Abstract
Purpose
Previous studies suggest tetracycline and other antibiotics lessen the severity of epidermal growth factor receptor (EGFR) inhibitor-induced rash. This study sought to confirm such findings.
Methods
Patients starting an EGFR inhibitor were eligible for this randomized, double-blinded, placebo-controlled study and had to be rash-free. They were then randomly assigned to tetracycline 500 mg orally twice a day for 28 days versus a placebo. Rash development and severity (monthly physician assessment and weekly patient-reported questionnaires), quality of life (SKINDEX-16), and adverse events were monitored during the 4-week intervention and then for an additional 4 weeks. The primary objective was to compare the incidence of grade 2 or worse rash between study arms; 32 patients per group provided a 90% probability of detecting a 40% difference in incidence with a type I error rate of 0.05 (two-sided).
Results
Sixty-five patients were enrolled, and groups were balanced on baseline characteristics. During the first 4 weeks, healthcare provider-reported data found that 27 tetracycline-treated patients (82%) and 24 placebo-exposed patients (75%) developed a rash. This rash was a grade 2+ in 17 (52%) and 14 (44%), respectively (p = 0.62). Comparable grade 2+ rash rates were observed during weeks 5 through 8 as well as with patient-reported rash data throughout the study period. Quality of life was comparable across study arms, and tetracycline was well tolerated.
Conclusion
Although previous studies suggest otherwise, this randomized, double-blinded, placebo-controlled study did not find that tetracycline lessened rash incidence or severity in patients who were taking EGFR inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
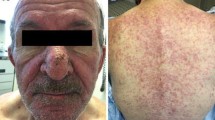
Rash occurs in greater than 50% of patients treated with epidermal growth factor receptor (EGFR) inhibitors [1, 2] Described as akin to acne, this rash can cause cutaneous burning and appears erythematous and pustular. It develops on the face, trunk, and upper extremities and also causes cutaneous discomfort. Although the typical EGFR inhibitor-induced rash does not appear to be fatal, it does negatively impact quality of life [3]. Interviewing 20 cancer patients who had developed such a rash, Wagner and Lacouture observed symptoms of worry, frustration, and depression that appeared to occur as a direct result of this drug-related adverse event [4].
Despite the negative quality of life implications of these rashes, few studies have focused on their prevention or palliation. The North Central Cancer Treatment Group (NCCTG) had previously reported on a 61-patient placebo-controlled trial that suggested tetracycline diminished rash severity [5]. Similarly, Scope and others also observed that minocycline might also decrease rash severity [6]. In view of such favorable, preliminary findings from the use of a single antibiotic as a means of rash palliation, the North Central Cancer Treatment Group undertook the current placebo-controlled trial with the goal of further assessing the role of tetracycline in decreasing the severity of EGFR inhibitor-induced rashes.
Methods
Overview
This study was conducted within the North Central Cancer Treatment Group. Institutional Review Boards from each study site approved the protocol prior to the enrollment of patients. All patients provided written informed consent.
Patient eligibility
The protocol outlined the following patient eligibility criteria: (1) patient age ≥18 years; (2) cancer diagnosis; (3) an epidermal growth factor receptor inhibitor (must have been gefitinib, cetuximab, erlotinib, or one of the other investigational agents within this class of drugs) to have been initiated less than 7 days prior to registration or less than 7 days after registration but prior to the initiation of the study intervention; (4) creatinine ≤2 mg/dL and total bilirubin ≤2 mg/dL within 14 days of trial registration; (5) patient able to take oral medications reliably; and (6) patient able to complete questionnaires with assistance if needed.
Patients were ineligible in the event of any one of the following: (1) a previous allergic reaction to or severe intolerance of tetracycline or one of its derivatives; (2) tetracycline use within 7 days of trial registration; (3) pregnant or nursing or of child-bearing potential and unwilling to employ contraception; (4) severe nausea or vomiting; (5) rash at study registration; or (6) a skin problem that might “flare” during cancer treatment.
Evaluations
Within 14 days of study registration, all patients underwent a history, physical exam, and assignment of a performance score by their healthcare provider. A blood draw for serum creatinine and total bilirubin were also obtained.
Patients were monitored for rash severity (assessed both by means of the physician reported National Cancer Institute Common Terminology Criteria (CTC) version 3 and a patient-reported questionnaire), rash incidence, quality of life as per the patient-completed SKINDEX-16 questionnaire and a series of Linear Analogue Self Assessment (LASA) scales [5, 7] and adverse events (both physician- (CTC, version 3) and patient-reported). Patients also completed a questionnaire on compliance with the EGFR inhibitor, as the study team recognized that stopping the EGFR inhibitor would likely lead to rash improvement. These questionnaires were to be completed at baseline and weekly for 8 weeks after starting tetracycline/placebo.
Although the intervention was to continue for a total of 4 weeks, monitoring continued for a total of 8 weeks. At the end of weeks 4 and 8, the treating oncologist performed a history and physical examination, assessment of performance status, and appraisal of adverse events, as per CTC, version 3.0. These criteria denote a symptomatic rash that involves <50 of the body surface area as grade 2, a symptomatic rash that involves ≥50% of the body surface area as grade 3, and a more generalized, exfoliative rash as a grade 4.
Treatment
The following stratification factors were utilized in determining the treatment assignment: (1) first-line cancer treatment: yes versus no; (2) EGFR inhibitor: gefitinib versus cetuximab versus other; (3) ongoing corticosteroid therapy: yes versus no. Thereafter, patients were randomly assigned to receive tetracycline 500 mg orally twice a day for 4 weeks versus an identical-appearing placebo at the same frequency and duration. Patients were instructed not to take antacids within 2 h of taking the tetracycline/placebo. Tetracycline dosing was based on favorable preliminary results from a previous NCCTG study as well as on favorable results in the treatment of acne [5, 8, 9]
The protocol required that tetracycline/placebo be discontinued in the event of grade 2 or worse nausea and/or vomiting. Otherwise, the treating oncologist was to utilize his/her clinical judgment in choosing to stop the intervention because of tetracycline/placebo-related side effects.
All other supportive care measures were allowed, and the protocol did not dictate any aspect of cancer therapy.
Statistical analyses
The primary study endpoint was to compare the incidence of a grade 2 or worse rash between the two study arms. An intent-to-treat approach was used; thus, patients who dropped out were considered to have developed a grade 2 or worse rash. A sample size of 30 patients per group provided a 90% probability of detecting a difference in the incidence of severe rash of 40% between the two study arms and of thereby rejecting the null hypothesis of equal proportions with a type I error rate of 0.05 by means of a Fisher’s exact test. This large effect size was sought and justified because previous NCCTG study data, as alluded to earlier, suggested such an effect size could be observed [5] and because, in the judgment of the study team, asking a patient to take an antibiotic twice a day for 1 month necessitated this degree of efficacy as a trade-off.
Overall rash incidence was analyzed similarly to the primary endpoint. Other secondary endpoints included comparisons across study arms to assess changes in the LASA quality of life scores from baseline and the incidence of adverse events by means of Wilcoxon rank-sum testing and Fisher’s exact testing, respectively.
Finally, a post hoc pooled analysis that utilized data from the current study and from a previous NCCTG placebo-controlled trial with tetracycline was also undertaken [5]. After pooling all patient data, the incidence of grade 2+ rash was compared between tetracycline-treated and placebo-exposed patients.
Results
Baseline characteristics
The target accrual of 65 patients was met between July of 2007 and February of 2008. Thirty-three patients were assigned to the tetracycline arm and 32 to placebo. Baseline characteristics appear in Table 1 and indicate that patient characteristics in each study arm were well balanced.
Compliance
The time that patients remained on tetracycline or placebo was comparable. The median time-on-tetracycline was 29 days (range 4, 44 days) and time-on-placebo was 29 days (range 4, 45 days; p = 0.94). Reasons for stopping tetracycline and placebo included completion of the protocol requirements in 61% and 59% of patients, respectively; patient declined further protocol therapy for otherwise unspecified reasons in 15% and 6%, respectively; an adverse event halted protocol participation in 9% and 22%, respectively; cancer progression prompted stoppage in 3% in both arms (Fig. 1).
In terms of compliance, three patients on the tetracycline arm and two on the placebo arm stopped the EGFR inhibitor during the study intervention. All these patients except one who was assigned to the tetracycline arm stopped the EGFR inhibitor because of rash development.
Incidence of rash and rash severity
The cumulative incidence of grade 2 or worse rash was comparable across study arms (Table 2, Fig. 2). In looking at healthcare provider-reported rash, during the first 4 weeks, 27 tetracycline-treated patients (82%) and 24 placebo-exposed patients (75%) developed a rash. This rash was a grade 2 or worse in 17 (52%) and 14 (44%) of patients, respectively (p = 0.62). Between 5 and 8 weeks, when patients were no longer taking the tetracycline/placebo and when patient dropout rates were high, 31 tetracycline-treated patients (94%) and 26 placebo-exposed patients (81%) had a rash (p = 0.15). Thus, there was no statistically significant difference in the development of grade 2 or worse rashes between the treatment groups.
Patient-reported rates of severe rash provided similar conclusions. During the first 4 weeks, patient-reported data found that 30 tetracycline-treated (91%) and 26 (81%) placebo-exposed developed a rash (p = 0.30). During this same period, five (19%) tetracycline-treated patients reported that the rash covered between 25% and 50% of their body surface area and five (20%) of placebo-exposed patients said the same. No tetracycline-treated and one placebo-exposed patient reported a rash that covered >50% of body surface area. In all, these differences were not statistically significant between study arms (p = 0.99). Between weeks 5 and 8, when patients were no longer taking the antibiotic/placebo, there were no reported statistically significant differences in patient-reported rash severity (p = 0.99).
The SKINDEX-16 questionnaire, which examines a variety of skin-related quality of life issues [7], such as skin burning and irritation, cutaneous pain, emotional, and social aspects of skin-related disease, did not observe differences between treatment arms at any point over the 8-week study period.
As expected, the tetracycline was well tolerated with no statistically significant differences in adverse events between the two study arms (Table 3).
Pooled results
An exploratory pooled analysis that examined efficacy of tetracycline versus placebo and that included data from an earlier-reported, 61-patient study from this same study team showed no statistically significant differences in rash incidence or rash severity at any time point [5].
Discussion
This randomized, double-blinded, placebo-controlled trial sought to confirm earlier data that suggested that tetracycline diminished rash severity in patients treated with EGFR inhibitors [5]. The findings reported here are disappointing. Both healthcare provider-reported and patient-reported incidence and severity of rash were not improved with the use of tetracycline. Based on these data, we do not recommend that prophylactic tetracycline, as prescribed in this trial, be used to prevent or attenuate a rash caused by an EGFR inhibitor.
How might we reconcile the negative findings observed here with the other positive findings that preceded this trial? Although previous reports suggest that the EGFR inhibitor-induced rash does not tend to become infected, a growing literature does in fact point to the possibility of superinfection [10–12]. Under such circumstances of superimposed cutaneous infection, antibiotics may conceivably play a role in palliating the appearance and symptoms associated with these rashes. This possibility of superinfection might perhaps account for the divergent results observed in the current trial as compared to others. Moreover, superinfection does suggest one scenario in which the initiation of antibiotic might be of value in treating an EGFR inhibitor-induced rash [5, 6].
Perhaps the most important conclusion to be drawn from the current report is the fact that there remains a strong need to investigate palliative strategies for the EGFR inhibitor-induced rash. Although previous studies suggest that rash may predict a better cancer prognosis, the negative impact of this rash on quality of life, as alluded to earlier, underscores the importance of attempting to palliate this rash in same manner that we palliate a variety of other treatment-induced side effects, such as nausea and vomiting. Moreover, although the recently reported STEPP trial provides some promising results with respect to rash palliation, some of the interventions included in this multi-interventional approach—such as the use of prophylactic antibiotics—do not consistently appear to yield efficacious results [13]. A clear and concise approach to rash palliation is needed.
References
Shepherd FA, Rodriques Pereira J, Ciuleanu T et al (2005) Erlotinib in previously treated non-small cell lung cancer. N Engl J Med 353:123–132
Saltz LB, Meropol NJ, Loehrer PJ et al (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Jatoi A, Nguyen PL (2008) Do patients die from rashes from epidermal growth factor receptor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist 3:1201–1204
Wagner LI, Lacouture ME (2007) Dermatologic toxicities associated with EGFR inhibitors: the clinical psychologist’s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology 21(suppl 5):34–36
Jatoi A, Rowland K, Sloan JA et al (2008) Tetracycline to prevent epidermal growth factor receptor inhibitor-induced rashes: results from a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB). Cancer 113:847–853
Scope A, Agero AL, Dusza SW et al (2007) Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol 25:5390–5396
Chren MM, Lasek RJ, Sahay AP, Sands LP (2001) Measurement properties of Skindex-16: a brief quality of life measure for patients with skin diseases. J Cutan Med Surg 5:105–110
Gammon WR, Meyer C, Lantis S et al (1986) Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double blind study. J Am Acad Dermatol 14:183–186
Meynadier J, Alirezai M (1998) Systemic antibiotics for acne. Dermatology 196:135–139
Li J, Peccerillo J, Kaley K, Saif MW (2009) Staphylococcus aureus bacteremia related with erlotinib skin toxicity in a patient with pancreatic cancer. JOP 10:338–340
Cholongitas E, Pipili C, Ioannidou D (2009) Malassezia folliculitis presented as acneiform eruption alter cetuximab administration. J Drugs Dermatol 8:274–275
Grenader T, Gipps M, Goldberg A (2008) Staphylococcus aureus bacteremia secondary to severe erlotinib skin toxicity. Clin Lung Cancer 9:59–60
Mitchell EP, Lacouture M, Shearer H et al (2009) Final STEPP results of the prophylactic versus reactive skin toxicity treatment for panitumumab-related skin toxicity in patients with metastatic colorectal cancer. J Clin Oncol 27:18s, supplement; abstract CRA4027
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35195, CA-35090, CA-35113, CA-35103, CA-35415, CA-35431, CA-35103, CA-35269, CA-35101, CA-37417, CA-63848, CA-63849, and CA-35267.
Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014 (Roscoe F. Morton, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.); Medcenter One Health Systems, Bismarck, ND 58506 (Edward J. Wos, M.D.); Rapid City Regional Oncology Group, Rapid City, SD 57709 (Richard C. Tenglin, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (Patrick J. Flynn, M.D.); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.) Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, M.D.); Hematology & Oncology of Dayton, Inc., Dayton, OH 45415 (Howard M. Gross, M.D.)
Rights and permissions
About this article
Cite this article
Jatoi, A., Dakhil, S.R., Sloan, J.A. et al. Prophylactic tetracycline does not diminish the severity of epidermal growth factor receptor (EGFR) inhibitor-induced rash: results from the North Central Cancer Treatment Group (Supplementary N03CB). Support Care Cancer 19, 1601–1607 (2011). https://doi.org/10.1007/s00520-010-0988-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0988-5