapy protocol to the date of death from any cause. Non-parametric tests (Mann–Whitney U) were used to test associations between variables (CRP, albumin, GPS, NLR and serum cytokines). Relevant data were dichotomised into binary variables according to either median values (cytokines), upper limits of normal (CRP) or other established categories (ECOG performance status, GPS). GPS was calculated as previously described [29, 31]. Patients with both elevated CRP level (>10 mg/ml) and hypoalbuminaemia (<35 g/l) were allocated a score of 2. Patients with only one of these biochemical abnormalities were allocated a score of 1 and patients with none of these abnormalities were allocated a score of 0. An NLR cut-off of greater than 5 was considered to be elevated based on previous trials [18, 19, 31]. Survival analysis was performed using the Kaplan–Meier method with log-rank test in univariate analysis. Cox regression analysis was used for multivariate survival analysis and for calculation of hazard ratios.
Results
Patient characteristics
Baseline patient characteristics are shown in Table 1. Sixty eight patients were enrolled in the study with relevant baseline clinical information and adequate follow up for analysis. The median age was 63 years (range: 28–85). Lung cancer the most common type of malignancy (30%) followed by head and neck (21%) and breast cancer (19%). The majority (64%) of patients had excellent performance status (0 or 1) with more than two-thirds of patients receiving prior chemotherapy for metastatic disease. A third of patients had GPS 0 with more than 50% of patients with NLR > 5.
Predictors of OS
At the time of analysis, all patients were deceased. The median OS was 6.3 months (95% confidence interval [CI] 4.7–7.9). In univariate analysis, variables predicting improved OS included primary site of disease, ECOG performance status 0 or 1, NLR ≤ 5, low or normal CRP levels, absence of hypoalbuminaemia, GPS 0 and low IL-6 levels (Table 2). Sub-group analysis was unable to be performed for cancer type due to the small sample sizes. In multivariate analysis, NLR > 5 and GPS 1 and 2 predicted for worse OS [hazard ratio 2.9 (95% CI 1.2–3.3) and hazard ratio 4.1 (2.2–7.7), respectively] (Table 3).
Inflammation-based score using GPS and NLR
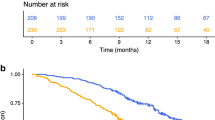
Based on the results of the multivariate analysis, an inflammation-based score using GPS and NLR was constructed (Table 4). Using this combined score, patients were categorised into three categories with patients with a score of 0 predicting improved OS (15.8 months, 95% CI 6.0–25.6) compared to a score of 1 (7.0 months, 95% CI 5.6–8.4) or 2 (4.3 months, 95% CI 2.7–7.9) (p < 0.0001) (Fig. 1).
Association between NLR and CRP, albumin, GPS and cytokines
The median NLR values for patients with CRP <10 was 4.3 (1.4–16.0) and was significantly lower compared to patients with elevated CRP levels (6.5; range 1.9–44.3) (p = 0.03). The median NLR values for patients with hypoalbuminaemia was 6.5 (range 3.1–44.0) and 6.1 (range 1.4–44.3) for patients with normal or high levels of albumin and not statistically significantly different (p = 0.15). In patients with GPS 0, the median NLR was 4.3 (range 1.4–16.0) and 6.5 (range 1.9–44.3) for patients with GPS 1 or 2 although the difference was not statistically significant (p = 0.094). There was no statistically significant association between NLR and IL-6 or TNF-alpha levels with NLR (p = 0.42 and 0.95, respectively).
Normalisation of NLR after three doses of docetaxel chemotherapy
Patients were categorised into the following categories (1) patients with NLR ≤ 5 at baseline (n = 31), (2) NLR > 5 at baseline and 1 week after three doses of docetaxel chemotherapy and prior to starting the fourth dose (n = 27) and (3) NLR >5 at baseline with normalisation to less or equal to 5 one week after three doses of docetaxel chemotherapy (n = 10). Patients with NLR normalisation after three doses of chemotherapy had a significantly improved OS of 10.3 months (95% CI 8.0–12.7) compared to patients without normalisation of NLR after three doses (4.3 months, 95% CI 2.7–6.0; p = 0.013). Although the median OS of patients with normalised NLR was better compared to those with NLR ≤ 5 at first cycle of chemotherapy (median OS 10.3 months [95% CI 8.0–12.7] versus 7.2 months [95% CI 4.9–9.4]), this did not approach statistical significance (p = 0.992, Fig. 2).
Discussion
The results of this study support the use of inflammation based scores (GPS or NLR) for predicting OS in patients with advanced cancer receiving docetaxel chemotherapy. Furthermore, a combination score using both these indices was able to categorise patients into three categories with significant differences in OS for patients with normal inflammatory status as defined by normal CRP levels, lack of hypoalbuminaemia and NLR ≤ 5 (median OS 15.8 months) compared to patients with a heightened systemic inflammatory response (median OS 4.3 months), a clinically significant difference of 11 months. However, this combined score requires further validation in independent study populations before it use in the clinical setting could be accepted. This study confirms the utility of GPS in predicting clinical outcomes in patients with advanced cancer receiving chemotherapy or immunotherapy as reported previously [16, 17, 30] but also the use of a simple, inflammatory score using components of the white cell count in predicting clinical outcomes in patients receiving chemotherapy for advanced cancer.
Previous studies, including one from our group, have reported the ability of NLR to predict surgical outcomes or outcomes from patients receiving first-line chemotherapy [18, 21, 25]. This study supports the use of NLR in patients who have received prior chemotherapy with 71% of patients having received at least one line of treatment. More than 50% of patients had a pre-chemotherapy NLR value >5, compared to our previous study of patients receiving first-line chemotherapy for colorectal cancer, where only a third of patients had an elevated NLR [25]. This may reflect the more advanced nature of disease of patients in this trial or be related to the different tumour types represented here including lung and head and neck cancers, resulting in a raised systemic inflammatory response.
This study strengthens the evidence for the role of the systemic inflammatory response on predicting outcomes for patients with advanced cancer receiving chemotherapy. The presence of inflammation is a strong predictor of the pharmacokinetics of docetaxel and thus drug exposure in the weekly [27] and three-weekly setting [32]. In both studies the acute phase reactant, α1 acid glycoprotein, the main determinant for protein binding, correlated with docetaxel pharmacokinetics. Furthermore, studies from our laboratory have demonstrated that tumour-mediated pro-inflammatory cytokines down-regulate the CYP3A4 enzyme; the main detoxifying metabolising enzyme for docetaxel [33]. Although there was no correlation between cytokine levels and NLR demonstrated in this study, this may have been due to the small sample size and lack of standard cut-off scores for serum cytokine measurements, hence confirming the difficulty of using cytokine measurements outside of a research setting. The association between high NLR values and elevated CRP levels, however, confirms the value of using NLR as a valid biomarker of systemic inflammatory response.
This study reports the investigation of the utility of NLR during the course of chemotherapy, in particular the normalisation of NLR, to predict early responses to treatment. In this cohort, a subset of patients with normalisation of NLR after three doses of treatment had substantially improved OS compared to those without normalisation of NLR after three weekly doses (10.3 versus 4.3 months). This additional information early during the course of treatment prior to radiological assessment may be useful in decision-making regarding further treatment or for identifying patients with worse prognosis who may be potentially suitable for anti-inflammatory mediators.
In conclusion, the results of this study have provided evidence for the use of NLR in the advanced cancer setting for patients receiving chemotherapy, introduced the concept of a combined inflammation score and the use of NLR early during a treatment course to provide additional information for prognostication. The use of a cheap, simple and easily accessible plasma marker such as the NLR should be validated in a larger patient population to confirm its utility.
References
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Balkwill F, Mantovani A (2010) Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther 87:401–406
Anderson GM, Nakada MT, DeWitte M (2004) Tumor necrosis factor-[alpha] in the pathogenesis and treatment of cancer. Curr Opin Pharmacol 4:314–320
Michalaki KS, Charles P, Waxman J (2004) Serum levels of IL-6 and TNF-a correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 91:1227
Scambia UT, Benedetti Panici P, Foti' E, Martucci R, Gadducci A, Perillo A, Facchini V (1995) Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer 71:354–356
Blay J-Y, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, Mercatello A, Ravault A, Tourani J-M, Moskovtchenko J-F, Philip T, Favrot M (1992) Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res 52:3317–3322
Chung YC, Chang CY (2003) Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol 83:222–226
De Vita F, Romano C, Orditura M, Galizia G, Martinelli E, Lieto E, Catalano G (2001) Interleukin-6 serum level correlates with survival in advanced gastrointestinal cancer patients but is not an independent prognostic indicator. J Interferon Cytokine Res 21:45–52
Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R (1995) Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol 13:575–382
Andrews BEN, Shariat SF, Kim J-H, Wheeler TM, Slawin KM, Lerner SP (2002) Preoperative plasma levels of interleukin-6 and its soluble receptor predict disease recurrence and survival of patients with bladder cancer. J Urol 167:1475–1481
Ledur AFC, David B, Hamberger C, Cavaillon JM (1995) Variable estimates of cytokine levels produced by commercial ELISA kits: results using international cytokine standards. J Immunol Methods 186:171–179
Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27:3437–3444
Polterauer S, Grimm C, Tempfer C, Sliutz G, Speiser P, Reinthaller A, Hefler LA (2007) C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol 107:114–117
Lee JG, Cho BC, Bae MK, Lee CY, Park IK, Kim DJ, Ahn SV, Chung KY (2009) Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer 63:106–110
Murri AMA, Bartlett JMS, Canney PA, Doughty JC, Wilson C, McMillan DC (2006) Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 94:227–230
Sara R, Gavin WAL, Michael A, John G, Donald CM (2007) Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer 109:205–212
Sharma R, Hook J, Kumar M, Gabra H (2008) Evaluation of an inflammation-based prognostic score in patients with advanced ovarian cancer. Eur J Cancer 44:251–226
Kishi Y, Kopetz S, Chun Y, Palavecino M, Abdalla E, Vauthey J-N (2009) Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 16:614–622
Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JPA (2008) Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol (EJSO) 34:55–60
Gomez D, Farid S, Malik H, Young A, Toogood G, Lodge J, Prasad K (2008) Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 32:1757–1762
Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E (2009) Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 137:425–428
Nakahara K, Monden Y, Ohno K, Fujii Y, Hashimoto J, Kitagawa Y, Kawashima Y (1987) Importance of biologic status to the postoperative prognosis of patients with stage III nonsmall cell lung cancer. J Surg Oncol 36:155–160
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91:181–184
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P (2009) Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 197:466–472
Chua WCK, Baracos VE, Clarke SJ (2011) Neutrophil–lymphocyte ratio (NLR) predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 104:1288–1295
Charles KA, Rivory LP, Stockler MR, Beale P, Beith J, Boyer M, Clarke SJ (2006) Predicting the toxicity of weekly docetaxel in advanced cancer. Clin Pharmacokinet 45(6):611–612
Slaviero KA, Clarke SJ, McLachlan AJ, Blair EYL, Rivory LP (2004) Population pharmacokinetics of weekly docetaxel in patients with advanced cancer. Br J Clin Pharmacol 57:44–53
Slaviero KA, Read JA, Clarke SJ, Rivory LP (2003) Baseline nutritional assessment in advanced cancer patients receiving palliative chemotherapy. Nutr Cancer 46:148–157
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ (2003) Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 89:1028–1030
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ (2004) Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 90:1704–1706
Halazun K, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, WItkowski P, Stegel AB, Brown RS, Emond JC (2009) Negative impact of neutrophil lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 250:141–151
Alexandre J, Rey E, Girre V, Grabar S, Tran A, Montheil V, Rabillon F, Dieras V, Jullien V, Hacrait P, Pons G, Treluyer JM et al (2007) Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Surg Oncol 18:168–172
Charles KA, Rivory LP, Brown SL, Liddle C, Clarke SJ, Robertson GR (2006) Transcriptional repression of hepatic cytochrome P450 3A4 gene in the presence of cancer. Clin Cancer Res 12:7492–7497
Acknowledgements
We would like to thank Jenny Peat for assistance with statistical analysis. Wei Chua was supported by an NSW Cancer Institute Fellowship (Australia) and a Pfizer Australia Cancer Research Grant.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chua, W., Clarke, S.J. & Charles, K.A. Systemic inflammation and prediction of chemotherapy outcomes in patients receiving docetaxel for advanced cancer. Support Care Cancer 20, 1869–1874 (2012). https://doi.org/10.1007/s00520-011-1289-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1289-3