Summary
Aim
The aim of the present study was to evaluate the effect of crizotinib on visceral organs in an experimental rat model.
Methods
Eighteen Wistar albino rats were divided into three groups: experimental toxicity was induced with crizotinib (10 mg/kg) administered for 28 days (Group 1), 42 days (Group 2) orally by gavage. Control group received only distilled water. Rats in Group 1 and Group 2 were sacrificed after the collection of blood and tissue samples on the 28th and 42nd days, respectively.
Results
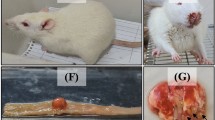
Subjects in Group 1 and Group 2 had abnormal histology mainly in lung and liver. There were intraalveolar hemorrhage in lungs; mild portal inflammation, perivenular focal and confluent necrosis in liver; inflammatory reaction in renal pelvis and periureteral areas, and focal pancreatitis in pancreas.
Conclusion
This study is the first to evaluate the histopathological features of toxicity of crizotinib in a rat model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of deaths related to cancer worldwide [1]. In 2014, the estimated incidence of lung cancer in the USA was approximately 224,210 and 159,260 of deaths attributed to this malignancy [2]. The 5-year survival rate for lung cancer is around 15 %. More than 70 % of lung cancers are locoregionally advanced or metastatic at the time of diagnosis. Lung cancers are clinically classified in two major groups: non-small cell lung cancer (NSCLC), which accounts for about 85 % of all lung cancers, and small cell lung cancer (SCLC) [3, 4]. Patients with metastatic NSCLC have a poor prognosis. Although cytotoxic chemotherapy remains the mainstay of treatment for the majority of the patients with advanced NSCLC, molecular targeted therapy has assumed to play an increasingly important role. Molecular targeted therapies have been developed for patients with activating epidermal growth factor receptor (EGFR) mutations or with the presence of EML4-ALK fusion proteins [5–7]. Anaplastic lymphoma kinase (ALK) is a well-known oncogenic driver in NSCLC, and approximately 5 % of patients harbor ALK gene rearrangements [7, 8]. Crizotinib is an oral small-molecule tyrosine kinase inhibitor targeting ALK, MET, and ROS1 tyrosine kinases [7, 9]. In previous studies, crizotinib showed marked (60 %) response rate in advanced NSCLC patients with ALK rearrangements. Median progression-free survival (PFS) was 8.1 months (95 % CI 6.8–9.7; [10]).
Common adverse effects of crizotinib were mild to moderate transient visual disorders, nausea, vomiting, diarrhea or constipation, fatigue, peripheral oedema, and neutropenia. The most serious adverse effects were interstitial lung disease and QT interval prolongation in electrocardiograms. Pulmonary toxicity is a rare but potentially life-threatening adverse reaction. Two deaths attributed to treatment-related pneumonitis were reported. Treatment-related grade 3 or 4 elevated aminotransferase levels were reported in 16 % of patients [11].
Although crizotinib is recognized as a relatively safe oral tyrosine kinase inhibitor, pulmonary toxicity, acute hepatitis, fulminant hepatitis have been reportedly associated with the therapy as serious adverse events [12–13]. Crizotinib is a relatively new drug developed for clinical usage, however its effects on normal tissues are not fully understood yet. In this study, we aimed to evaluate the histopathological changes and biochemical alterations of crizotinib related toxicity in an experimental rat model.
Materials and methods
Methods
This study was carried out in the Laboratory of Experimental Animal Studies, following the approval from the Animal Studies Ethics Comittee of Faculty of Medicine, Gazi University (19 December 2013; 219-28134). Biochemical tests were performed in the Department of Biochemistry and pathological evaluation in Medical Pathology.
Eighteen male adult Wistar albino rats (each 8-weeks-old), each weighing 200–300 g, were used in our study. Animals were kept at constant environmental and nutritional conditions throughout the experimental period which was a room temperature of 23°C ± 2 and with a 12-h-on/off-light schedule. Standard food and water were given to animals during the experiment.
Study groups
After acclimation for a week, the rats were randomly divided into three groups:
(i) Control group: The sham group, which only received distilled water (n = 6), (ii) Group 1: Experimental group which was treated for 28 days and then sacrificed after the collection of blood and tissue samples (n = 6), and (iii) Group 2: Experimental group which was treated for 42 days and then sacrificed after the collection of blood and tissue samples (n = 6).
Crizotinib was administered orally by gavage, as a single dose of 10 mg/kg according to a study of Smith et al. in the literature [14]. On day 28, all rats in Group 1 and the rats in control group, numbering 1, 2 and 3; and on day 42, all rats in Group 2 and the rats in control group, numbering 4, 5 and 6 were sacrificied after the collection of blood and tissue samples, properly. In this stage, the rats were anesthesized with 50 mg/kg ketamine (Ketalar, Eczacıbaşı, Türkiye) and 5 mg/kg xylazine HCl. Blood samples were collected from the heart and centrifuged for 5 min. Hematologic parameters were studied. Liver functions were evaluated by measuring the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) in blood. To evaluate nephrotoxicity, blood urea nitrogen (BUN), creatinine, and uric acid were analyzed. Lipid profile (including total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and triglycerides) and cardiac markers (including troponin and creatinine kinase, CK) were processed. After blood sampling process, the rats were euthanized by the intracardiac exsanguination method under anesthesia. Then, laparotomy and thoracotomy were performed. The lungs, liver, kidneys, pancreas, and heart were removed from each animal. All tissue specimens were fixed in 10 % formaldehyde solution, sampled, underwent tissue processing, and then embedded in liquid paraffin in order to obtain tissue blocks. The tissue blocks were cut as 3-µm-thick sections by using a rotary microtome and then stained for routine hematoxylin and eosin (H&E) stains. Histopathological examination was conducted mainly focusing on the effects of the crizotinib on normal tissues.
Statistical analysis
Statistical analysis was performed using the SPSS version 21 (SPSS Inc., Chicago, IL). Data were expressed as median (minimum–maximum) values. Comparisons were made among three study groups: control group (received no medicine), Group 1 (received crizotinib for 28 days), and Group 2 (received crizotinib for 42 days). Kruskal–Wallis tests were conducted to compare parameters among threse groups. The Mann–Whitney U test was performed to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons. An overall 5 % type 1 error level was used to infer statistical significance.
Results
No deaths or abnormal clinical findings were noted in any of the experimental groups. There were no significant difference in the body weight alterances among the three groups. The hematological parameters were almost constant among the groups. The median values of the biochemical tests are demonstrated in Table 1. We did not find any significant difference in biochemical parameters concerning these groups (Table 1).
Histopathological findings
All rats in Sham group had normal histological features; however, none of the rats in Group 1 and Group 2 showed completely normal histology.
Liver
In Group 1, we observed perivenular focal necrosis in liver parenchyme with hepatocytes showing focal acinar transformation, hydropic degeneration, and rare mitotic figures. A few apoptotic bodies were seen. In sinusoidal areas mild dilatation, congestion, and enlarged Kupffer cells were observed. These findings were noticed particularly around the central veins. In Group 2, the findings mentioned above were more prominent and besides these findings Kupffer cell hyperplasia, mild portal inflammation, and perivenular focal and confluent necrosis were also observed (Fig. 1).
Lungs
Related to the pulmonary parenchyme, we observed emphysematous alveolar changes, nonspecific interstitial inflammation, bronchial dilatation and peribronchial inflammation, vascular congestion with perivascular inflammation and in some areas pigment-laden macrophages, focal parenchymal foamy histiocytes, focal granuloma formation containing foreign body-type multinucleated giant cells in both study groups, but more prominent in Group 2. In addition to these findings, in Group 2, we also observed intraalveolar hemorrhage and marked peribronchial inflammatory cell infiltration (Fig. 2).
Kidneys
We observed inflammatory reaction in renal pelvis and periureteral areas (Fig. 3).
Pancreas
The Sham group and Group 1 showed normal histologic findings. In Group 2, we observed focal pancreatitis characterized with panlobular inflammation and atrophic change limited to only one lobular structure (Fig. 4).
Heart
No histological abnormality was noted in any of the experimental groups.
Discussion
Crizotinib is an adenosine triphosphate-competitive small molecule oral inhibitor of ALK, c-MET/hepatocyte growth factor receptor (HGFR), and ROS receptor tyrosine kinases [9, 15]. A phase I trial in advanced ALK-positive NSCLC (PROFILE 1001) reported a marked 60.8 % response rate and PFS of 9.7 months. PROFILE 1005, a phase II study confirmed its clinical activity [10, 13]. In PROFILE 1007, crizotinib was compared with standard second line chemotherapy [11]. Second line therapy with crizotinib improved PFS and response rate when compared with single agent therapy (either docetaxel or pemetrexed). On the basis of the data from PROFILE 1001 and 1005, crizotinib is approved by Food and Drug Administration in 2011 for the treatment of ALK rearranged NSCLC. Crizotinib is generally well tolerated, with most adverse events within grades 1 or 2 [11]. Pneumonitis, QT interval prolongation, and liver toxicity are the most severe side effects. In PROFILE 1005 the most common grade 3/4 adverse events were elevated ALT level (3.9 %) and neutropenia (5.5 %). Permanent discontinuation because of the hepatic toxicity occured in 1.3 % of patients. Pneumonitis is another serious toxicity resulting from permanent discontinuation of the drug [10, 13]. A meta-analysis of 6 studies was performed to evaluate the efficacy and safety of crizotinib. Dose reduction or cessation of drug because of crizotinib toxicity was observed in 6.5 % of patients [16]. Crizotinib-related histopathologic changes in patients are unknown. In our study, we found that substantial histopathologic changes were observed in lung, liver, kidney, and pancreas. There was no significant difference in biochemical test analysis between medicated groups by crizotinib and control group.
Hepatotoxicity associated with tyrosine kinase inhibitors and monoclonal antibodies has been reported [17, 18]. Drug-induced hepatotoxicity has been described with the use of imatinib, gefitinib, erlotinib, and gemtuzumab ozogamicin. Imatinib hepatotoxicity was presented with hepatic necrosis and has been reported to induce hypersensitivity reaction. Gemtuzumab ozogamicin may cause sinusoidal obstruction syndrome. Mechanisms involved in gemtuzumab related liver toxicity are targeting of CD33 cells in the sinusoids of the liver, activation of stellate cells, sinusoidal endothelial cell damage, sinusoidal vasoconstriction, and ischemic hepatocyte necrosis [17]. Lapatinib is the first orally active dual inhibitor of HER1 and HER2 and may cause liver damage which can be severe or life threatening. Demirci et al. evaluated the histopathological features of hepatic toxicity of lapatinib in an experimental rat model. Parenchymal acinar transformation, sinusoidal dilatation, hydropic degeneration of hepatocytes, and mild inflammation were observed [18]. Ripault et al. reported the first case of acute hepatitis induced by crizotinib. In this case, isolated transaminase elevation without liver failure and cholestasis were observed. Two months after discontinuation of crizotinib, the liver test results returned to normal level. The patient was rechallenged with crizotinib at the half of initial dose. The rapid relapse of transaminase elevation was observed. Liver biopsy was not performed and histopathological features associated with drug toxicity were unknown [19]. Sato et al reported a case of fatal fulminant hepatitis due to crizotinib administration. The patient did not respond to supportive therapies and died on the 36th day of treatment [20]. Hepatic failure and serious hepatitis have been reported with imatinib and gemtuzumab ozogamicin [21, 22]. In our study, histopathological evaluation of the liver demonstrated perivenular focal necrosis, venous congestion and sinusoidal dilatation, mild hydropic degeneration of hepatocytes, rare mitosis, rare apoptotic bodies, focal acinar transformation, and, particularly in Group 2 with extended exposure period, Kupffer cell hyperplasia, mild portal inflammation, perivenular focal and confluent necrosis. These findings were marked around the central veins (Zone 3 area of the liver parenchyme). We did not observe liver fibrosis or hepatic failure associated with crizotinib. The histologic changes observed in our study were not reflected by any change in liver enzymes. The reason of normal findings on liver function tests is unknown.
In PROFILE 1001 and 1005, severe pneumonitis was reported in 1.6 % of patients (4 of 255). Pneumonitis was typically occured within 2 months after initiation of the treatment [10, 13]. Ono et al. presented the first case report of an acute life-threatening lung injury associated with crizotinib therapy. After 7 weeks of crizotinib treatment, the patient developed acute pneumonitis and died due to acute respiratory distress syndrome. In his autopsy fibroproliferative phase of diffuse alveolar damage (DAD) was observed [23]. The mechanisms of drug-related lung damage are direct pulmonary toxicity and indirect effects via stimulation of several inflammatory reactions. The inflammatory reactions of lung are divided into five subtypes: DAD, hypersensitivity reaction, nonspecific interstitial pneumonia, organizing pneumonia, and eosinophilic pneumonia [24, 25]. The underlying mechanism of the crizotinib-related alveolar damage remains unknown. In our study, lung injury was observed in all rats treated with crizotinib. In Group 1, emphysematous changes, interstitial inflammation, bronchial dilatation and peribronchial inflammation, vascular congestion and perivascular inflammation were seen. Pulmonary intraalveolar hemorrhage, marked peribronchial inflammatory cell infiltrates were observed with extended drug exposure in Group 2. Lung toxicity was also reported associated with anti-EGFR agents, although the underlying mechanism is unclear. EGFR is expressed on type 2 pneumocytes and has a role in alveolar repairment. It is thought that anti-EGFR therapy cause lung damage by interrupting alveolar repair mechanisms [26]. Gefitinib-induced interstitial lung disease, that was detected in an autopsy examination, has been found to show a pattern of diffuse alevolar damage (DAD; [27]). Bleomycin is a well-known chemotherapeutic agent that induces lung injury. Oxidative damage, relative deficiency of the deactivating enzyme bleomycin hydrolase, and genetic susceptibility were considered as the mechanisms of bleomycin-induced pulmonary toxicity [28]. Further investigations are required to elucidate the mechanisms of crizotinib-related lung toxicity.
Drug induced acute pancreatitis is not commonly described in experimental animals. The early phase of acute pancreatitis in experimental rat is characterized by focal necrosis, acute parenchymal inflammation, hemorrhage, and oedema. In Group 2, we observed focal pancreatitis, and panlobular inflammation and atrophy in one lobule. The clinical relevance of these observations is unknown. Previous study revealed 91 cancer patients with acute pancreatitis receiving molecular-targeted therapy. Of these patients, 11 had focal and 4 had diffuse pancreatitis detected on imaging. Molecular-targeted therapy associated pancreatitis was mild, focal, and can be managed conservatively with discontinuation of targeted therapy [29].
Renal impairment was not observed in phase II and phase III trial of crizotinib [11, 13]. Complex renal cysts were reported very infrequently in patients treated with crizotinib [30]. Martin Martorell et al. reported a case who presented with worsening renal functions secondary to crizotinib treatment [31]. Borsnan et al. revealed a study including 38 patients who were treated with crizotinib. They found that EGFR was decreased by 23.9 % compared with baseline [32]. In our study, we observed inflammatory reaction in renal pelvis and periureteral areas. The toxicological significance of this effect was unknown.
Our study has some limitations including lack of data on the influence of crizotinib therapy on inflammatory cytokines which may have a substantial impact on organ function, and small number of rats investigated in each group. There is individually a wide variation spectrum related to the tolerance of crizotinib among patients, with some individuals tolerating the drug without any complication, while others develop severe toxicity.
To our knowledge, this is the first study to demonstrate the toxic effects of crizotinib in a rat model. Revealing the histopathological evaluation of crizotinib-related toxicity, we observed that crizotinib causes primarily intraalveolar hemorrhage in lungs; mild portal inflammation, perivenular focal and confluent necrosis in liver, inflammatory reaction in renal pelvis and periureteral areas, and also focal pancreatitis in pancreas. The clinical implication of these histopathological findings in patients who developed severe toxicity due to crizotinib therapy is that they may give an idea associated with and an opportunity to estimate the histopathological changes, especially within those particular organs mentioned above, in the absence of biopsy.
Compliance with ethical standards
References
Jemal A, Bray F, Center MM, Ferlay J, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Collins LG, Haines C, Perkel R, et al. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75(1):56–63.
Alberts WM. Diagnosis and management of lung cancer executive summary: ACCP evidence-based clinical practice guidelines (2nd Edition). Chest. 2007;132(3 Suppl):1S–19S.
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39.
Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500.
Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703.
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–6.
Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9.
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Stahel RA, Rosell R, Thatcher N, Soria JC. ALK translocation and crizotinib in non-small cell lung cancer: an evolving paradigm in oncology drug development. Eur J Cancer. 2012;48(7):961–73.
Crinò L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29 (suppl; abstr 7514).
Smith EB, Goulet L, Walker GS, et al. Metabolism and excretion of Crizotinib in rats and dogs. 17th North American Regional International society for the study of xenobiotics Meeting. (P82).
Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70.
Qian H, Gao F, Wang H, et al. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: a meta-analysis of clinical trials. BMC Cancer. 2014;14:683.
Loriot Y, Perlemuter G, Malka D, et al. Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. Nat Clin Pract Oncol. 2008;5(5):268–78.
Demirci U, Buyukberber S, Yılmaz G, et al. Hepatotoxicity associated with lapatinib in an experimental rat model. Eur J Cancer. 2012;48(2):279–85.
Ripault MP, Pinzani V, Fayolle V, et al. Crizotinib-induced acute hepatitis: first case with relapse after reintroduction with reduced dose. Clin Res Hepatol Gastroenterol. 2013;37(1):e21–3.
Sato Y, Fujimoto D, Shibata Y, et al. Fulminant hepatitis following crizotinib administration for ALK-positive non-small-cell lung carcinoma. Jpn J Clin Oncol. 2014;44(9):872–5.
Pariente A, Etcharry F, Cales V, et al. Imatinib mesylate-induced acute hepatitis in a patient treated for gastrointestinal stromal tumour. Eur J Gastroenterol Hepatol. 2006;18(7):785–7.
Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99(7):2310–4.
Ono A, Takahashi T, Oishi T, et al. Acute lung injury with alveolar hemorrhage as adverse drug reaction related to crizotinib. J Clin Oncol. 2013;31(26):e417–9.
Muller NL, White DA, Jiang H, et al. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004;91:S24–S30.
Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–56.
Higenbottam T, Kuwano K, Nemery B, et al. Understanding the mechanisms of drug-associated interstitial lung disease. Br J Cancer. 2004;91(Suppl 2):S31–7.
Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361(9352):137–9.
Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617–24.
Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology. 2013;13(5):461–7.
Rothenstein JM, Letarte N. Managing treatment-related adverse events associated with Alk inhibitors. Curr Oncol. 2014;21(1):19–26.
Martín Martorell P, Huerta Alvaro M, Solís Salguero MA, et al. Crizotinib and renal insufficiency: a case report and review of the literature. Lung Cancer. 2014;84(3):310–3.
Brosnan EM, Weickhardt AJ, Lu X, et al. Drug-induced reduction in estimated glomerular filtration rate in patients with ALK-positive non-small cell lung cancer treated with the ALK inhibitor crizotinib. Cancer. 2014;120(5):664–74.
Conflict of interest
O. Gumusay, G. Esendagli-Yilmaz, A. Uner, B. Cetin, S. Buyukberber, M. Benekli, M. N. Ilhan, U. Coskun, O. Gulbahar, and A. Ozet declare that there are no actual or potential conflicts of interest in relation to this article. The authors received no additional financial support or national funding for this study. There is no substantial contribution provided by a person different from the authors. This submission has not been published anywhere previously and that it is not simultaneously being considered for any other publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gumusay, O., Esendagli-Yilmaz, G., Uner, A. et al. Crizotinib-induced toxicity in an experimental rat model. Wien Klin Wochenschr 128, 435–441 (2016). https://doi.org/10.1007/s00508-016-0984-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-016-0984-y