Abstract
Fatigue is a frequent problem after surgical treatment of solid tumours. Aerobic exercise and psychosocial interventions have been shown to reduce the severity of this symptom in cancer patients. Therefore, we compared the effect of the two therapies on fatigue in a randomised controlled study. Seventy-two patients who underwent surgery for lung (n=27) or gastrointestinal tumours (n=42) were assigned to an aerobic exercise group (stationary biking 30 min five times weekly) or a progressive relaxation training group (45 min three times per week). Both interventions were carried out for 3 weeks. At the beginning and the end of the study, we evaluated physical, cognitive and emotional status and somatic complaints with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core Module (EORTC-QLQ-30) questionnaire, and maximal physical performance with an ergometric stress test. Physical performance of the training group improved significantly during the programme (9.4±20 watts, p=0.01) but remained unchanged in the relaxation group (1.5±14.8 watts, p=0.37). Fatigue and global health scores improved in both groups during the intervention (fatigue: training group 21%, relaxation group 19%; global health of both groups 19%, p for all ≤0.01); however, there was no significant difference between changes in the scores of both groups (p=0.67). We conclude that a structured aerobic training programme improves the physical performance of patients recovering from surgery for solid tumours. However, exercise is not better than progressive relaxation training for the treatment of fatigue in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many patients undergoing cancer treatment experience a substantial loss of energy and a severe impairment of physical performance. Furthermore, up to 30% of cancer survivors report a reduced performance status even years after treatment [20, 27]. This problem has been linked to several factors including nutritional status, protein turnover, anaemia, sleep disturbances, increased production of pro-inflammatory cytokines, psychosocial situation, mood disorders and amount of physical activity [31]. However, the causes of fatigue in cancer patients are not fully understood. This symptom is also a substantial problem of patients undergoing surgery [5]. In this setting, fatigue has been related to decreased muscle strength and impaired cardiorespiratory function [6, 7]. In fact, low physical performance has frequently been postulated to be a substantial contributor to cancer fatigue [30]. However, fatigue represents only one side of the impairment of performance status experienced by cancer patients. Cancer is usually accompanied by an “asthenic syndrome” consisting of two components, one objective (loss of physical performance) and one subjective (fatigue). Indeed, patients who report “feeling tired” may experience a spectrum of symptoms that includes diminished energy, hypersomnia, cognitive dysfunction (forgetfulness, impaired short-term memory, reduced concentration), post-exertional malaise and reduced ability to carry out activities involving physical effort [24]. Due to the complexity of the cancer-related fatigue syndrome, therapeutical programmes for the treatment of this symptom have evaluated different approaches including exercise, psychotherapy, progressive relaxation training and cognitive behavioural therapy [24]. In most studies, endurance and resistance exercise programmes during and after treatment have resulted in improvements of physical performance, quality of life and mood [8–13]. Moreover, a recent meta-analysis showed positive effects of progressive relaxation training on the emotional adjustment and treatment-related symptoms in non-surgical cancer patients [23]. These results suggest that different therapeutical approaches may have positive effects on cancer-related fatigue. However, studies about the effects of other treatments (i.e. individual and group psychotherapy) have yielded contradictory results [2, 8, 18].
To our knowledge, the effectiveness of exercise and psychosocial interventions for the treatment of this symptom has yet not been compared. While an exercise programme can improve functional status and therefore reduce fatigue, behavioural and psychological therapies, which have no effect on physical performance, may effectively address the mental component of this symptom. Based on these considerations, we compared the effect of two interventions, aerobic exercise and progressive relaxation training, on the fatigue, quality of life and physical performance of cancer patients recovering from surgical treatment of solid tumours.
Patients and methods
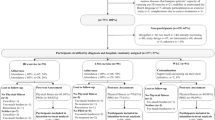
A consecutive series of 72 patients undergoing surgery for a solid tumour (lung, n=27, stomach, n=13; colon, n=16; sigmoid, n=13; and rectum, n=3) participated in the study (see Table 1). Inclusion criteria were age between 30 and 75 years, Eastern Cooperative Oncology Group (ECOG) score 0–2, surgical intervention for a histologically confirmed lung or gastrointestinal tumour and an understanding of written German. Exclusion criteria were bone metastasis, diabetes mellitus, impaired left ventricular function, coronary heart disease, liver or kidney dysfunction, psychiatric or rheumatic disease, haemoglobin concentration <10 g/dl, and ongoing chemo-, radio- or immune therapy. The study was approved by the institutional ethics committee, and all patients provided informed consent. Eighteen patients had received adjuvant chemotherapy, and 11 had undergone post-operative radiation. All patients had concluded therapy before recruitment into the study.
Patients were included in the study a mean of 120 days after surgery (Table 1). On the first day, maximal physical performance was assessed with a stress test on an ergometer with continuous ECG monitoring. The test was started with 25 watts and increased by 25 watts every 3 minutes until exhaustion. Quality of life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core Module (EORTC QLQ-C30 version 2). This instrument consists of 30 questions and allows an evaluation of emotional, cognitive, physical and social functioning (function scales) and of the severity of fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss and constipation/diarrhoea (symptom scales). While higher scores in the function scales show a better functional status, higher scores in the symptom scales indicate more severe complaints.
Patients were stratified according to the tumour localisation (lung or gastrointestinal cancer) and randomly assigned to an aerobic exercise or relaxation training group. Randomisation was carried out using a computer-generated random number list. The randomisation sequence was concealed until assignment of interventions.
Aerobic exercise training: The exercise programme consisted of biking on a stationary bike for 30 min daily 5 days per week following an interval-training pattern. During the first week, exercise duration was 5×3 min per day. Exercise duration was increased to 4×5 min per day in the second week and to 3×8 min in the third week. Patients were instructed to keep a pedalling frequency of about 50 cycles per min. Training intensity corresponded to a heart rate of about 80% of the maximal heart rate in the stress test. Heart rate during training was continuously assessed with a heart-rate monitor. The subjective intensity of effort was evaluated with the Borg Rate of Perceived Exertion scale, a visual analogue scale ranging from 6,”the effort is very light”, to 20 “the effort is very, very hard” [3]. Intensity effort during training corresponded to 13–14 (“somewhat hard”). As exercise heart rate decreased due to training adaptation, workload was increased to maintain training intensity. During workouts, patients were continuously supervised by a physician.
Relaxation training: The progressive muscle relaxation technique (Jacobson method) consists of a systematic programme of contraction and relaxation of muscle groups (face, neck, shoulders, arms, forearms, hands, back, abdomen, buttocks, thighs, legs and feet [19]. During training, participants tightened each muscle group progressively; after reaching a maximal contraction, tension was held for about 5 s; then, participants relaxed for 30 s while focussing on breathing. This procedure was repeated for each muscle group. Sessions lasted approximately 45 min and were repeated on Mondays, Thursdays and Fridays for 3 weeks.
A second assessment of maximal physical performance with an ergometric test and of quality of life with the EORTC questionnaire was carried out at the end of the programme. All tests were carried out between 9:00 a.m. and 12:00 noon. Three patients in the aerobic exercise group were admitted to hospital for the treatment of a concurrent disease (thrombosis, infection) and dropped out of the programme. Therefore, no assessment of physical performance or quality of life could be obtained at the end of the study. The data of these patients were evaluated using the “worst rank assumption” [21].
Statistical analysis: A preliminary sub-analysis of both strata was carried out. It included data at baseline and changes in physical performance, global quality of life scores and severity of dyspnea, pain and fatigue. This analysis showed no difference between patients with gastrointestinal tumours and with lung cancer regarding baseline scores and changes after the intervention. Therefore, data of both strata (gastrointestinal tumours and lung cancer) were considered together in order to increase the power of the statistical analysis.
Maximal physical performance of all patients was compared with tables of normal values for aerobic power tests for healthy adults [1]. According to these tables, patients were assigned to one of six functional categories (very poor, poor, fair, good, excellent and superior). These categories correspond approximately to a functional capacity lower than 50%, 50–54%, 55–65%, 66–70%, 71–75% and higher than 76% of the maximal values recorded in healthy persons of corresponding age and gender.
Primary endpoint of the study was the reduction in fatigue scores during the intervention. A difference of 30% or more between the two groups was considered to be clinically relevant. To detect this difference with a probability of an α- and a β-error of 5% and 10%, at least 30 patients were required in each group. Statistical analyses were carried out with the Wilcoxon, Mann-Whitney U, and Fisher tests. To evaluate the association between physical performance and fatigue, we compared changes in both parameters during the interventions with the Spearman test. A value of p<0,05 was considered to be statistically significant, and a value of r>0.30 to show a relevant association. Values are expressed as mean ± standard deviation. Expected maximal heart rate was calculated using the formula 220 minus age (in years). Maximal physical performance in METs (metabolical equivalents) was calculated according to the guidelines of the American College of Sports Medicine [1].
Results
Haemoglobin concentration: There were no differences in the haemoglobin concentrations of the two groups at recruitment or at the end of the study (relaxation training group pre: 13.0±1.4; post 13.2±1.3; exercise training: pre 13.4±1.4; post 13.6±1.3, p for all not significant).
Quality of life and symptom scores: Assessment of cognitive, physical, role, social and emotional functioning, global health, severity of dyspnea, fatigue, pain and sleep disorders showed no difference in the scores of the two groups at recruitment (p for all not significant, see Table 1). At the end of the study, both groups showed significant improvement in the sub-scales fatigue (6±33% versus 9±25%), emotional functioning (8±34% versus 11±30%) and global health (20±36 versus 17±51%, p for all differences before-after <0.05). However, the reduction of these scores did not differ significantly between groups (p for the difference of scores between groups not significant). While pain scores of the relaxation training group fell significantly during the study (p=0.02), pain scores of the aerobic exercise training group remained unchanged (see Table 1, Fig. 1).
Physical performance: The subjective effort in the tests before and after the programme was comparable for both groups (heart rate median Borg scale scores 17–18 for both groups before and after the programme, meaning “the effort was very hard”). After 3 weeks, the maximal physical performance of the training group increased significantly (before: 116±35 watts, after: 125±39 watts, p<0.05); however, the maximal physical performance of the relaxation group remained unchanged (before: 96±32 watts; after: 97±34 watts, p:0.56). At this point, maximal physical performance of 68% of patients in the aerobic exercise group and 75% of patients in the relaxation training group was “very poor” or “poor” (see Table 2).
A correlation analysis with the Spearman test showed no significant association between increase of maximal physical performance and reduction of fatigue scores (r: 0.13, p: 0.27) or improvement of global health status (r=0.07, p=0.56).
Discussion
Results of the present study show that a daily aerobic training programme results in a substantial and clinically relevant improvement of maximal physical performance in cancer patients after surgery. However, the fatigue reduction after an aerobic exercise training programme was not greater than after progressive relaxation training. Furthermore, the reduction of fatigue scores was not related to the change in maximal physical performance.
Cancer-related fatigue is defined as an unusual and persistent sense of tiredness that can occur during or after treatment, may affect both physical and mental ability and is not relieved by rest [4]. However, the perception of “tiredness” is subjective and may therefore differ between patients. In fact, while fatigue may be intuitively associated with physical exhaustion, the ICD-10 diagnostic criteria of cancer-related fatigue include several symptoms that are most likely independent of performance status (i.e. diminished concentration or attention and hypersomnia). This shows that the cancer-related fatigue syndrome consists of several components and that different therapeutic approaches may thus be required for treating this problem.
Recent studies have yielded a considerable amount of information about the pathogenesis of reduced physical performance in cancer patients [15, 22]. However, it has been reported that the perception of fatigue in this setting may change with time and may be independent of the reduction of performance status [28]. Furthermore, we have reported a lack of association between maximal physical performance and fatigue in cancer patients after chemotherapy [12]. Moreover, previous studies about the effect of an exercise programme in cancer patients have sometimes shown a substantial improvement of physical function but only a marginal reduction of fatigue scores [26]. These results suggest that the two problems, tiredness and impairment of physical performance, are related but not identical phenomena. Hence, the different aspects of the cancer-related fatigue syndrome may require diverse therapeutic approaches.
Therapies for cancer-related fatigue may address an identified cause (anaemia, hypothyroidism, depression) or be symptom-directed. In this case, exercise, psychotherapy and cognitive behaviour techniques have been shown to reduce fatigue in cancer patients. However, these therapies have a substantially different mechanism of action. Impaired physical performance is a main cause of fatigue in cancer patients [15]. This limitation can result in increased dependence, reduced self-esteem, limitations in social activities and in family life and in a pessimistic mood. Furthermore, low physical performance can be interpreted by the patient as a sign of poor health and thus increase his or her psychological distress. Hence, improving performance status with an exercise programme may increase quality of life and reduced fatigue and psychological stress. In fact, we have reported a substantial improvement of physical performance and emotional stability in cancer patients participating in an aerobic training programme [10, 13, 14]. Psychotherapy and relaxation techniques, on the other hand, may help reduce stress, anxiety and depression. These problems are strongly associated with fatigue [12]. Thus, therapies that reduce global stress and anxiety and improve mood may decrease fatigue.
The prospective evaluation of fatigue, the presence of a control group, the randomised design and the simultaneous evaluation of mental status and physical performance are strengths of our study. However, there are also some methodological limitations. Fatigue is a multi-dimensional problem that may affect physical, cognitive and affective areas. The severity of this symptom may be assessed by several questionnaires. However, these tools evaluate different aspects of the fatigue syndrome. While the EORTC QLQ-30 is an established and reliable tool, its fatigue sub-scale primarily assesses physical impairments and yields no information about cognitive or motivational deficits. Therefore, we cannot draw any conclusions about the effects of the endurance and relaxation training programmes on these areas. A critical point of the present study is the sample size. The significance of differences in pre and post QLQ-C30 scores can be interpreted in terms of small, moderate or large changes in quality of life, where on a 100-point scale, mean changes of 5–10 points express small, 10–20 moderate and more than 20 very large differences [25]. However, these figures represent absolute differences within a group. For the calculation of sample size, we considered a difference of 30% or more between groups to show a clinically relevant difference in quality of life scores. Hence, results were independent from effect size (i.e. 30% is the same from 6 to 8, an absolute difference of 3 points, as for 51–68, an absolute difference of 17 points). Therefore, our study was able to detect differences as small as 2 points between groups.
While most patients feel fatigued after surgery, the severity of this symptom usually decreases in the following weeks. Since our study did not include a control group without therapy for fatigue, it is difficult to separate the effects of exercise and relaxation training from the spontaneous improvement of this symptom. On the other hand, physical performance of patients in the relaxation training group remained unchanged during the 3-week intervention. Since impaired physical performance is a cardinal problem of cancer patients, our results underscore the need for appropriated rehabilitation strategies for oncological patients undergoing surgery. Several months after operation, the performance status of 68% of patients in the aerobic exercise group and 75% of patients in the relaxation training group was still “poor” or “very poor” according to reference values for age and gender [1]. This loss of physical performance has been shown to correlate with impaired mood and increased morbidity [12]. Furthermore, severely reduced physical ability usually causes long-lasting self-perpetuating fatigue [13, 30].
We have previously reported that physical activity may reduce pain in cancer patients during high-dose chemotherapy with autologous stem cell rescue [11]. However, in the present study, pain scores of patients in the exercise group did not change during the intervention. Since operations had been carried out several weeks before the study, the findings suggest that physical activity may have different effects on acute and chronic pain. On the other hand, pain scores of patients in the progressive relaxation training group were significantly lower at the end of the study, indicating that progressive relaxation training may be superior for the treatment of pain in this setting.
A further critical issue in our study was the method used to assess maximal physical performance. The most accurate indicator of physical fitness is maximal oxygen uptake (VO2max). This method is the gold standard when different stress test protocols or different exercise types are compared. However, this index depends on the maximal workload and hence has a high correlation with the maximal effort in watts during a stress test [1]. The assessment of maximal workload in watts is one of the usual methods for determining maximal physical performance and has high reliability and validity when used to compare two populations or to evaluate changes in physical performance with time [16].
We conclude that aerobic exercise and progressive relaxation techniques are effective therapies for the treatment of fatigue in cancer patients after surgical interventions. However, only endurance training improves the performance status of patients in this setting. Furthermore, progressive relaxation training may result in a better pain control. Thus, multi-modal approaches including several therapies are required to address the different problems of cancer patients after surgery.
References
American College of Sports Medicine (1995) Guidelines for exercise testing and prescription, 3rd edn, Lea & Feibiger, Philadelphia
Bordeleau L, Szalai JP, Ennis M et al (2003) Quality of life in a randomized trial of group psychosocial support in metastatic breast cancer: overall effects of the intervention and an exploration of missing data. J Clin Oncol 21:1944–1951
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scandinavian Journal of Rehabilitation Medicine 3:92–98
Cella D, Davis K, Breitbart W et al (2001) Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 19:3385–3391
Christensen T, Hjortso NC, Mortensen E et al (1986) Fatigue and anxiety in surgical patients. Acta Psychiatr Scand 73:76–79
Christensen T, Kehlet H, Vesterberg K et al. Fatigue and muscle amino acids during surgical convalescence. Acta Chir Scand (1987) 153:567–570
Christensen T, Stage JG, Galbo H et al (1989) Fatigue and cardiac and endocrine metabolic response to exercise after abdominal surgery. Surgery 105:46–50
Courneya KS, Friedenreich CM, Sela RA et al (2003) The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: Physical fitness and quality of life outcomes. Psychooncology 12:357–374
Courneya KS, Mackey JR, Bell GJ et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol (2003) 21:1660–1668
Dimeo F, Bertz H, Finke J et al (1996) An aerobic exercise program for patients with haematological malignancies after bone marrow transplantation. Bone Marrow Transplant 18:1157–1160
Dimeo F, Fetscher S, Lange W et al (1997) Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood 90:3390–3394
Dimeo F, Stieglitz RD, Novelli-Fischer U et al (1997) Correlation between physical performance and fatigue in cancer patients. Ann Oncol 8:1251–1255
Dimeo F, Rumberger BG, Keul J (1998) Aerobic exercise as therapy for cancer fatigue. Med Sci Sports Exerc 30:475–478
Dimeo F, Stieglitz RD, Novelli-Fischer U et al (1999) Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 85:2273–2277
Dimeo FC (2001) Effects of exercise on cancer-related fatigue. Cancer 92:1689–1693
Fletcher GF, Balady G, Froelicher VF et al (1995) Exercise standards. A statement for healthcare professionals from the American Heart Association. Circulation 91:580–615
Forester B, Kornfeld DS, Fleiss JL (1985) Psychotherapy during radiotherapy: effects on emotional and physical distress. Am J Psychiatry 142:22–27
Forester B, Kornfeld DS, Fleiss JL et al (1993) Group psychotherapy during radiotherapy: effects on emotional and physical distress. Am J Psychiatry 150:1700–1706
Jacobson E (1938) Progressive relaxation. University of Chicago Press, Chicago
Jereczek-Fossa BA, Marsiglia HR, Orecchia R (2002) Radiotherapy-related fatigue. Crit Rev Oncol Hematol 41:317–325
Lachin JM (1999) Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials 20:408–422
Lucia A, Earnest C, Perez M (2003) Cancer-related fatigue: can exercise physiology assist oncologists? Lancet Oncol 4:616–625
Luebbert K, Dahme B, Hasenbring M (2001) The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology 10:490–502
National Comprehensive Cancer Network (2003) Cancer-related fatigue. http://www.nccn.org (Cited 8 Aug 2004)
Osoba D, Rodrigues G, Myles J et al (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Segal RJ, Reid RD, Courneya KS et al (2003) Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 21:1653–1659
Smets EMA, Garssen B, Schuster-Uitterhoeve ALJ et al (1993) Fatigue in cancer patients. Br J Cancer 68:220–224
Sprangers MA, Van Dam FS, Broersen J et al (1999) Revealing response shift in longitudinal research on fatigue—the use of the thentest approach. Acta Oncol 38:709–718
Stone P, Richards M, Hardy J (1998) Fatigue in patients with cancer. Eur J Cancer 34:1670–1676
Winningham ML (1992) The role of exercise in cancer therapy. In: Watson R, Eisinger M, (eds) Exercise and Disease CRC Press, Boca Raton, p. 63
Winningham ML, Nail LM, Barton B et al (1994) Fatigue and the cancer experience: the state of the knowledge. Oncology Nurse Forum 21:23–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimeo, F.C., Thomas, F., Raabe-Menssen, C. et al. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery. A randomised controlled trial. Support Care Cancer 12, 774–779 (2004). https://doi.org/10.1007/s00520-004-0676-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0676-4