Summary
Objective
The purpose of this study was to examine whether gestational and lactational exposure to environmental endocrine disrupting chemical, nonylphenol (NP), in pregnant dams would lead to the alterations in hormone levels in the body, apoptosis and glial fibrillary acidic protein (GFAP) in hippocampus during weaning and sexual maturity periods in pups of rats.
Methods
Dams were gavaged with NP at dose levels of 25 mg/kg/day (low dose), 50 mg/kg/day (middle dose), 100 mg/kg/day (high dose) and groundnut oil alone (vehicle control) respectively from gestational day 6 to postnatal day (PND) 21.
Results
At PND 21, serum testosterone (TT) level significantly decreased in the 50, 100 mg/kg NP-treated groups compared with the control (p < 0.01). Serum estradiol (E2) level was increased with the increase in the NP concentration; a dose–effect relationship was revealed (r = 0.462, p < 0.01). At both PND 21 and PND 60, pups exposed to 100 mg/kg/day NP had an obviously higher apoptotic rate than control did. We observed a significant positive correlation between the dose of NP and the apoptotic rate (r = 0.836, p < 0.05). The number of GFAP-positive cells in rat hippocampus and integral optical density (IOD) of 100 mg/kg/day NP-treated group were much higher than the control group. GFAP mRNA expressions increased at high dose (100 mg/kg/day) (p < 0.05), and positive correlations between the GFAP mRNA expressions and NP level was observed (r = 0.586, 0.737, p < 0.05). Both the number of growth-associated protein (GAP)-43 positive cells and IOD were much lower at high dose (100 mg/kg/day) than the control at both PND 21 and PND 60 (p < 0.05). The number of GAP-43 positive cells was negatively correlated with the NP exposure dose (r = − 0.562, − 0.649, p < 0.05) at these two time points. GAP-43 mRNA expressions in the hippocampus of pups decreased dramatically at high dose (100 mg/kg/day) at both PND 21 and PND 60 compared with the control (p < 0.05).
Conclusion
High exposure to NP might inhibit neuronal development and differentiation as indicated by the reduction of the neurotrophic factor GAP-43.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonylphenol (NP) is an endocrine-disrupting chemical with weak estrogenic activity [1]. NP is a breakdown product of NP ethoxylates, which are used in a variety of industrial, agricultural, household cleaning, and beauty products [2]. Since previous studies have reported that NP interferes with the development and function of endocrine-reproductive systems through binding to the estrogen receptors [3–5], NP and other environmental contaminants have been recently categorized into endocrine disrupting chemicals. Accumulating data suggested that NP has adverse effects on the reproductive, digestive and immune systems in F1 generation rats [6–8], the toxic effects of NP exposure via placenta on central nervous system (CNS), including disrupting neuroendocrine homeostasis, altering cognitive function, and neurotoxicity of tissues of offspring in rat, were reported sparsely, particularly when NP’s disruption occurs during critical developmental window of brain.
In recent years, a few studies have reported the effects of perinatal NP exposure on the behavioral traits in brain of the offspring of experimental animals [4–6]. In our previous report using rats, we have demonstrated that exposure to NP during gestation was shown to lead to alterations in behavior, learning and memory capacity in the male offspring of rats [3]. Negishi et al. (2004) [9] reported the estrogen-like actions of NP, as displayed by the incapacity of offspring to react to certain behavioral stimuli. The CNS is highly sensitive to exogenous chemicals. Exogenous chemicals such as NP may break homeostasis and affect normal brain functions. Prior evidence demonstrated neurotoxicity of NP to mothers and offspring’ nervous system [4–6], the precise mechanisms behind the neurotoxicity of NP has not been elucidated.
In the present study, the authors’ focus was to shorten NP exposure time into the embryo developmental sensitive period of nerve system (from pregnancy day 6 to postnatal day 21) of filial generation (F1) rats. The aim of the present study was to investigate whether exposure to NP would induce the alterations in hormone levels, apoptosis and glial fibrillary acidic protein (GFAP) in hippocampus during weaning and sexual maturity periods in F1 male rats.
Materials and methods
Reagents
The NP was purchased from the Tokyo Chemical Co., Ltd (Tokyo, Japan). The testosterone – 19 checkerboard was purchased from the Beijing North Biotechnology Institute (Beijing, China). The TUNEL kit was purchased from Roche Molecular Biochemicals (Meylan, France). The rabbit anti-mouse GFAP and GAP-43 were purchased from Beijing Zhongshan Biotechnology Reagent Company (Beijing, China). The goat anti-rabbit IgG was purchased from Beijing Zhongshan Golden Bridge Biotechnology Company (Beijing, China). DAB kit was purchased from DAKO (Glostrup, Denmark); Trizol was purchased from Invitrogen (Carlsbad, USA). RNA extraction and reverse transcription kit was purchased from Takara (Dalian, China). All other chemicals were commercially available. All chemical purities were at least 99 %.
Animals
Two female rats were mated with one male rat in one cage. Vaginal smears were examined daily; a sperm-positive smear determined gestational day (GD) 0. A total of 31pregnant dams were randomly assigned to four groups, each group had 7–8 dam. The treatment groups received gavage with NP at dose levels of 25 mg/kg/day (low dose), 50 mg/kg/day (middle dose), 100 mg/kg/day (high dose) and groundnut oil alone (vehicle control), respectively. The pregnant dams were housed individually and fed intragastrically. NP exposure time was limited from gestational day 6 to postnatal day 21. Dams were subjected to spontaneous parturition, and the pups were breastfed. After parturition (PND 0), the pups were counted and weighed. Pups remained with their biological mother. Male pups in the litter (n4–5/group) were subjected to the measurement of neurotoxic indicators. Male pups were selected on the basis of evidences that NP had toxic impacts on male pups in our prior studies [7–8].
Serum hormone measurement
Blood samples were collected from PND 21 and PND 60 male rats by decapitation and allowed to coagulate on ice. Serum samples were prepared by centrifuging the whole blood samples at approximately l000 x g and 4 °C for 30 min and stored below − 20 °C until hormone assay. Serum total testosterone was assayed in duplicate by using a Coat-A-Count radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, CA). The concentration of serum estradiol was determined using the commercial ELISA kit (Alpha Diagnostic, San Antonio, TX).
TUNEL staining
For visualization of DNA fragmentation, a marker of apoptotic cell death, TUNEL staining was performed using In Situ Cell Death Detection Kit (Meylan, France). To begin the procedure, the sections were post-fixed in ethanol-acetic acid (2:1) and rinsed. Then the sections were incubated with proteinase K (100 μg/mL), rinsed, incubated in 3 % H2O2, permeabilized with 0.5 % Triton X-100, rinsed again, and incubated in TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.02 % 3,3´-diaminobenzidine (DAB). Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used for counter-staining, and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount [12].
Total TUNEL-positive cell numbers were determined by the average from three different sections of each animal. Nuclei/field was calculated from nuclei counting of 20 fields from each animal using the same magnification. Apoptotic rate was expressed as the percentage of TUNEL-positive cells per nuclei.
Immunohistochemistry for GFAP and GAP-43 protein expression
Briefly, the sections were incubated in phosphate-buffered saline (PBS) for 10 min and washed three times with PBS, and then incubated in 1 % hydrogen peroxide (H2O2) for 30 min. Next, the sections were incubated overnight with mouse anti- GFAP antibody (Chemicon, Temecula, CA, USA) at a dilution of 1:2,000 for visualization of astrocytes. The sections were then incubated for 1 h with anti-mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Bound secondary antibody was then amplified with a Vector Elite ABC kit (Vector Laboratories). The sections were subsequently incubated with avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1 h at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05 % 3,3´-diaminobenzidine (DAB) and 0.01 % H2O2 in 50 mM Tris buffer (pH 7.6) for approximately 3 min. The sections were then mounted on gelatin-coated glass slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount [13].
Pictures that were taken under high magnification fields of view (400 ×) were analyzed using the professional image analysis software, ImagePro Plus 6.0 (IPP6.0). First, we used IPP6.0 to analyze a group of pictures with positive staining areas and typical significance. According to the characteristics of positive immunohistochemical staining of each protein, we chose the definite areas of these pictures and calculated the IOD in these areas. After the adjusting for operation, we confirmed and saved the installation parameters used for measurement. Then the operative procedure and installation parameters would be saved as a “setting”. By operating this setting, we could use the photodensitometry to analyze all the pictures at the same installation parameters. We used the IOD to represent the grain density of each protein in those pictures, so it could reflect and represent the expression of each protein.
RNA preparation and RT-PCR for GFAP and GAP-43 mRNA expression
Total RNA was isolated from the brain using the TRIzol reagent (Carlsbad, USA), then separated with chloroform (J.T. Backer) and precipitated with isopropanol (J.T. Backer). The obtained RNA pellet was washed once with 75 % ethanol and once with 100 % ethanol to ensure complete dehydration. The RNA was then dissolved in diethylpyrocarbonate-treated water, and its concentration was determined by measuring the absorbance at 260 and 280 nm using a spectrophotometer (ND-1000, NanoDrop). First-strand cDNA was reverse-transcribed using 1 mg of RNA template, Moloney murine leukemia virus reverse transcriptase and appropriate oligo-dT primers. Quantitative real-time polymerase chain reaction (PCR) was performed using a Bio-Rad iQ5 real-time detection machine (Bio-Rad Laboratories Inc., Hercules, CA). The reactions were carried out in a final volume of 12.5 ml containing the cDNA template, forward and reverse primers for each gene and SYBRH Green Real-time PCR Master Mix (TOYOBO). The primers used for amplification were listed in Table 1–2 [14].
Statistical analysis
The statistical analyses were performed with SPSS software, version 18.0 for Windows (SPSS Inc., Chicago, IL). Values of all variables are presented as mean and standard deviation. One-way analysis of variance (ANOVA) with Tukey’s HSD as posthoc test and LSD-t test were used to determine the effects of different treatments. A P-value <0.05 was considered as the level of statistical significance. The litter was used as the statistical unit.
Results
Effects of NP on body weight and level of hemoglobin in pups
Compared with the negative control, the body weight of pups significantly decreased in the 25, 50 or 100 mg/kg NP-treated groups on PND 3, PND 7 (week 1), PND 14 (week 2), PND 21 (week 3), PND 42 (week 6), and PND 56 (week 8) (p < 0.05) (Fig. 1).
In contrast to the negative control group, the levels of hemoglobin in pups significantly decreased in the 100 mg/kg NP-treated group on PND 30 and PND 60 (p < 0.05) (Fig. 2).
Effects of NP on serum hormone levels in pups
At PND 21, serum testosterone (TT) level decreased with the increase in the NP exposure dose in a dose-dependent manner (r = − 0.417, p < 0.05). Compared with the negative control, serum TT level significantly decreased in the 50, 100 mg/kg NP-treated groups (p < 0.01). Estradiol (E2) level was increased with the increase of NP-treated concentration, a dose–effect relationship was revealed (r = 0.462, p < 0.01), oral exposure to NP at doses 100 mg/kg/day showed statistically significant effect on E2 level in pups compared with the control (p < 0.05) (Table 3).
Effects of NP on apoptosis in hippocampus of pups
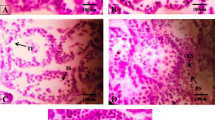
Based on visual estimation, at both PND 21 and PND 60, about 90 % of the cells stained with trypan blue, brownish apoptotic cells were rarely present in hippocampus in the control and low-dose NP treatment group, whereas increased number of brownish apoptotic cells were present in the high-dose NP treatment group (Fig. 3–4).
At both PND 21 and PND 60, offspring rats exposed to 100 mg/kg/day NP revealed higher rates of apoptosis than the control (p < 0.01). At these two time points, both positive correlations between NP exposure dose and apoptotic rates were observed (r = 0.836, 0.521, p < 0.05). Apoptotic rates in different treatment groups at PND 21 were greater than that at PND 60 (t = 3.331, p < 0.05) (Table 4).
Effects of NP on GFAP in the hippocampus of pups
Alterations in GFAP protein expression
GFAP positive cells was rarely present with shorter cell processes in both control and low-dose experimental animals. In contrast, the astrocytes in high-dose experimental animals displayed larger cell body, increased number of GFAP positive cells distributed densely with spider-like shape, longer cell processes, dark stained (Fig. 5–6). With the increase of dose, the number of GFAP positive cells increased gradually with brown stain in cytoplasm, resembling “spider-like”, at PND 60 compared with PND 21 (Fig. 7).
Both the number of GFAP positive cells and IOD were much higher in animals exposed to 100 mg/kg/day NP than that found in the control animals at both PND 21 and PND 60; a dose–effect relationship between NP exposure dose and the number of GFAP positive cells was found (r = 0.736, 0.679, p < 0.05); similarly, positive correlation between NP exposure dose and IOD was also observed (r = 0.802, 0.603, p < 0.05). The number of GFAP positive cells at PND 21 in different treatment groups was lower than that at PND 60 (t = − 5.525, p < 0.05) (Table 5).
Detection of GFAP mRNA expression by real-time PCR
In contrast to the control group, GFAP mRNA expressions increased at high dose (100 mg/kg/day) at both PND 21 and PND 60, differences were statistically significant (p < 0.05). There was a positive correlation between GFAP mRNA expression and NP exposure dose at PND 21 or PND 60 (r = 0.586, 0.737, p < 0.05) (Table 6).
Alterations in GAP-43 protein expression
Approximately 80 % of GAP-43 positive cells were present with brown stain in cytoplasm, distributed densely, in both control and low-dose NP-treated group. In contrast, fewer GAP-43 positive cells were present with light stain in cytoplasm at high doses (100 mg/kg/day). With the increase of dose, the number of GAP-43 positive cells in hippocampus of pups decreased increasingly (Fig. 8–9).
Both the number of GAP-43 positive cells and IOD were much lower at high dose (100 mg/kg/day) than the control at both PND 21 and PND 60, differences were statistically significant (p < 0.05). The number of GAP-43 positive cells was negatively correlated with the NP exposure dose (r = − 0.562, − 0.649, p < 0.05) at these two time points. Inverse dose–effect relationships between IOD and NP exposure dose were observed at both PND 21 and PND 60 (r = − 0.814, − 0.605, p < 0.05) (Table 7).
Detection of GAP-43 mRNA expression by real-time PCR
GAP-43 mRNA expressions in the hippocampus of pups decreased dramatically at high dose (100 mg/kg/day) at both PND 21 and PND 60 compared with the control (p < 0.05), there was no significant difference between low- and middle-dose NP-treated groups and the control (p < 0.05). Negative correlations between GAP-43 mRNA expression and NP exposure dose were discovered at both PND 21 and PND 60 (r = 0.616, − 0.598, p < 0.05) (Table 8).
Discussion
In the present study, we carried out a series of toxicological tests and demonstrated the weak estrogen-like effects of NP could induce adverse alterations in brain development in F1 rats. Exposure to NP can inhibit neuronal development and differentiation such as neurite growth and synapse formation, resulting in reductions in neurotrophic factor-GAP-43 expressions. Additionally, NP can cause GFAP overexpression and alterations in the morphological structure of astrocytes, these changes lead further to neuronal apoptosis in hippocampus of pups. We conclude that these adverse alterations induced by NP contribute to NP neurotoxicity in F1 rat.
Our findings of a negative effect of NP on sex hormone levels (i.e., E2 and TT) in pups were in accordance with our previous reports [8]. Prior studies [8, 10, 11, 15] demonstrated that long-term exposure to low-dose hormones could have the potential to perturb neurobehavioral system (e.g., emotion and cognitive behaviors) of the offspring of experimental animals. Combining the changes of dam exposed to 100 mg/kg/day with the earlier study we postulate that alterations in TT and E2 level in hippocampus of pups can induce changes in hormone levels, these changes have further effects on nerve cell differentiation such as neurite outgrowth, synaptogenesis, neurotransmitter expression, and cell proliferation and death. Changes in hormone levels may contribute to the abnormal expression of certain genes in nerve cells, which might be implicated in the CNS dysfunction.
Our results showed that apoptotic rate was positively correlated with NP exposure dose, whereas an inverse correlation between NP concentration and learning and memory capacity in pups was observed. Previous studies have suggested that NP can cause apoptosis in sperm in vitro, thymic cells and human embryonic stem cells [16–18]. NP neurotoxicity was associated with apoptosis in rat brain cells [19], the deleterious effects of NP on nerve cells in rat offspring have not been elucidated. The influence of memory ability is thought to involve the nervous system. It is clear that learning and memory and hippocampus appear to be closely associated. The hippocampus plays an important role in long-term memory, learning processes and spatial navigation. Increase in apoptotic rate and decrease in learning and memory capacityin the hippocampus of pups can be associated with NP exposure [3]. We postulate that NP can pass through the placenta and/or blood–testis barriers to induce impairment of neurocytes, and result in the influence on learning and memory function in male offspring rats.
GFAP is a major protein of astrocyte intermediate filaments and a specific marker for astrocytes. Changes in levels of GFAP can reflect pathological regulation of neuronal function, survival and abnormal synaptogenesis, and neurotransmission. Exposure to NP can lead to increase in the number of astrocytes, resulting in astrocytes dysfunction. The proliferous astrocytes can interfere with functional interactions between neurons and astrocytes, which cause signal transmission efficiency reduction. Previous studies related to the adverse effects of NP on GFAP were sparse; only one study conducted by Yokosuka et al. (2008) [20], the results indicated that not only E2 but also the environmental estrogenic chemicals, NP and bisphenol A (BPA), affect development of fetal rat hypothalamic cells in vitro. Yokosuka’s study focused on the effects of the joint exposure of multiple endocrine disruptors rather than exclusive NP exposure on GFAP in hippocampus. In our study, the number of GFAP positive cells in the hippocampus of pups at PND 60 in exclusive NP-treated groups was higher than that at PND 21; the results demonstrated that the negative effects of NP on CNS in F1 rats still remain notwithstanding the lack of NP exposure during the weaning period. NP can cause irreversible astrocytes damage. Our study observed a decrease in learning and memory function in pups during weaning period. These neurobehavioral changes were consistent with GFAP positive cells expression alterations in pups. The results have clearly demonstrated that neurotoxicity of NP in F1 rats following subchronic (GD6~PND21) high-dose oral exposure might be related to an increase in GFAP expression.
Many previous studies in animals have reported that GAP-43 played a major role in neuronal growth, synapse formation, and signal transduction [21, 22]. GAP-43 was closely associated with learning and memory function [23]. However, effects of NP exposure on GAP-43 have not been reported so far. In order to explore the neurotoxicity of NP to CNS, especially among offspring, from the perspective of growth-associated nutritional factors, this is the first study reporting the effects of NP on GAP-43 mRNA expression in hippocampus in F1 rats. Exposure to NP during gestation and lactation induced the decreases in GAP-43 mRNA expression. A negative correlation between GAP-43 mRNA expression in hippocampus and NP exposure dose was discovered. The mechanism that involves the effects of GAP-43 expression on learning and memory capacity is still obscure, we postulated that the mechanism may result from the following: GAP-43 is an intrinsic determinant of neuronal development and plasticity. GAP-43 has important roles as a marker of age-dependent deterioration of synaptic plasticity in the body, especially in those areas of the brain involved in memory and emotional behavior [24]. Additionally, GAP-43 plays an important role in the process of Ca2+-induced noradrenaline release [25]. Exposure to NP during gestation might interfere with many links of the growth and development of neurones via inhibiting GAP-43 mRNA and protein expression. These links include signal transduction, release of neurotransmitters, synaptic plasticity, Ca2+ signal transduction, phospholipid metabolism, and release of monoamine transmitters. These interferences can lead to learning and memory impairment in male rat offspring. These impairments induced by NP in CNS in childhood could persist into adulthood, GAP-43 expression in hippocampus in pups could not return to the normal levels after NP exposure has ceased, the learning and memory capability could not fully recovered either. This illustrates that at the cessation of NP exposure in pups after weaning, the accumulation concentrations of NP in pups’ brains could not be completely removed until adulthood. The adverse effects of NP on pups’ nervous systems might persist into adulthood.
References
White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135(1):175–82.
Jie X, Jianmei L, Zheng F, Lei G, Biao Z, Jie Y. Neurotoxic effects of nonylphenol: a review. Wien Klin Wochenschr. 2013;125(3–4):61–70.
Jie X, Yang W, Jie Y, Hashim JH, Liu XY, Fan QY, Yan L. Toxic effect of gestational exposure to nonylphenol on f1 male rats. Birth Defects Res B Dev Reprod Toxicol. 2010;89(5):418–28.
Park K, Kwak IS. Gene expression of ribosomal protein mRNA in Chironomus riparius: effects of endocrine disruptor chemicals and antibiotics. Comp Biochem Physiol C Toxicol Pharmacol. 2012;156(2):113–20.
Roig B, Cadiere A, Bressieux S, Biau S, Faure S, de Santa Barbara P. Environmental concentration of nonylphenol alters the development of urogenital and visceral organs in avian model. Environ Int. 2014;62:78–85.
Y Liu, LI Zhe, ZW Duan, LI Hai-Shan, YM Zhang. Effects of nonylphenol on reproduction of pregnant rats and behavior development of their offspring. Ind Health Occ Dis. 2007;33:140–43.
Kumar V, Majumdar C, Roy P. Effects of endocrine disrupting chemicals from leather industry effluents on male reproductive system. J Steroid Biochem Mol Biol. 2008;111:208–16.
Xu J, Fan Q-Y, Luo J-M. Effects of nonylphenol on immune function of F1 male rats after gestation exposure. Chin J Public Health. 2008;24:611–12.
Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112:1159–64.
Xu J, Fan Q-Y, Zhou Y-Z. Study on reproductive toxicity in F1 male rats associated with the pregnant rats exposed by nonylphenol. J Toxicol. 2008;22:223–5.
Jie Y, Fan QY, Binli H, Biao Z, Zheng F, Jianmei L, Jie X. Joint neurodevelopmental and behavioral effects of nonylphenol and estradiol on F1 male rats. Int J Environ Health Res. 2013;23(4):321–30.
Tulsulkar J, Shah ZA. Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int. 2013;62(2):189–97.
Renno WM, Al-Khaledi G, Mousa A, Karam SM, Abul H, Asfar S. (-)-Epigallocatechin-3-gallate (EGCG) modulates neurological function when intravenously infused in acute and, chronically injured spinal cord of adult rats. Neuropharmacology. 2014;77:100–19.
Fassunke J, Majores M, Ullmann C, Elger CE, Schramm J, Wiestler OD, Becker AJ. In situ-RT and immunolaser microdissection for mRNA analysis of individual cells isolated from epilepsy-associated glioneuronal tumors. Lab Invest. 2004;84(11):1520–25.
Couderc M, Gandar A, Kamari A, Allain Y, Zalouk-Vergnoux A, Herrenknecht C, Le Bizec B, Mouneyrac C, Poirier L. Neurodevelopmental and behavioral effects of nonylphenol exposure during gestational and breastfeeding period on F1 rats. Neurotoxicology. 2014;44:237–49. (pii:S0161-813 × (14)00131–4).
Yuan J. Apoptosis in the nervous system. Nature. 2000;407(5):802–13.
Aoki M, Kurasaki M, Saito T, Seki S, Hosokawa T, Takahashi Y, Fujita H, Iwakuma T. Nonylphenol enhances apoptosis induced by serum deprivation in PC12 cells. Life Sci. 2004;74(18):2301–12.
Yao G, Hu Y, Liang J, Hou Y. Nonylphenol-induced thymocyte apoptosis is related toFas/FasL pathway. Life Sci. 2005;77(26):3306–20.
Kim SK, Kim BK, Shim JH, Gil JE, Yoon YD, Kim JH. Nonylphenol and octylphenol-induced apoptosis in human embryonic stem cells is related to Fas-Fas ligand pathway. Toxicol Sci. 2006;9(19):1832–35.
Yokosuka M, Ohtani-Kaneko R, Yamashita K, Muraoka D, Kuroda Y, Watanabe C. Estrogen and environmental estrogenic chemicals exert developmental effects on rat hypothalamic neurons and glias. Toxicol In Vitro. 2008;22(1):1–9.
Tolner EA, van Vliet EA, Holtmaat AJ, Aronica E, Witter MP, da Silva FH, Gorter JA. GAP-43 mRNA and proteinexpression in the hippocampal and parahippocampal region during the coupe of epileptogenesis in rats. Eur J Neurosci. 2003;17(11):2369–80.
Wang XZ, Zhang JT. Effects of ginsenoside Rgl on synaptic plasticity offreely moving rats and its mechanism of action. Acta Pharmacol Sin. 2001;22(7):657–62.
Karimi-Abdolrezaee S, Verge VM, Schreyer DJ. Developmental down-regulation of GAP-43 expression andtiming of target contact in rat corticospinal neurons. Exp Neurol. 2002;176(2):390–401.
Casoli T, Spagna C, Fattoretti P, Gesuita R, Bertoni-Freddari C. Neuronal plasticity and aging:aquantitative immunohistochemistry study of GAP-43 distribution in discrete regions of the rat brain. Brain Res. 1996;714(1–2):111–7.
Hens JJ, De Wit M, Boomsma F, Mercken M, Oestreicher AB, Gispen WH, De Graan PN. N terminal specificanti B 50 GAP-43antibodies inhibit Ca2 + induced noradrenaline release, B-50 phosphorylation and dephosphorylation, and calm odulin binding. J Neurochem. 1995;64(3):1127–36.
Acknowledgments
This work was supported by foundation of: National Natural Science Foundation of China (NO. 81360439) and (NO. 81560527); Youth Foundation of Department of Education of Guizhou Province (KY[2013]198); Foundation of Department of Health of Guizhou Province (gzwkj20131127); Doctor Startup Fund of Zunyi Medical University of China (ZMKD2012-002); Bidding project of Zunyi Medical University of China (2013F-68); College Student Innovation Program of Zunyi Medical University of China (20136509).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Jie, Y., Xuefeng, Y., Mengxue, Y. et al. Mechanism of nonylphenol-induced neurotoxicity in F1 rats during sexual maturity. Wien Klin Wochenschr 128, 426–434 (2016). https://doi.org/10.1007/s00508-016-0960-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-016-0960-6