Abstract
Sexual reproductive success is essential for the survival of all higher organisms. As the most prosperous and diverse group of land plants on earth, flowering plants evolved highly sophisticated fertilization mechanisms. To adapt to the terrestrial environment, a tubular structure pollen tube has been evolved to deliver the immobile sperm cells to the egg and central cell enclosed within the ovule. The pollen tube is generated from the vegetative cell of the pollen (male gametophyte), where two sperm cells are hosted. Pollen tube elongation in the maternal tissue and navigation to the ovule require intimate cell–cell interactions between the tube and female tissues. Questions on how the single-celled pollen tube accomplishes such task and how the female tissues accommodate the tube have attracted many plant biologists. Here, we review recent progresses and concepts in understanding the molecular mechanisms governing pollen tube growth and its interactions with the female tissues. We will also discuss the future perspective in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During evolution, siphonogamy, a phenomenon depicting the delivery of the immotile sperms to the egg by a pollen tube (PT), emerged in flowering plants. The male gametophyte often contains two sperms enclosed in a large vegetative cell, which germinates the PT to deliver the sperms to the female gametophyte (embryo sac) embedded within the ovule in the ovary for double fertilization. The embryo sac (ES) is a seven-celled structure consisting of an egg, a central cell, two synergids and three antipodal cells (Yang et al. 2010). Upon pollination, pollen land on the stigma and germinate PTs that navigate through the maternal tissues to the ovule (Li and Yang 2016). Once it reaches a certain distance from the ovule, the tube is attracted by signals emanated from the ovule to leave the transmitting tract and grows on the funiculus surface toward the ovule. Finally, the embryo sac-secreted attractants guide the tube to the micropyle where it meets and bursts in the synergid of the embryo sac (Takeuchi and Higashiyama 2011; Higashiyama and Takeuchi 2015) (Fig. 1). It is worth to note that only fully developed ES attracts and receives PT in a one-to-one manner.
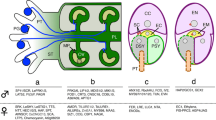
Schematic of an Arabidopsis flower and the fertilization process. a A flower. b A pollen grain germinates a pollen tube which penetrates the stigma, style, transmitting tact sequentially and then turns to the septum surface and is attracted to the micropylar opening of the ovule. The pollen tube grows to the embryo sac composed of two synergid cells, one egg cell, one central cell and three antipodal cells which degenerate before fertilization, and releases the sperm cells within one of the synergid cell
As above, the intimate interplay between the PT and female tissue is critical for sperm delivery. Intercellular crosstalk between the PT and cells of the maternal tissues, and then the ES has been studied extensively during the past two decades (Higashiyama and Yang 2017). In this review, we summarize the key molecules and signaling pathways critical for pollen tube growth in the maternal tissues and pollen tube guidance when approaching the ovule. The signaling of self-incompatibility is not within the scope of this review.
Signaling during pollen tube growth
Extracellular peptides and RLK signaling
Pollen tube growth initiates after pollen germination on the stigma surface. During pollen germination, secretory vesicles accumulate at the emerging tube tip to sustain its rapid growth. The crosstalk between pollen tube and stylar tissue is critical for its in vivo growth although PTs grow unilaterally in a self-sustainable fashion, inasmuch as their growth and shape are maintained on synthetic media in vitro. The stylar tissues not only supply nutrients, signals and cell wall materials for the tube growth, but also are essential for the priming of the tube to respond to ovular attractants (Higashiyama et al. 1998). For solid-style species, the style renders nutritive and signaling support to the compatible pollen tubes and also acts as physical barriers for alien PTs. In species with a hollow stigma and style, PT adhesion to the style surface is essential for its growth. A number of maternal factors, especially the secreted peptides and extracellular glycoproteins, have been identified to mediate pollen tube growth in the style and transmitting tract (Higashiyama 2010). Correspondingly, the pollen tube also secretes peptides, glycoproteins, receptor-like kinases (RLKs) and cell wall-modifying enzymes or other proteins crucial for its growth.
Stylar factors crucial for in vivo pollen tube growth have been identified in the past two decades. Arabinogalactan proteins (AGPs) TTS were first shown to be abundant in the extracellular matrix of transmitting tract to promote PT growth and attraction (Cheung et al. 1995). Later, cysteine-rich peptides (CRPs), such as stigma/style cysteine-rich adhesion (SCA) (Lord 2000) and chemocyanin (Kim et al. 2003) in lily, plantacyanin (Dong et al. 2005) and lipid transfer protein 5 (LTP5) (Chae et al. 2009) in Arabidopsis and LeSTIG1 in tomato (Tang et al. 2004) were identified. More recently, small molecules secreted from the style and transmitting tract, like GABA (Palanivelu et al. 2003) and AMOR (Mizukami et al. 2016), were also found to promote PT growth and guidance. These data suggest that the style plays an active role to modulate pollen tube growth, although it is still unclear how the pollen tube recognizes and responds to these maternal factors.
In plants, plasma membrane and extracellular proteins, such as receptors, peptides and polysaccharides-modified proteins, play key roles during intercellular crosstalk. Pollen-specific RLKs (PRKs) containing an extracellular leucine-rich repeat (LRR) domain were shown to regulate PT growth firstly in petunia and tomato (Mu et al. 1994; Muschietti et al. 1998) and then in Arabidopsis where they act likely through activating the small G proteins GEF-RAC/ROP switch (Kim et al. 2002; Zhang and McCormick 2007). LeSTIG1 is perceived by protein kinases LePRK1 and LePRK2 during PT growth in tomato (Huang et al. 2014), and overexpression of LePRK1 or truncated LePRK1 lacking its extracellular ligand-binding domain causes enlarged PT tips with “blebs”, indicating a role of PRK1 in exocytosis (Gui et al. 2014). Recent study shows that PRK6, one member of the PRK subfamily in Arabidopsis, not only promotes PT growth, but also acts as a receptor to perceive the ovular attractant during PT guidance possibly through activating ROP signaling and LIP1/2 kinases (Liu et al. 2013; Takeuchi and Higashiyama 2016). Disruption of PRK1, 3 and 6 almost totally abolished pollen tube growth (Takeuchi and Higashiyama 2016), indicating an essential and conserved role of PRK subfamily in pollen tube growth among species.
Besides LRR-RLKs, members of the CrRLK1L subfamily also play critical roles in pollen tube growth and integrity. Loss of function of the CrRLK1L proteins ANX1/2 or BUPS1/2 causes premature pollen tube burst before reaching the synergid (Boisson-Dernier et al. 2009; Miyazaki et al. 2009; Ge et al. 2017). BUPS1/2 was recently shown to be able to form heterodimers with ANX1/2 and bind RAPID ALKALINIZATION FACTOR (RALF) peptides RALF4/19 secreted by the pollen tube (Ge et al. 2017), suggesting that the CrRLK1L receptors maintain pollen tube integrity by perceiving the autocrine peptides RALF4 and 19. Intriguingly, RALF4/19 has the activity of prohibiting pollen tube germination in vitro and loss of RALF4 function expedites pollen tube growth (Morato et al. 2014; Mecchia et al. 2017). Similarly, the pollen-expressed RALF also inhibits PT elongation in tomato (Covey et al. 2010), validating a conserved self-autonomous role of RALFs in PT growth and integrity (Wang et al. 2008; Leydon et al. 2017). However, very little is known about RALF-BUPS/ANX signaling pathway. Recently, the reactive oxygen species (ROS)-producing NADPH oxidases or “respiratory burst oxidase homologs” (Rboh) RBOHH and RBOHJ were shown to act downstream of ANXs to regulate ROS production during pollen tube growth (Boisson-Dernier et al. 2013). Furthermore, the tube burst phenotype of both anx1anx2 and rbohh rbohJ can be suppressed by a point mutation (MRIR240C) in the receptor-like cytoplasmic kinase MARIS (MRI) (Boisson-Dernier et al. 2015), while RALF4 and 19 were evidenced to function upstream of MARIS (Mecchia et al. 2017). These suggest most likely that RALF-BUPS/ANX complex regulates downstream RBOHH, RBOHJ and MRI to maintain pollen tube integrity during its growth. Interestingly, mutations in pectin methylesterase (PME) and its inhibitor (PMEI) also induce pollen tube burst (Jiang et al. 2005; Woriedh et al. 2013), suggesting that cell wall integrity is critical. Consistently, RALF4 and 19 are also shown to bind cell wall extensin LRX8, LRX9 and LRX11 expressed in pollen tube and play vital role in deposition of cell wall materials (Mecchia et al. 2017). Additionally, ANX1 overexpression retards PT elongation and induces deposition of cell wall components (Boisson-Dernier et al. 2013), indicating an active role of ANX1-mediated secretion in pollen tube growth and integrity. Together, these suggest a positive role of cell wall in pollen tube growth. Pollen tube growth exhibits oscillatory growth which is underlined by both positive and negative molecular feedback loops to balance growth and cell wall integrity. Hence, it is reasonable to speculate that RALF4/19 can function to slow down pollen tube growth in an oscillatory manner by direct binding to the cell wall extensin. RALF’s inhibition on the PT elongation through extracellular alkalization and cell wall monitoring plays a negative role during the oscillatory growth to avoid overgrowth, which may cause uncoupling between growth and cell wall buildup and even rupture. In this scenario, maintenance of pollen tube integrity is part of the coordinated signaling network of pollen tube growth that is shared with other cell types. RALFs can rapidly increase extracellular pH and inhibit cell expansion in general (Murphy and De Smet 2014), but whether these processes are executed through the BUPS/ANX receptor complex in pollen tube and/or cell wall LRXs is not yet clear.
What makes the growth regulation more complex is the finding that ion homeostasis is also indispensable for pollen tube growth and integrity. In rice and maize, K+ homeostasis is suggested to be the key event in pollen tube integrity. Loss of RUPO, a homologue of BUPSs in rice, causes PT burst after germination (Liu et al. 2016). Further study suggested that RUPO inhibits the precocious PT burst through direct interaction with K+ transporter HAK1. In maize, the peptide ZmES4 secreted from the embryo sac acts through K+ channel KZM1 to induce pollen tube burst in the synergid, suggesting a role of K+ channels or transporters in maintaining pollen tube integrity. Interestingly, loss of the K+ channel SPIK in Arabidopsis also causes precocious PT burst and retarded growth as well, implying a conserved role of K+ homeostasis in pollen tube growth and burst (Mouline et al. 2002). Although BUPSs and RALFs are conserved among species, the link between RALF4/19 signaling and K+ flux is still lacking. K+ homeostasis may also actively participate in pollen tube guidance to the ovule. Loss of function of the endoplasmic reticulum-localized cation/H+ exchanger CHX21 and 23 impairs the pollen tube navigation to the ovule (Lu et al. 2011). K+ flux has been evidenced to be directly regulated by Ca2+ signaling (Zhao et al. 2013). K+ as an important osmolyte, its homeostasis is essential for PT growth and its deregulation leads to cell burst. Therefore, K+ flux must be precisely regulated during pollen tube growth.
Except for K+, ROS has also been unveiled to be affected by ANXs (Boisson-Dernier et al. 2013). rbohh rbohj mutant pollen tubes show strong fluctuations in amplitude and frequency of ROS which ultimately cause pollen tube burst (Lassig et al. 2014). RBOHH and RBOHJ were revealed to slow down pollen tube growth to couple the rate of exocytosis and cell expansion possibly through the Ca2+ influx and K+ efflux (Lassig et al. 2014; Kaya et al. 2014). The impaired amplitude and frequency of ion oscillation are detrimental to the precise coupling of tip growth and cell wall dynamics. Additionally, as discussed earlier, cell wall composition is also essential for pollen tube integrity which is regulated by cell wall-modifying enzymes and ROS, except for extensin (Jiang et al. 2005; Woriedh et al. 2013). Based on these evidences, the ANX-RBOH pathway is acknowledged to regulate the controlled cell expansion or growth which couples the external cell wall dynamics with intracellular components (Nissen et al. 2016). Whether the same regulating pathway is also employed in rice or other species still needs further investigation. A comparison of the signaling pathway of pollen tube integrity in Arabidopsis, rice and maize is summarized in Fig. 2.
Ca2+ signaling
It has been well verified that a tip-focused oscillated calcium gradient is essential for the tip growth of pollen tubes among species. Both the extra- and intracellular Ca2+ are required for PT growth and orientation. Existence of multiple Ca2+ channels, different layers of regulation and the multiple Ca2+ sensors in the PTs makes the Ca2+ signaling network largely intricate. Although several Ca2+ channels are expressed in pollen, phenotypically CNGC18 appears to be the essentially required Ca2+ channel in PT growth and guidance (Frietsch et al. 2007). With the identification of two point-mutated cngc18 mutants, the cyclic nucleotide (cNMP) activation of CNGC18 is likely involved in ovular guidance, but not PT growth (Gao et al. 2016). From this result, one would assume that Ca2+ influx regulates the PT growth and guidance in a biphasic manner: basal influx for PT growth and the cNMP-activated influx for PT reorientation. An interesting and totally unanswered question is whether pollen tubes generate cyclic nucleotides and if so, how they are generated in response to ovular attractants. Therefore, the identification of potential guanylyl cyclases functioning during pollen tube guidance will substantially advance our understanding of the functional divergence of different Ca2+ signatures in pollen tube growth and guidance.
PTs extract Ca2+ and extracellular matrix components from the maternal tissues to sustain their growth. In rice and Arabidopsis, the development of stigma and transmitting tract requires the Ca2+ channel OsCNGC13 and AtCNGC2, respectively, to regulate style development and secretion of extracellular matrix for PT growth (Chaiwongsar et al. 2009; Xu et al. 2017). The Ca2+ pump ACA9 expressed in the PT was shown to regulate pollen tube growth and rupture in the synergid (Schiott et al. 2004). In the synergid, FERONIA-dependent ROS generation and Ca2+ flux also contribute to the pollen tube–synergid interaction (Duan et al. 2014; Hamamura et al. 2014; Ngo et al. 2014). These imply a shared signaling module at both the male and female side. Nonetheless, vacuole acidification of the synergid mediated by ADAPTOR PROTEIN 1G (AP1G) and V-ATPases regulates PT reception in parallel with FERONIA-Ca2+ pathway (Wang et al. 2017), indicating multiple signaling pathways and multiple cellular events are involved.
Downstream of Ca2+, a large number of components decoding and relaying Ca2+ signaling have been identified to be required for optimal PT behavior. Multiple Ca2+ sensors, such as calmodulin (CaM), CaM-like (CML), CPK and CBL-CIPK, can bind cytosolic Ca2+ and transduce different Ca2+ signals. For example, CML24, CML25, CPK24, CPK11, CIPK12 and 19 are involved in pollen germination and tube growth (Yang et al. 2014; Wang et al. 2015; Steinhorst et al. 2015; Zhou et al. 2015). In the PT, Ca2+ and phosphatidylinositol contribute to ROS generation which is essential for pollen tube growth and integrity as mentioned above (Potocký et al. 2012). In addition, a reciprocal crosstalk between ROS production and Ca2+ signaling exists during the tube growth (Kobayashi et al. 2007; Kaya et al. 2014). ROS functions by modifying proteins, possibly including the Ca2+ channels and cell wall components to regulate cell growth, but its exact targets in the PT are still unknown (Mangano et al. 2016). Based on these studies, the molecular regulation of the tip growth is multilayered and intersected by diverse peptide–receptor combinations, ROP, Ca2+, K+, ROS, phospholipids, vesicle trafficking, cell wall dynamics, cytoskeleton and featured by feedback loops. In this process, the transmembrane ion flux contributes to both signaling, electric potential and osmotic homeostasis, which further complicates the molecular network.
Turgor pressure
Turgor pressure has been considered a basic force driving cell expansion in plant cells. Although turgor is not considered the major driving force of pollen tube growth, the general consensus is that a basal turgor pressure is crucial for mechanical force for cell wall deposition (Michard et al. 2017). Turgor is formed by the transport of water, ions and protons and is actively adjusted according to the growth state. During rapid pollen tube growth, cell wall hardening and deposition as well as osmotic water influx are all supposed to be interlaced with turgor pressure (Vogler et al. 2012; Winship et al. 2011). As mentioned above, K+ may contribute to turgor pressure and its dysregulation can cause pollen tube burst. However, the molecular regulation of turgor pressure is still unclear.
Recently, TurgOr regulation Defect 1 (TOD1) was identified as a turgor pressure regulator during PT growth (Chen et al. 2015). TOD1 is essential for PT growth in planta, but not in vitro, suggesting that TOD1 is involved in crosstalk between the pollen tube and maternal tissues. Interestingly, tod1 PTs have higher turgor pressure than the wild type. TOD1 is preferentially expressed in PTs and guard cells, and encodes a Golgi-localized alkaline ceramidase that converts ceramide into sphingosine-1-phosphate (S1P) at alkaline conditions (Chen et al. 2015). Remarkably, exogenously applied S1P can rescue the defective growth of tod1 pollen tubes in the pistil, implying a direct involvement of this small molecule in the PT–pistil interaction. Intriguingly, Ca2+ level is decreased in tod1 PTs indicating a correlation between Ca2+ flux and S1P level. Consistently, S1P can promote pollen tube growth probably by inducing cytosolic Ca2+ increase which prohibits K+ influx (Wu et al. 2014). The defective tube growth of tod1 in pistil can also be restored by the mutation of GAUT13, which is involved in pectin biogenesis during PT growth (Wang et al. 2013). This suggests that the high turgor pressure can be balanced by cell wall changes in the tube. Hence, TOD1 couples turgor pressure and cell wall to balance the tip growth and cell wall buildup through S1P.
Other lipids generated at the Golgi also contribute to PT growth; for instance, the ER- and Golgi-localized lipid-modifying enzyme PLA2 paralogs play key roles in pollen germination and tube growth (Kim et al. 2011). Similar to S1P, the functional mechanism of the products of PLA2 is unknown. Membrane is the basis of all the cellular events. The products of PLA and sphingolipid can contribute to membrane organization, vesicle budding and fission and even act as signaling molecules. But hitherto, the importance of the complex lipid derivatives in a wide scope of plant development and reproduction may be underestimated. Pollen exhibits a unique lipid composition (Ischebeck 2016). The functional identification of the large diversity of lipids in pollen is challenging, but will be promising to help get a comprehensive understanding of pollen tube growth.
Pollen tube guidance to the ovule
Peptides and RLKs signaling
Embryo sac has been genetically evidenced to be essential for pollen tube reorientation from the transmitting tract, as ovules lacking functional embryo sacs do not attract any pollen tubes. Different molecular attractants have been identified among species to be expressed in and secreted from egg apparatus (synergid and egg cell) or synergid cells. In maize, the secreted peptide ZmEA1 containing an EA1-box is the first identified pollen tube attractant with species specificity (Márton et al. 2005). Of note, peptides containing EA1-box are confined to Gramineae. On the other hand, in dicot Torenia and Arabidopsis, a group of defensin-like peptide LUREs, containing six cysteine residues, was uncovered to be the ovular pollen tube attractant with species preferentiality even between closely related species, such as between Torenia fournieri and T. conclor/T. billonii, Arabidopsis and A. lyrata (Okuda et al. 2009; Takeuchi and Higashiyama 2012). Although TfLURE and AtLURE sharing the same cysteine pattern, they do not show any sequence similarity, indicating this kind of peptides is under fast evolution during plant specification to avoid crossing.
Since the identification of the female attractants, different approaches have been employed to identify their receptors. Recently, PRK6/PRK3 and MDIS1/MIK complexes have been identified as receptors for LUREs in Arabidopsis (Takeuchi and Higashiyama 2016; Wang et al. 2016a, b). Of note, PRK6 has been structurally evidenced to directly recognize LURE, but the activation mechanism is still obscure (Zhang et al. 2017). The landscape of how attractants are sensed is still far from clear. As lack of LUREs and their receptors does not lead to seed abortion and with the unveiling of the large number of CRPs and RLKs expressed in the embryo sac and the PT (Huang et al. 2015; Loraine et al. 2013), other players in PT guidance need to be explored. It was shown that PRK6 is relocated toward the LURE gradient, while MDIS1 is induced to undergo endocytosis and degradation on the media containing LURE1 in vitro. This might imply that the two receptors act independently, or on the other hand, whether they form a large complex through the transmembrane or kinase domain in specific lipid raft still awaits further investigation (Higashiyama and Yang 2017). Alternatively, gradient and homogenous LURE1 functions in distinctly manner, or a concentration-dependent sensing mechanism exists. The biological significance of MDIS1 endocytosis upon LURE binding is still unclear, although endocytosis of the receptor–ligand is essential for the downstream cellular events and receptor recycling in other signaling pathways (Robatzek et al. 2006). In maize, the attractant ZmEA1 can be internalized to vesicles for degradation in the PT (Uebler et al. 2014). It is proposed that endocytosis would make the PT under a constant sensitized status for continuous sensing. Of note, along the growth path from the septum surface and the funiculus, to the micropyle, the concentration of LURE1 is increasing. Hence, whether different concentration of LURE1 can induce different cellular events during pollen tube guidance still needs further investigation. Given the sampling limitation of crystal structural analysis, the understanding of the molecular binding of low affinity, transient activation or with requirement for membrane context, still wait for emergence of new techniques.
Mechanisms about how these receptor complexes are activated upon ligand binding and their downstream signaling pathways need to be investigated. Except for LIP1/2 and ROP signaling module, mitogen-activated protein kinases MPK3 and 6 are also involved in pollen tube navigation, but specifically in funicular guidance as well as pollen development (Guan et al. 2014).
Signaling on the ER
ER is the entry organelle of all membrane and secretory proteins, wherein the RLKs and the secreted peptides are synthesized and modified before being delivered to the cell surface for cell–cell communication (Li and Yang 2012). ER-localized proteins have been shown to play specific roles in processing RLKs involved in both development and immunity (Li and Yang 2012). Recently, several ER-localized proteins are revealed to play specific roles in PT guidance, including the K+ transporter CHX21/23 (Lu et al. 2011) as mentioned above and POD1 (Li et al. 2011). POD1 interacts with the ER chaperone CRT3 and thus may be involved in the folding or processing of glycosylated proteins. CRT3 has been demonstrated to regulate the maturation of several LRR-RLK receptors (Li and Yang 2012). It would be interesting to explore whether POD1, together with CRT3, regulates the folding or secretion of the attractant receptors during PT guidance. Furthermore, multiple BiP genes, which function as the ER chaperones, are also involved in PT growth, suggesting that protein folding and ER homeostasis are also essential for the tube growth (Maruyama et al. 2014). ABNORMAL POLLEN TUBE GUIDANCE1 (APTG1) is an ER-localized putative mannosyltransferase that regulates the biogenesis of GPI anchors. Loss of APTG1 causes defective PT guidance and embryo development (Dai et al. 2014). As one of the substrates of APTG1, the GPI-anchored protein COBRA-LIKE10 (CBL10) functions in PT growth and guidance (Li et al. 2013). In cbl10 pollen tubes, the pectin cap is dampened, indicative of failed modulation or secretion of the cell wall. Since CBL10 contains a cell wall-binding motif, it may control PT growth through regulating the cell wall state and then indirectly the guidance process or CBL10 itself can acts as receptor chaperones.
What are the commonality and difference between pollen tube growth and guidance is an intriguing yet open question. Components functioning in both growth and guidance, or specifically on either process, have been identified. For a clearer comparison, we summarize these two groups of factors mentioned in this article in Table 1 and Fig. 3. As our knowledge on molecular pathway of the female signal perception and response as well as the molecular machinery of pollen tube growth is still limited, any speculation or explanation based on limited number of functional studies may be partial or dogmatic.
Model illustrating the signaling of components regulating pollen tube growth and guidance. a Pollen tube growing in the transmitting tract perceives the autocrine (self-generated) and paracrine (transmitting tract secreted) signals and transduces different signals inside the cell. Ion channels, cell wall components as well as other basic cellular components regulating exocytosis are also involved. b A pollen tube is attracted to the embryo sac through the funiculus
Intercellular signaling within the embryo sac in PT attraction
The ovule secretes guidance cues to direct the pollen tube growth. The embryo sac has hitherto been proved to secrete peptide signals, which are affected by the sporophytic tissues (Wang et al. 2016b). Angiosperm evolved multicellular embryo sac with a characterized central cell that is devoid in other plant phylum. The central cell has long been considered as the precursor of endosperm without a positive role in the pre-fertilization process. With the discovery of CENTRAL CELL GUIDANCE (CCG), it becomes clear that the central cell plays an active role in PT guidance. CCG is specifically expressed in the central cell, and loss of CCG function abolishes micropylar PT guidance to the ovule, but does not affect the embryo sac formation (Chen et al. 2007). Recently, it was shown that CCG-binding protein CBP1 recruits the Mediator complex and RNA Pol II with CCG in the central cell (Li et al. 2015). Transcriptome profiling revealed that a large number of genes expressed in the central cell and synergid are downregulated in ccg ovules, including MYB98 which regulates transcription of LUREs. Among the regulated genes, secreted peptides are overrepresented (Li et al. 2015). Except for LUREs, other classes of peptide subfamiles, such as RALFs and ECA1s, are also overrepresented. RALFs in Solanaceous species are involved in sporophyte–gametophyte communication during embryo sac development (Chevalier et al. 2013), but its role in Arabidopsis ovule is still obscure. RALF34 was recently revealed to be expressed predominantly in the inner integument and has an activity to induce pollen tube burst (Ge et al. 2017). Interestingly, the synergid-expressed MYB98 and LUREs are downregulated in ccg ovules, suggesting that CCG somehow regulates gene expression in the synergid likely via cell–cell communication. Our unpublished data showed that ectopic expression of CCG in the synergid couldn’t alleviate the fertility defect of ccg ovule, suggesting that some non-cell autonomous mechanisms, e.g., symplastic signaling or/and extracellular molecular recognition, likely exist.
There indeed exists a dynamic symplastic connection, which allows free flow of molecules below 10 kDa between the central cell and the synergid before/and at anthesis in Torenia (Han et al. 2000). Similarly, in Arabidopsis mature embryo sac, molecules at least smaller than 20 kDa can move from the central cell into the egg apparatus (Erdmann et al. 2017). However, earlier experiment showed that the ablation of the synergid, but not the central cell, disrupts PT attraction in Torenia (Higashiyama et al. 2001). Recently, the ablation of the central cell at FG5 stage influences the synergid function in Torenia (Susaki et al. 2015). This suggests that the signaling between the central cell and synergid are dynamically and developmentally regulated. It can be conceived that the intercellular signaling is confined within a short time-window after cellularization during cell differentiation and thereafter its significance is undermined. Whether extracellular molecular binding contributes to central cell-synergid signaling is totally unknown. Peptides from gamete cells have been show to mediate the cell fate or development of accessory cells in Arabidopsis and maize (Kagi et al. 2010; Krohn et al. 2012). Hence, whether some central cell-expressed peptides regulated by CCG are perceived by the synergid cell surface still needs further investigation.
Central cell maturation also appears important, since embryo sac maturity is positively correlated with PT guidance. On one hand, MAGATAMA3 (MAA3) is required for both polar nuclei fusion and PT attraction (Shimizu et al. 2008). On the other hand, mutations of ER-localized BiP proteins impair the polar nuclei fusion and the pollen tube competitiveness, but do not affect PT attraction (Maruyama et al. 2010, 2014). Similarly, loss of AGL80 or AGL61 disrupts central cell development, but does not impair PT attraction (Portereiko et al. 2006; Steffen et al. 2008). It seems that the regulation of the central cell is more complex than previously anticipated and identification of the direct players involved in central cell–synergid interaction will be helpful to clarify the basic principle of the non-cell autonomous regulation of pollen tube attraction.
Concluding remarks and future perspectives
Although substantial progresses have been made in the past decades, the information on pollen tube growth and guidance is still fragmented. There is still a gap in our knowledge of the molecular connection between different signaling modules and components. For example, how the different membrane receptors integrate in response to different ligands in pistil? How the signaling modules crosstalk with each other? At which step different signaling pathways converge to sustain the oscillated polar growth? To what extent, the molecular components governing pollen tube growth and guidance are conserved among species? What are the intercellular signals regulating the cell differentiation within the embryo sac? Upcoming challenges that need to be addressed include fully deciphering the signaling between different cell types and to overcome the functional redundancy of diverse gene families. With new approaches such as translatomes (Lin et al. 2014), single-cell-based transcriptome and CRISPR/CAS9 technology, the functional identification of novel components and connections will be greatly advanced.
Author contribution statement
HJL and WCY contribute equally to the writing, and JGM contributes to the figures.
References
Boisson-Dernier A, Roy S, Kritsas K, Grobei MA, Jaciubek M, Schroeder JI, Grossniklaus U (2009) Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136:3279–3288
Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S, Grossniklaus U (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11:e1001719
Boisson-Dernier A, Franck CM, Lituiev DS, Grossniklaus U (2015) Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. PNAS 112:12211–12216
Chae K, Kieslich CA, Morikis D, Kim SC, Lord EM (2009) A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization. Plant Cell 21:3902–3914
Chaiwongsar S, Strohm AK, Roe JR, Godiwalla RY, Chan CW (2009) A cyclic nucleotide-gated channel is necessary for optimum fertility in high-calcium environments. New Phytol 183:76–87
Chen YH, Li HJ, Shi DQ, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang WC (2007) The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19:3563–3577
Chen LY, Shi DQ, Zhang WJ, Tang ZS, Liu J, Yang WC (2015) The Arabidopsis alkaline ceramidase TOD1 is a key turgor pressure regulator in plant cells. Nat Commun 6:6030
Cheung AY, Wang H, Wu HM (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82:383–393
Chevalier E, Loubert-Hudon A, Mátton DP (2013) ScRALF3, a secreted RALF-like peptide involved in cell-cell communication between the sporophyte and the female gametophyte in a Solanaceous species. Plant J 73:1019–1033
Covey PA, Subbaiah CC, Parsons RL, Pearce G, Lay FT, Anderson MA, Ryan CA, Bedinger PA (2010) A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiol 153:703–715
Dai XR, Gao XQ, Chen GH, Tang LL, Wang H, Zhang XS (2014) ABNORMAL POLLEN TUBE GUIDANCE1, an endoplasmic reticulum-localized mannosyltransferase homolog of GLYCOSYLPHOSPHATIDYLINOSITOL10 in yeast and PHOSPHATIDYLINOSITOL GLYCAN ANCHOR BIOSYNTHESIS B in human, is required for Arabidopsis pollen tube micropylar guidance and embryo development. Plant Physiol 165:1544–1556
Dong J, Kim ST, Lord EM (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138:778–789
Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5:3129
Erdmann RM, Hoffmann A, Walter HK, Wagenknecht HA, Groß-Hardt R, Gehring M (2017) Molecular movement in the Arabidopsis thaliana female gametophyte. Plant Reprod 30:141–146
Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104:14531–14536
Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF (2016) Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 113:3096–3101
Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, Luo X, Ruan H, García-Valencia LE, Zhong S, Hou S, Huang Q, Lai L, Moura DS, Gu H, Dong J, Wu HM, Dresselhaus T, Xiao J, Cheung AY, Qu LJ (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358:1596–1600
Guan Y, Lu J, Xu J, Mcclure B, Zhang S (2014) Two mitogen-activated protein kinases, MPK3 and MPK6, are required for funicular guidance of pollen tubes in Arabidopsis. Plant Physiol 165:528–533
Gui CP, Dong X, Liu HK, Huang WJ, Zhang D, Wang SJ, Barberini ML, Gao XY, Muschietti J, McCormick S, Tang WH (2014) Overexpression of the tomato pollen receptor kinase LePRK1 rewires pollen tube growth to a blebbing mode. Plant Cell 26:3538–3555
Hamamura Y, Nishimaki M, Takeuchi H, Geitmann A, Kurihara D, Higashiyama T (2014) Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat Commun 5:4722
Han YZ, Huang BQ, Zee SY, Yuan M (2000) Symplastic communication between the central cell and the egg apparatus cells in the embryo sac of Torenia fournieri Lind. before and during fertilization. Planta 211:158–162
Higashiyama T (2010) Peptide signaling in pollen-pistil interactions. Plant Cell Physiol 51:177–189
Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66:393–413
Higashiyama T, Yang WC (2017) Gametophytic pollen tube guidance: attractant peptides, gametic controls, and receptors. Plant Physiol 173:112–121
Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1998) Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10:2019–2032
Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293:1480–1483
Huang WJ, Liu HK, McCormick S, Tang WH (2014) Tomato pistil factor STIG1 promotes in vivo pollen tube growth by binding to phosphatidylinositol 3-phosphate and the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 26:2505–2523
Huang Q, Dresselhaus T, Gu H, Qu LJ (2015) Active role of small peptides in Arabidopsis reproduction: expression evidence. J Integr Plant Biol 57:518–521
Ischebeck T (2016) Lipids in pollen—they are different. Biochim Biophys Acta 1861:1315–1328
Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17:584–596
Kagi C, Baumann N, Nielsen N, Stierhof YD, Gross-Hardt R (2010) The gametic central cell of Arabidopsis determines the lifespan of adjacent accessory cells. Proc Natl Acad Sci USA 107:22350–22355
Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, Takayama S, Abe M, Kuchitsu K (2014) Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26:1069–1080
Kim HU, Cotter R, Johnson S, Senda M, Dodds P, Kulikauska R, Tang W, Ezcura I, Herzmark P, McCormick S (2002) New pollen-specific receptor kinases identified in tomato, maize and Arabidopsis: the tomato kinases show overlapping but distinct localization patterns on pollen tubes. Plant Mol Biol 50:1–16
Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100:16125–16130
Kim HJ, Ok SH, Bahn SC, Jang J, Oh SA, Park SK, Twell D, Ryu SB, Shin JS (2011) Endoplasmic reticulum- and Golgi-localized phospholipase A2 plays critical roles in Arabidopsis pollen development and germination. Plant Cell 23:94–110
Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19:1065–1080
Krohn NG, Lausser A, Juranic M, Dresselhaus T (2012) Egg cell signaling by the secreted peptide ZmEAL1 controls antipodal cell fate. Dev Cell 23:219–225
Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T (2014) Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 78:94–106
Leydon AR, Weinreb C, Venable E, Reinders A, Ward JM, Johnson MA (2017) The molecular dialog between flowering plant reproductive partners defined by SNP-informed RNA-sequencing. Plant Cell 29:984–1006
Li HJ, Yang WC (2012) Emerging role of ER quality control in plant cell signal perception. Protein Cell 3:10–16
Li HJ, Yang WC (2016) RLKs orchestrate the signaling in plant male-female interaction. Sci China Life Sci 59:867–877
Li HJ, Xue Y, Jia DJ, Wang T, Hi DQ, Liu J, Cui F, Xie Q, Ye D, Yang WC (2011) POD1 regulates pollen tube guidance in response to micropylar female signaling and acts in early embryo patterning in Arabidopsis. Plant Cell 23:3288–3302
Li S, Ge FR, Xu M, Zhao XY, Huang GQ, Zhou LZ, Wang JG, Kombrink A, McCormick S, Zhang XS, Zhang Y (2013) Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J 74:486–497
Li HJ, Zhu SS, Zhang MX, Wang T, Liang L, Xue Y, Shi DQ, Liu JA, Yang WC (2015) Arabidopsis CBP1 is a novel regulator of transcription initiation in central cell-mediated pollen tube guidance. Plant Cell 27:2880–2893
Lin SY, Chen PW, Chuang MH, Juntawong P, Bailey-Serres J, Jauh GY (2014) Profiling of translatomes of in vivo-grown pollen tubes reveals genes with roles in micropylar guidance during pollination in Arabidopsis. Plant Cell 26:602–618
Liu J, Zhong S, Guo X, Hao L, Wei X, Hou Y, Shi J, Huang Q, Wang C, Gu H, Qu LJ (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23:1–6
Liu L, Zheng C, Kuang B, Wei L, Yan L, Wang T (2016) Receptor-like kinase RUPO interacts with potassium transporters to regulate pollen tube growth and integrity in rice. PLoS Genet 12:e1006085
Loraine AE, Mccormick S, Estrada A, Patel K, Qin P (2013) RNA-seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiol 162:1092–1109
Lord E (2000) Adhesion and cell movement during pollination: cherchez la femme. Trends Plant Sci 5:368–373
Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23:81–93
Mangano S, Juarez SP, Estevez JM (2016) ROS regulation of polar growth in plant cells. Plant Physiol 171:1593–1605
Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307:573–576
Maruyama D, Endo T, Nishikawa S (2010) BiP-mediated polar nuclei fusion is essential for the regulation of endosperm nuclei proliferation in Arabidopsis thaliana. Proc Natl Acad Sci USA 107:1684–1689
Maruyama D, Sugiyama T, Endo T, Nishikawa S (2014) Multiple BiP genes of Arabidopsis thaliana are required for male gametogenesis and pollen competitiveness. Plant Cell Physiol 55:801–810
Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Martínez-Bernardini A, Fabrice TN, Ringli C, Muschietti JP, Grossniklaus U (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358:1600–1603
Michard E, Simon AA, Tavares B, Wudick MM, Feijo JA (2017) Signaling with Ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol 173:91–111
Miyazaki S, Murata T, Sakurai-Ozato N, Kubo M, Demura T, Fukuda H, Hasebe M (2009) ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr Biol 19:1327–1331
Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D, Iwai H, Garénaux E, Sato C, Kitajima K, Tsumuraya Y, Mori H, Yamaguchi J, Itami K, Sasaki N, Higashiyama T (2016) The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr Biol 26:1091–1097
Morato do Canto A, Ceciliato PH, Ribeiro B, Ortiz Morea FA, Franco Garcia AA, Silva-Filho MC, Moura DS (2014) Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis. Plant Physiol Biochem 75:45–54
Mouline K, Véry AA, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud JB, Sentenac H (2002) Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev 16:339–350
Mu JH, Lee HS, Kao TH (1994) Characterization of a pollen-expressed receptor-like kinase gene of Petunia inflata and the activity of its encoded kinase. Plant Cell 6:709–721
Murphy E, De Smet I (2014) Understanding the RALF family: a tale of many species. Trends Plant Sci 19:664–671
Muschietti J, Eyal Y, McCormick S (1998) Pollen tube localization implies a role in pollen-pistil interactions for the tomato receptor-like protein kinases LePRK1 and LePRK2. Plant Cell 10:319–330
Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U (2014) A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell 29:491–500
Nissen KS, Willats WG, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trend Plant Sci 21:516–5271
Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, Kawano N, Sakakibara T, Namiki S, Itoh K, Otsuka K, Matsuzaki M, Nozaki H, Kuroiwa T, Nakano A, Kanaoka MM, Dresselhaus T, Sasaki N, Higashiyama T (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458:357–361
Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114:47–59
Portereiko MF, Lloyd A, Steffen JG, Punwani JA, Otsuga D, Drews GN (2006) AGL80 is required for central cell and endosperm development in Arabidopsis. Plant Cell 18:1862–1872
Potocký M, Pejchar P, Gutkowska M, Jiménez-Quesada MJ, Potocká A, Alché Jde D, Kost B, Žárský V (2012) NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J Plant Physiol 169:1654–1663
Robatzek S, Chinchilla D, Boller T (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20:537–542
Schiott M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101:9502–9507
Shimizu KK, Ito T, Ishiguro S, Okada K (2008) MAA3 (MAGATAMA3) helicase gene is required for female gametophyte development and pollen tube guidance in Arabidopsis thaliana. Plant Cell Physiol 49:1478
Steffen JG, Kang IH, Portereiko MF, Lloyd A, Drews GN (2008) AGL61 interacts with AGL80 and is required for central cell development in Arabidopsis. Plant Physiol 148:259–268
Steinhorst L, Mähs A, Ischebeck T, Zhang C, Zhang X, Arendt S, Schültke S, Heilmann I, Kudla J (2015) Vacuolar CBL-CIPK12 Ca2+-sensor-kinase complexes are required for polarized pollen tube growth. Curr Biol 25:1475–1482
Susaki D, Takeuchi H, Tsutsui H, Kurihara D, Higashiyama T (2015) Live imaging and laser disruption reveal the dynamics and cell-cell communication during Torenia fournieri female gametophyte development. Plant Cell Physiol 56:1031–1041
Takeuchi H, Higashiyama T (2011) Attraction of tip-growing pollen tubes by the female gametophyte. Curr Opin Plant Biol 14:614–621
Takeuchi H, Higashiyama T (2012) A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol 10:e1001449
Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531:245–248
Tang W, Ezcurra I, Muschietti J, McCormick S (2002) A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14:2277–2287
Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J 39:343–353
Uebler S, Dresselhaus T, Márton ML (2014) Species-specific interaction of EA1 with the maize pollen tube apex. Plant Signal Behav 8:e25682
Vogler H, Draeger C, Weber A, Felekis D, Eichenberger C, Routier-Kierzkowska AL, Boisson-Dernier A, Ringli C, Nelson BJ, Smith RS, Grossniklaus U (2012) The pollen tube: a soft shell with a hard core. Plant J 73:617–627
Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH (2008) Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol 148:1201–1211
Wang L, Wang W, Wang YQ, Liu YY, Wang JX, Zhang XQ, Ye D, Chen LQ (2013) Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol Plant 6:1131–1148
Wang SS, Diao WZ, Yang X, Qiao Z, Wang M, Acharya BR, Zhang W (2015) Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ 38:2372–2386
Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC (2016a) A receptor heteromer mediates the male perception of female attractants in plants. Nature 531:241–244
Wang JG, Feng C, Liu HH, Ge FR, Li S, Li HJ, Zhang Y (2016b) HAPLESS13-mediated trafficking of STRUBBELIG is critical for ovule development in Arabidopsis. PLoS Genet 12:e1006269
Wang JG, Feng C, Liu HH, Feng QN, Li S, Zhang Y (2017) AP1G mediates vacuolar acidification during synergid-controlled pollen tube reception. Proc Natl Acad Sci USA 114:E4877–E4883
Winship LJ, Obermeyer G, Geitmann A, Hepler PK (2011) Pollen tubes and the physical world. Trends Plant Sci 16:353–355
Woriedh M, Wolf S, Márton ML, Hinze A, Gahrtz M, Becker D, Dresselhaus T (2013) External application of gametophyte-specific ZmPMEI1 induces pollen tube burst in maize. Plant Reprod 26:255–266
Wu J, Qin X, Tao S, Jiang X, Liang YK, Zhang S (2014) Long-chain base phosphates modulate pollen tube growth via channel-mediated influx of calcium. Plant J 79:507–516
Xu Y, Yang J, Wang Y, Wang J, Yu Y, Long Y, Wang Y, Zhang H, Ren Y, Chen J, Wang Y, Zhang X, Guo X, Wu F, Zhu S, Lin Q, Jiang L, Wu C, Wang H, Wan J (2017) OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet 13:e1006906
Yang WC, Shi DQ, Chen YH (2010) Female gametophyte development in flowering plants. Annu Rev Plant Biol 61:89–108
Yang X, Wang SS, Wang M, Qiao Z, Bao CC, Zhang W (2014) Arabidopsis thaliana calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca2+ concentration. Plant Mol Biol 86:225–236
Zhang Y, McCormick S (2007) A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA 104:18830–18835
Zhang X, Liu W, Nagae TT, Takeuchi H, Zhang H, Han Z, Higashiyama T, Chai J (2017) Structural basis for receptor recognition of pollen tube attraction peptides. Nat Commun 8:1331
Zhao LN, Shen LK, Zhang WZ, Zhang W, Wang Y, Wu WH (2013) Ca2+-dependent protein kinase 11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 25:649–661
Zhou L, Lan W, Chen B, Fang W, Luan S (2015) A calcium sensor-regulated protein kinase, CALCINEURIN B-LIKE PROTEIN-INTERACTING PROTEIN KINASE19, is required for pollen tube growth and polarity. Plant Physiol 167:1351–1360
Acknowledgments
This work is supported by the Grant from National Natural Science Foundation of China (31571385, 31622010 to H. L. and 31330053 to W. Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflicts of interest.
Additional information
Communicated by Tetsuya Higashiyama.
A contribution to the special issue ‘Plant Reproduction Research in Asia’.
Rights and permissions
About this article
Cite this article
Li, HJ., Meng, JG. & Yang, WC. Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod 31, 31–41 (2018). https://doi.org/10.1007/s00497-018-0324-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-018-0324-7