Abstract
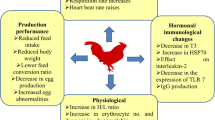
Heat stress is one of the greatest challenges for the global livestock industries as increased environmental temperature and humidity compromises animal production during summer leading to devastating economic consequences. Over the last 30 years, significant developments have been achieved in cooling and provision of shade and shelter to mitigate heat stress reducing some of the losses associated with heat stress in farm animals. However, the recent increase in the incidence of heat waves which are also becoming more severe and lasting longer, due to climate change, further accentuates the problem of heat stress. Economic losses associated with heat stress are both direct due to loss in production and animal life, and indirect due to poorer quality products as a result of poor animal health and welfare. Animal health is affected due to impaired immune responses and increased reactive oxygen species production and/or deficiency of antioxidants during heat stress leading to an imbalance between oxidant and antioxidants and resultant oxidative stress. Research over the last 20 years has achieved partial success in understanding the intricacies of heat stress impacts on oxidative stress and immune responses and developing interventions to ameliorate impacts of heat stress, improving immune responses and farm animal health. This paper reviews the body of knowledge on heat stress impacts on immune response in farm animals. The impacts of heat stress on both cell-mediated and humoral immune responses have been discussed identifying the shift in immune response from cell-mediated towards humoral response, thereby weakening the immune status of the animal. Both species and breed differences have been identified as influencing how heat stress impacts the immune status of farm animals. In addition, crosstalk signaling between the immune system and oxidative stress has been considered and the role of antioxidants as potential nutritional strategies to mitigate heat stress has been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global temperature is predicted to increase by 1.5 °C because of continued global warming between 2030 and 2052 (IPCC 2014). Climate change over recent decades has impacted the global ecosystem which is indicative of the sensitivity of our ecosystems to climate change. A range of challenges have been predicted to occur for the livestock industries that will be affected by these changes in environmental temperature (Osei-Amponsah et al. 2019). Increased environmental temperature and high relative humidity during summer can compromise the thermoregulatory mechanisms of ruminants affecting heat loss and leading to increased heat load on animals, resulting in heat stress (HS). While farm animals experience several stressors throughout their lifetime, HS is the most frequent and difficult to manage (Aggarwal and Upadhyay 2012; Joy et al. 2020). In farm animals, as with other homeotherms, the balance between heat produced (metabolic activities) and heat lost from body is crucial to maintain core body temperature (Berman 2011) and HS results when this balance is disturbed because the animal is not able to dissipate heat to the environment (Bernabucci et al. 2014). Environmentally induced periods of HS compromise animal welfare and decrease animal productivity leading to devastating economic consequences for animal agriculture across the globe (Bernabucci et al. 2010). While there have been considerable advances in management, housing, and cooling technologies (Schütz et al. 2011; Schütz et al. 2014) to mitigate HS impacts, HS still remains an incredibly costly issue for livestock production. For instance, in the USA alone, it was reported that heat stress caused losses of $ US 900 million/year for dairy production and > $ US 300 million/year for both beef and swine production almost two decades ago (St-Pierre et al. 2003). Most recently, these losses have been estimated to $ US 1.5 billion for dairy and $ US 1 billion for the swine industry (Key and Sneeringer 2014; Mayorga et al. 2020). Annual heat–related production losses in the Australian feedlot industry were estimated to be $ AUD 16.5 million/year (MLA 2010). This clearly highlights the need to develop strategies to reduce the impact of HS on animal agriculture. Animal agriculture in developing countries is predicted to sustain the future of global food production. However, many challenges are faced by livestock producers in these countries, with climatic factors being most crucial and limiting, especially in the tropical and subtropical countries (Berman 2011; Renaudeau et al. 2012) as HS is known to negatively influence animal performance and animal health.
Animal health may be affected directly by HS causing metabolic alterations (Rhoads et al. 2009), oxidative stress (Chauhan et al. 2014a; Chauhan et al. 2014c), and immune suppression, and may ultimately lead to death (Lacetera 2018). For example, there was a high mortality in dairy cattle (30,000 cows) during the 2006 heat wave in California (California Department of Food and Agriculture 2006) and similarly a heat wave in Iowa during 2011 caused an estimated 4000 beef cattle deaths (Drovers 2011; Baumgard and Rhoads 2013). The immune responses play a most important role in an animal’s defense against invading pathogens. However, the immune responses in animals are profoundly altered from the prenatal stage to the productive phases (Dahl et al. 2020) by HS which is a consequence of the imbalance between heat gained and heat lost from the body leading to core body temperature exceeding the range specified for normal activity (Bernabucci et al. 2010).
Heat stress has been reported to shift the adaptive immune function of the animal from the normal cell mediated to humoral immunity (Sophia et al. 2016) and such a shift would therefore weaken the immune status of the animal increasing their susceptibility to several pathogens (Vandana et al. 2019). In addition, HS hampers the immune capabilities of the animal to tackle emerging and re-emerging pathogens and vector-borne diseases (Sophia et al. 2016). Furthermore, the immune response and the health status of an animal is influenced by oxidative stress (OS) (decreased antioxidant status/increased production of free radicals) (Sordillo and Aitken 2009) and HS has been reported to cause oxidative stress (OS) in dairy cattle (Bernabucci et al. 2002), sheep (Chauhan et al. 2014a; Chauhan et al. 2016), pigs (Liu et al. 2016), and poultry (Mujahid et al. 2005; Shakeri et al. 2019; Shakeri et al. 2020a), increasing their susceptibility to several pathogens and production diseases. Therefore, dietary antioxidant supplementation offers an avenue to mitigate HS and improve the immune response during HS. In this paper, we review various immunological markers such as Toll-like receptor-2 (TLR2), TLR3, TLR4, TLR8, interleukin-8 (IL-8), IL-10, and tumor necrosis factor-α (TNF-α), affected by HS in farm animals. Furthermore, various signaling pathways such as TLR, NF-κB, interferon signaling, mitogen-activated protein kinase (MAPK), and stress induction of heat shock protein (HSP) and associated changes in immune system of large ruminants have been discussed. In addition, the impact of HS on oxidative balance and potential implications for animal health are considered. Finally, potential nutritional strategies to mitigate these negative impacts to improve animal health are presented.
Impacts of heat stress on immune responses in farm animals
Heat stress has detrimental effects on the immune system of livestock by altering the immune function and increasing susceptibility to diseases (Lacetera et al. 2006; do Amaral et al. 2009). Heat stress increases the secretion of glucocorticoids which acts as inhibitor of the pro-inflammatory cytokines such as TNF-α, IL-6, IL-8 initiating the innate immune responses by the inhibition of the p38 MAPK pathway which maintains the stability of the immune system in animals (Abraham et al. 2006). While there are common responses across species, there are also some species-specific variations in responses to HS. The detailed species-specific effects of HS on immune response in farm animals are discussed in subsequent sections.
Heat stress effects on immunity in cattle and buffalo
Recent research has shown that HS inhibits the pro-inflammatory cytokines required to initiate the innate immune response in the post partem period and in lactating cows, respectively (Safa et al. 2019; Sun et al. 2018). Previous experiments on thermal stress in in utero heat stressed calves have reported a decrease in relative weights of immune organs like the spleen and thymus (Ahmed 2017) which can potentially affect the immune response. Heat stress in general has been implicated to weaken the animal immune action to microbial intrusion by shifting the adaptive immune function to humoral immunity from cell-mediated immunity. Studies in both dry and lactating dairy cows suggest that HS suppresses the innate immune function by decreasing some of the vital immune responses, such as decreased proliferation of immune cells, cytokine production, and migration of lymphocytes to the udder and cell viability, and increasing the incidence of production diseases such as mastitis and metritis in dairy cattle (Steele 2016).
Heat stress has been suggested to negatively influence the health status of lactating and dry dairy cows and their calves (Dahl and Collier 2017). Previous studies in lactating cows have demonstrated the negative effects of HS on leukocyte migration to mammary gland following a chemotactic challenge (Elvinger et al. 1992). Increased ambient temperature and humidity are known to cause significant endocrine perturbations to facilitate increased heat loss from the body. However, these endocrine alterations may influence immune functions. For example, the coexistence of both prolactin and cortisol exerts negative impact on immune-associated genes notably with the help of HSPs during chronic heat stress (Collier et al. 2008).
Serum concentrations of TNF-α and IL-10 were increased in healthy uniparous mid-lactation Holstein cows during exposure to HS (Zhang et al. 2014). Furthermore, the expression pattern of the IL-10 was increased in HS and varied between the breeds and the level of exposure in the peripheral blood mononuclear cells of transition Sahiwal and Karan Fries cows incubated in vitro (Sheikh et al. 2016). Tao et al. (2013) reported increased TNF-α expression in heat HS cows during the transition period. Furthermore, clusters of infiltrating cells were observed in the mucosa and submucosa of German Holstein cows during HS (Koch et al. 2019). Thus, in this study, HS triggered the migration of infiltrating cells into the mucosa and submucosa cells while the jejunal morphology remained unaltered (Koch et al. 2019).
The immune system–related pathway analysis in German Holstein cows subjected to HS revealed upregulation of distinct cytokines such as IL5, IL4, IL6, IL2, and TGFB1 and lipoprotein polysaccharide (LPS) which are responsible for the activation of the signaling pathway (Koch et al. 2019). The LPS, a constituent of bacterial cell walls, enters the mucosa more readily during HS because of gut damage due to OS and hypoxia (Cronje 2005) and activates the immune response via TLRs (Koch et al. 2019). Thompson et al. (2014) reported increased IL-10 expression and no change in the expression levels of IL1-β, IL6, IL8, or TNF-α in HS multiparous Holstein dairy cows followed by Streptococcus uberis challenge by intra mammary infusion. Bharati et al. (2017) reported that HS-associated changes in expression patterns of TLR 2, TLR4, IL-2, and IL-6 could possibly play a vital role in assessing the greater thermo-tolerance of Tharparkar cattle.
Generally, the immune status of the Murrah buffalo calves was lower during HS exposure mediated by the increased concentration of blood cortisol (Mishra et al. 2011). In another study in Murrah buffalo calves, Mishra et al. (2011) reported a multifold increase in serum HSP70 concentrations during exposure to HS resulting in modulation of the immune response in buffaloes. In another study, Mukherjee et al. (2011) reported increased concentration of HSP70 both in vivo as well as in lymphocytes. These authors also observed that the increased concentration of HSP70 did not cause immune protection at physiological levels. Furthermore, these authors also observed that exposing lymphocytes to high temperatures in vitro can depress the cell-mediated immunity response in buffaloes (Mukherjee et al. 2011).
Heat stress effects on immunity in sheep
Gomes da Silva et al. (1992) assessed the impact of HS in Polwarth sheep and observed a reduction in the blood neutrophil and eosinophil count when compared to their control counterparts. In another study conducted by Wojtas et al. (2014), Merino sheep were subjected to simulated HS in climatic chambers and observed a reduction in white blood cell count.
Heat shock proteins are biological markers that act as protein chaperones and that increase during various stress conditions and also play a vital role in antigen processing and presentation, especially the HSP70 and 90 (Archana et al. 2017). Increased HSP expression could be inferred as an adaption in antigen processing and presentation in addition to greater potential of activating the innate and acquired immune system directly (Colaco et al. 2013). Apart from this, HSP70 inhibits the nuclear factor-κB signaling cascade activated by LPL, thus reducing IL-6 production (Chen et al. 2006). HSP70 has also been proven to increase cellular IL-1β secretion thereby facilitating the transport of antigens to T cells (Zininga et al. 2018). RNA-Seq analysis of heat stressed Hu sheep revealed increased expression of IL1R1, IL1R2, and HSPA2 (which belongs to HSP70 family) in the liver samples when compared to their respective control group (Lu et al. 2019). These data thereby suggest the potential role of these genes in immunoregulatory process of sheep. Cortisol supplementation in hyperthermia-induced in vitro sheep peripheral blood mononuclear cell (PBMC)–cultured cells reduced the concentration of IL-6, IL-1β, and IL-10 reflecting the immune modulatory role of cortisol in sheep (Caroprese et al. 2018).
Heat stress effects on immunity in goats
A study conducted by Sophia et al. (2016) showed greater expression pattern of TLR1 in Osmanabadi goats when exposed to HS. Furthermore, Tirumurugaan et al. (2010) reported that TLR1 was over expressed in organs such as the uterus, skin, lymph node, PBMC, and lungs of Kanni goats during HS. All the TLR mRNAs (1–10) showed increased expression in the lymph node while the spleen showed lower expression of TLR 6 and TLR 10 mRNAs (Tirumurugaan et al. 2010). Furthermore, PBMC and lung expressed increase in all TLR mRNAs except TLR 10 mRNA (Tirumurugaan et al. 2010). Furthermore, Tirumurugaan et al. (2010) also reported that tissues such as uterus, lung, and skin, which generally exhibit lower amounts of TLR 2, had higher amounts of TLR 6 mRNAs.
Malabari goats were reported to be extremely resilient to HS in terms of being able to maintain the innate immune response (Vandana et al. 2019). This was evident in the studies conducted by Vandana et al. (2019) in Malabari goats wherein the immune response genes TLR3, TLR7, TLR8, TLR9, and TLR10 had no significant expression despite the animals being subjected to HS. Furthermore, the study also indicated the sensitivity of TLR1, TLR4, TLR5, and TLR6 genes to HS effects and the lower expression of these genes indicates the partially compromised immune status in HS Malabari goats (Vandana et al. 2019). The TLR-2 expression pattern was considered an important biological marker for reflecting the immune competency during HS in goats (Vandana et al. 2019). In the same study, the IL-18, TNF-α, IFN-β, and IFN-γ expression patterns all were downregulated in Malabari goats exposed to HS (Rashamol et al. 2019). Furthermore, the histopathological changes of mesenteric lymph node showed a paucity of lymphocyte distribution in follicular areas as well as decreased density of lymphocytes in the germinal centers of the HS group suggests that the animal’s immunity will be compromised during chronic HS (Rashamol et al. 2019).
Another study by Madhusoodan et al. (2019) reported lower expression of TLR 2 in Salam Black goats during HS. The expression pattern of IL 2, IL 6, IL 18, TNF-α, and IFN-β in hepatic tissue was lower in Salem Black goats during HS (Madhusoodan et al. 2019) and these data indicate the compromised growth and immune status of Salem Black goats probably occurs to support life-sustaining activities to adapt to HS challenges. In addition, Paul et al. (2015) concluded that TLR1, which was highly expressed during summer in Black Bengal goats, contributed to the immune suppressive effect of HS in these goats. Paul et al. (2015) similarly concluded that the HS-induced increase in TLR 2 expression in Black Bengal goats resulted in immunosuppressive effects in this breed. Furthermore, Paul et al. (2015) concluded that HS induced TLR2 expression to increased innate immune response to pathogen-associated molecular patterns by differentially mediated immune response by promoting both TLR expression and signaling in immune cells during summer. A study by Savitha et al. (2019) indicated that the expression of TLR1, TLR4, TLR5, TLR6, and TLR10 genes did not significantly change in Osmanabadi goats but were significantly increased in Salam Black goats during HS. The higher expression of most of the cell surface TLRs in the Salam Black goats as compared to Osmanabadi goats indicates the superior resilient capacity of Salem Black goats to maintain immune status even during exposure to adverse environmental condition.
Heat stress effects on immunity in pigs
In two different studies assessing the impact of HS in Bama miniature pigs, the total white blood cells decreased while the percentage of eosinophils and monocytes were increased (Ju et al. 2011; Ju et al. 2014). Furthermore, the CD8 T cells were also found to be at higher concentration in HS pigs. It has also been established that the alteration in the ratio of CD4 and CD8 T cell could reflect the compromised immune status during HS (Ju et al. 2011). These authors also established that the expression of both TLR2 and TLR4 increased while the expression of IL-2 and IFN-γ were decreased in PBMC obtained from HS pigs (Ju et al. 2014). In another study, Chu and Song (2018) reported that the blood concentrations of platelets, IgG, IgM, and IFN-γ decreased while leukocyte concentrations increased during summer as compared to other seasons in finishing pigs. Plasma TNF-α, IL-6, and IL-8 concentrations were unchanged. Thus, decrease in the IFN-γ cytokine and no change in the IL-6 indicates compromised Th1 type immune response (Chu and Song 2018). A similar observation of a reduction in IgG concentrations during HS was also observed by Morrow-Tesch et al. (1994).
In a study conducted in crossbred pigs, Cui et al. (2016) reported compromised immune response after exposure to chronic HS. These authors also observed that the HS-induced compromised immune response in these pigs occurred irrespective of their nutritional status. In a similar study conducted in pigs, Pearce et al. (2013) observed increased stress and immune-related proteins such as stress-induced phosphoprotein 1, neurofibromin1, and Igγ-chain in ileum while observing decreased levels of protein Rho-GDP inhibitor 1, cofilin 1, and peptidyl-prolyl-cis-trans isomerase A expression in pigs exposed to HS. Huo et al. (2019) reported higher levels of HSP70 expression in both the spleen and intestinal mucosa of pigs and they attributed this to immune modulation in these animals after exposing them to HS. Likewise, in the same study, increased concentration of T cells was reported both in the spleen and small intestine attributing to inhibition of apoptosis during HS (Huo et al. 2019). These authors also observed increase in the number of CD3+ T cells after chronic HS exposure indicating the proliferation and differentiation of T cells. In addition, altered balance between the CD4 and CD8 ratio reflected serious immune dysfunction during chronic HS in pigs (Huo et al. 2019).
Heat stress effects on immunity in poultry
Heat stress has been identified to be the most critical limiting factor in poultry, affecting their vigor and vitality (Rostagno 2020). Apart from causing productive losses, HS also impairs the immune response of poultry. Thaxton et al. (1968) were the first to illustrate the detrimental effects of HS on development of the immune responses in chicken when they established that as an adoptive mechanism the pullets would develop innate as well as humoral immunity when exposed to HS. This was later validated by McDaniel et al. (2004) who correlated the damaged cells with the expression of immune responses in HS birds. Innate responses are the first line of defense exhibited by animals on exposure to HS (Bagath et al. 2019). Upon failure of the primary innate immune responses, birds were found to facilitate an array of secondary responses through migration of blood cells. In broiler chicks, HS-induced responses significantly influenced the innate immune responses pertaining to the higher heterophil-lymphocyte ratio, abdominal exudative cell phagocytic activities, and serum anti-SRBC titer (Tirawattanawanich et al. 2011). In agreement with this, Hirakawa et al. (2020) stated that extreme temperatures resulted in limited synthesis of lymphocytes along with suppression in phagocytic activities of leukocytes.
There are diverse reports on the development of humoral immune responses pertaining to exposure of poultry to higher ambient temperatures. Mashaly et al. (2004) reported that HS depressed the production of antibodies in chickens with the lowest production of IgM and IgG factors. This agreed with the observation of Bartlett and Smith (2003) that the humoral responses such as serum total antibody, IgM, and IgG concentrations were lower during HS. A reduction in antibody production can be attributed to the release of cytokines during HS, resulting in stimulation of hypothalamus to release corticotrophin releasing hormone, culminating with an increase in corticosterone secretion with an intermediate production of adrenocorticotropic hormone (Mashaly et al. 2004). Gross (1992) identified that presence of corticosterone hampers antibody production, resulting in impairment of the immune system. As birds attempt to maintain homeostasis during HS, a reduction in the expression of cytokine genes like IL-12, IFN-γ, IgA and TLR 2 occurs (Ohtsu et al. 2015; Quinteiro-Filho et al. 2017). Such reductions are of concern as the birds would therefore be prone to pathogens which could lead to deadly disease outbreaks in poultry.
Potential immunological biomarkers for heat stress in livestock
This section of the review provides an overview on the various immunological indicators to reflect the HS impact on the immune response in livestock. In depth research efforts in a few indigenous (to India) goat breeds established TLR2 expression in the spleen, liver, and mesenteric lymph node as a potential biomarker to reflect immunological changes during HS (Sophia et al. 2017; Vandana et al. 2019). Similar HS-induced higher expression of TLR2 was also reported in Tharparkar cattle in PBMC (Bharati et al. 2017) indicating that TLR2 could serve as potential biomarker for heat stress–associated changes in farm animals. There are also a few studies which suggested that TLR3 (spleen, mesenteric lymph node), TLR8, and TLR9 (mesenteric lymph node) could be reliable indicators of the severity of the effects of HS on immune responses in spleen farm animals (Sophia et al. 2017; Savitha et al. 2019).
Heat stressed lactating cows (Zhang et al. 2014) and pigs (Pearce et al. 2013 Ganesan et al. 2018, Gabler et al. 2018) exhibited lower leucocyte TNF-α or blood TNF-α expression, respectively. Likewise, both Malabari and Osmanabadi goats subjected to HS also displayed downregulation of TNF-α mRNA expression in mesenteric lymph node and hepatic tissues, respectively (Rashamol et al. 2019; Madhusoodan et al. 2019). However, Chauhan et al. (2014a) reported increased expression of TNF-α in skeletal muscle from HS Merino × Poll Dorset crossbred ewes. This indicates that level of TNF-α could be a reliable immunological marker to reflect immunological changes during HS exposure in livestock although there may be differences in expression in different tissues.
Interleukin-8, another pro-inflammatory cytokine, was decreased during HS in pigs in one study (Pearce et al. 2013) but not in another (Ju et al. 2014). On the other hand, HS combined with the transition period in dairy cows was associated with an increase in IL-8 expression (Tao et al. 2013). Similarly, IL1β was also reduced during HS in pigs (Pearce et al. 2013). During the transition period, heat-treated PBMC of Karan Fries cows showed increase in IL-10 concentrations compared to Sahiwal cattle (Sheikh et al. 2016). Similarly, in vivo studies in ewes (Caroprese et al. 2014) and cows (Thompson et al. 2014) subjected to HS showed increase in the circulating IL-10 concentrations. In contrast, in vitro studies showed that IL-10 production was negatively affected in both cattle and sheep PMBC cultures under HS (Zhang et al. 2014; Caroprese et al. 2018). Other interleukins such as IL34, IL27RA, IL6R, IL10RB, and IL1R1 were found be decreased in bovine mammary tissues of cows subjected to heat stress (Dado-Senn et al. 2018). In another study in goats, the expression of hepatic IL-2, IL-6, IL-18, mRNA was downregulated in Salem Black goats exposed to HS indicating compromised immune status (Madhusoodan et al. 2019). These results indicate that all the above discussed interleukins could serve as biomarkers to reflect changes associated with immune system in livestock.
Heat stress in crossbred ewes increased the skeletal muscle nuclear factor kappa B (NF-κB) (Chauhan et al. 2014a, 2014b, 2014c). Similarly, Ganesan and co-workers showed that the phosphorylated NF-κB was in abundance in the muscle of pigs subjected to HS (Ganesan et al. 2018). In a study on Malabari goats, Rashamol et al. (2019) reported decreased mesenteric lymph node interferon-β, and interferon-γ mRNA expression. Likewise, in another study in Salem Black goats, the expression of hepatic interferon-β mRNA was downregulated during HS (Madhusoodan et al. 2019). In contrast, Chen et al. (2018b) reported greater expression of IFN-γ during HS in Chinese Holstein dairy cows. Thus, IFN-γ could also be considered an important HS marker to reflect immunological changes in livestock although the diversity of responses needs to be clarified. The gene expression patterns of all above discussed genes are important in PBMC than tissues. This is because the level of invasiveness is not very severe in PBMC as compared to the target tissues which require slaughtering the animals. Therefore, from animal welfare and ethical point of view, targeting PBMC than tissues would be more appropriate and meaningful.
Immune pathways affected during heat stress in farm animals
Very limited research reports are available relating directly the impact of HS on immune pathways in farm animals. However, a few studies have correlated the immune system and other immune-related pathways with HS in farm animals. The pathways that are associated with HS-related impacts on immune response include (i) cytokines and inflammatory response; (ii) TNF-α and NF-κB signaling pathways; (iii) MAPK, FAS (Fas cell surface death receptor), and stress induction of HSP regulation; and (iv) apoptosis modulation by HSP70 in the bovine mammary epithelial cells (Kapila et al. 2016). In another study in Holstein calves, whole blood cells subjected to HS showed activation of B cell receptor, T cell receptor, antigen-presenting TNF, TLR, interferon, MAPK, NF-κB, and JNK signaling pathways (Srikanth et al. 2017). Direct HS in the lactating bovine model showed impaired intestinal barrier leading to penetration of the bacterial and toxic compound triggering the immune, cytokine, LPS, and phagocytic-related pathways (Koch et al. 2019). This study (Buffington et al. 1983) showed higher expression of CD163, CD68, MCR1 (CD206), CD14, and FCGR2B (CD32) genes related to macrophage/phagocyte/myeloid cells in dairy cows. Similarly, another study established an important upstream regulator for signaling pathway in infiltrating cells and involvement of cytokines like IL4, IL5, IL6, TGFB1, and LPS in the jejunal mucosa scrapings of HS cows (Koch et al. 2019).
Nutritional interventions to mitigate heat stress
Climate change and heat stress adaptation and mitigation strategies are required to reducing and managing the risks and economic losses to farm animal productivity. There are three main strategies for mitigation of HS, altering the animal’s environment, genetic selection for heat tolerance, and feeding strategies to reduce metabolic heat production or amelioration of negative impacts of HS, which were recognized about 35 years ago (Beede and Collier 1986). Comprehensive research and development has been done in the past on the physical modification of an animal’s environment (Collier et al. 1981; Buffington et al. 1983; Knapp and Grummer 1991; Armstrong 1994; Blackshaw and Blackshaw 1994; Hemsworth et al. 1995; Correa-Calderon et al. 2004; Berman 2005; Collier et al. 2006; Kendall et al. 2006; Smith et al. 2006; Tucker et al. 2008; Khongdee et al. 2010; Schütz et al. 2011; Schütz et al. 2014) by providing shade or direct/tunnel cooling of animals. For the short term, nutritional strategies for amelioration of HS would be most feasible and more rapidly incorporated strategies to be implemented as the genetic development of heat-tolerant breeds will be a long-term process (Osei-Amponsah et al. 2019). New insights into promising nutritional strategies have been developed recently for the alleviation of HS in farm animals and have been reviewed before (Dunshea et al. 2013; Rhoads et al. 2013; Cottrell et al. 2015; Gonzalez-Rivas et al. 2020; Zhang et al. 2020). Here we would focus mainly on antioxidant and betaine supplementation to mitigate HS improving oxidative balance and potential benefits for immune responses in farm animals.
Oxidative stress and immune responses
Heat stress has been implicated to cause OS in livestock (Bernabucci et al. 2002; Mujahid et al. 2005; Chauhan et al. 2014b; Liu et al. 2016) and OS is one of the contributing factors of dysfunctional inflammatory responses in lactating dairy cows, more so during the periparturient period (Sordillo and Aitken 2009; Sordillo and Mavangira 2014). Dairy cattle are more susceptible to diseases (both severity and incidence are greater) during the periparturient period because of impaired immune mechanisms and inflammatory responses (Sordillo et al. 2011) caused by negative energy balance and changes in nutrient metabolism. Heat stress leads to negative energy balance due to reduced feed intake (Rhoads et al. 2009) and OS due to decreased antioxidant concentrations in dairy cattle during the transition period (Bernabucci et al. 2005). In transition dairy cows, OS may be the cause or effect of the decreased serum antioxidant levels, such as vitamin E (Vit E) and vitamin A (Vit A), reported earlier (Lean et al. 2013). Moreover, redistribution of blood supply away from visceral organs to extremities to increase heat dissipation from the body on exposure to HS leads to hypoxia in the gastrointestinal tract (GIT) (Cronje 2005; Lambert 2009) which triggers OS leading to oxidative damage of GIT epithelial cells causing leaky gut, which in turn can cause undesired inflammatory response (Liu et al. 2016). Inflammation is an important aspect of the immune response and refers to the various biological processes recruited by the body, in particular vascular tissue, to defend against invading pathogens or harmful stimuli (Chen et al. 2018a). However, immune responses may be impaired due to excessive or chronic, inflammatory responses leading to excessive reactive oxygen species (ROS) production. Although some ROS production is required to achieve the normal functions of cells, the excessive ROS generation can overwhelm the antioxidant defenses of cells leading to oxidative damage of tissue as a result of OS (Sordillo and Aitken 2009). Thus, there is crosstalk between nutrient metabolism, inflammation, and OS (Sordillo and Mavangira 2014) which is exacerbated by HS and therefore, additional feeding of antioxidant has been demonstrated to improve the cellular immune function (Rimbach et al. 2002; Rooke et al. 2004), by maintaining plasma and tissue antioxidant concentrations and reducing OS (Zhang et al. 2020).
Oxidative stress is defined as the imbalance of ROS generation and cellular antioxidant defense which may lead to oxidative damage of cellular DNA, proteins, and lipids and may result in apoptosis (Lykkesfeldt and Svendsen 2007). Oxidants are compounds capable of oxidizing target molecules and include free radicals (HO., NO., O2−.) or the highly reactive molecules hydrogen peroxide, peroxynitrite, and hypochlorus acid (Mates et al. 1999; Chauhan et al. 2014c). In general, natural antioxidant system counteracts the generation of free radicals or ROS under physiological conditions (Miller et al. 1993; Castillo et al. 2001). As mentioned previously, HS has been implicated in increased or induced OS (Bernabucci et al. 2002; Mujahid et al. 2005; Sivakumar et al. 2010) possibly leading to mitochondrial oxidative damage, as the mitochondria are generally accepted as the largest source of ROS (Rhoads et al. 2013). Whenever the equilibrium between oxidants and antioxidants is disturbed, progressive oxidation of biological molecules (proteins, lipids, DNA, etc.) occurs and OS is established and this may affect the health status directly via lipid peroxidation and indirectly via physiological perturbations and alteration of metabolic pathways or both (Miller et al. 1993; Davies 2000). The double bonds in polyunsaturated fatty acids (PUFA) are very susceptible to oxidative damage caused by free radicals and may lead to chain reaction of lipid peroxidation (Williams 2000). In lactating dairy cows, the effects of OS are more important as lactation itself presents a big challenge to maintain homeostasis (Bell 1995; Castillo et al. 2006). Dairy cows have been reported to have higher OS during the periparturient period than advanced pregnant cows and this could increase the susceptibility of transition cows to production diseases such as mastitis and retained placenta (Sharma et al. 2011).
In small ruminants such as goats, the hot season has more pronounced effects on the oxidative status of lactating animals than nutritional factors, for example, plasma ROS concentrations and levels of antioxidant enzymes (superoxide dismutase and glutathione peroxidase were increased during the hot season compared to spring (Di Trana et al. 2006)). However, OS in dairy goats can also be affected by plane of nutrition during the peripartum period (Celi et al. 2010). In sheep, OS and oxidative modification of lipids have been implicated in pathogenesis of pregnancy toxemia (Al-Qudah 2011) and there is a reduced total antioxidant status in pregnant animals (Rezapour and Taghinejad-Roudbaneh 2011). The production of free radicals is directly related to energy metabolism and decreased aerobic metabolism would mean reduced mitochondrial generation of oxygen molecules and reduced ROS generation (Jamme et al. 1995). As mentioned before, ROS in low concentrations is required to achieve normal cellular functions, including cell differentiation, cell immunity and defense mechanism, cell signaling, protein phosphorylation, and apoptosis (Miller et al. 1993; Agarwal et al. 2005). In contrast, high concentrations of ROS overwhelms the antioxidant system resulting in OS (Mates et al. 1999); therefore antioxidant, supplementation is required to maintain normal cellular function and animal health.
Antioxidant supplementation and immune responses
Antioxidants are the compounds that can scavenge free radicals and thus prevent/reduce oxidative damage (Halliwell 2007). Superoxide dismutase, glutathione peroxidase, thioredoxin reductase, and catalase are some of the most important high molecular weight antioxidant enzymes which play important roles to maintain cellular oxidant and antioxidant functions (Lykkesfeldt and Svendsen 2007). On the other hand, nutrients such as Vit E, Vit C, glutathione, beta-carotene, and selenium (Se) are low molecular weight non-enzymatic antioxidants which can either directly scavenge the free radicals or are integral component of antioxidant enzymes. Vitamin E plays the most important role in maintaining the cellular membrane integrity by preventing chain reaction and lipid oxidation, as it is lipid soluble and present in the membranes (Watson 1998). In addition, Vit E is essential for various productive (animal growth) and reproductive functions, and prevention of various diseases (McDowell et al. 1996). It plays a very important role in chronic diseases by reduction of ROS and reactive nitrogen species (RNS), generated as byproducts of normal aerobic metabolism, specifically formed during respiratory, phagocytic, and microsomal cytochrome P450 metabolism (Rooke et al. 2004). Immune cells are more likely to suffer oxidative damage as they produce large amount of ROS (respiratory burst) for phagocytising and killing pathogens. Furthermore, higher PUFA content of plasma membranes of immune cells makes them more sensitive to oxidative damage. Under normal physiological conditions, there is a homeostatic balance between oxidants and antioxidants. However, increased metabolic reactions in response to increased physiological demand or environmental stressors lead to excessive generation and accumulation of free radicals disturbing redox balance resulting in OS and increased susceptibility to diseases (Bernabucci et al. 2005; Castillo et al. 2005; Sordillo and Aitken 2009).
Given the fact that HS affects farm animal health because of the increased OS (Mitchell and Russo 1983; Bernabucci et al. 2002), supplementation of antioxidants to control OS holds promise for improving animal health during HS. Vitamin E and Se supplementation have been well studied and the beneficial effects of the combined supplementation of the two are well known. These beneficial effects are explained by the synergistic action of Vit E and Se to reduce lipid oxidation and prevent chain reaction of lipid peroxidation (Miller et al. 1993). Selenium plays an important role in antioxidant functions of glutathione peroxidase (GSH-Px) by participating in the oxidant antioxidant reaction catalyzed by GSH-PX (Rotruck et al. 1973) and protects against oxidative damage by reducing lipid hydroperoxides and H2O2 to water (Hefnawy and Tortora-Perez 2010). Se and Vit E thus play a major role to prevent oxidative damage by neutralizing ROS and maintain integrity of biological membranes. The combined supplementation of Vit E and Se has been reported to reduce the incidence of various production diseases such as endometritis, retained fetal membranes, and improved reproduction in various investigations in dairy cows (Hogan et al. 1993; Miller et al. 1993; Pavlata et al. 2004; Brozos et al. 2009; Moeini et al. 2009). Pre-partum (before calving) supplementation of Se (5mg/100 kg of body weight) and dl-alpha-tocopheryl acetate (25 IU/100 kg of body weight) improved colostrum as well as milk production; however, no additional effects of the improved Se status on the passive immunity and growth of calves were found (Lacetera et al. 1996). Conversely, pre-partum administration of Vit E (5 mg/kg body weight) and Se (0.1 mg/kg body weight) was reported to increase the activity of GSH-Px (139.5 vs. 86.3 U/ml of PCV) and concentration of blood neutrophils in subsequent lactation in dairy sheep (Morgante et al. 1999). Similarly, pre-partum (3 weeks before calving) administration of 1100 IU of Vit E (dl-alpha-tocopherol acetate) and Se (30 mg of sodium selenite) was reported to improve the oxidative status of crossbred dairy cattle and reduce the erythrocyte peroxide levels (Gupta et al. 2005). Earlier studies by Smith et al. (1997) had demonstrated the increased concentration of alpha-tocopherol acetate in the polymorphonucleocytes (PMN) following supplementation of Vit E and Se during transition period which helped to maintain the bactericidal action of PMN around parturition. Additionally, Vit E and Se supplementation have also been reported to reduce the incidence of reproductive disorders such as still births (dead calves carried to full term or calf mortalities within 24 h of birth) in dairy cows (Waller et al. 2007) and reduced OS indices in buffaloes affected by dystocia (difficulty in calving requiring assistance to deliver the calf) (Sathya et al. 2007).
In HS, dietary Vit E feeding has resulted in improved immune function in poultry (Niu et al. 2009b). Our laboratory has recently investigated Vit E and Se supplementation in various farm animals exposed to HS, and we found that dietary Se (1.0 ppm) and Vit E (200 IU kg−1) greater than those usually recommended for pigs reduced intestinal leakiness caused by HS-induced oxidative damage (Liu et al. 2016). Similarly, in sheep, inclusion of dietary Vit E and Se above recommended levels modulated the longissimus muscle expression of HSP, pro-inflammatory cytokine, and NF-ĸB transcription factor, protecting against HS and reducing OS (Chauhan et al. 2014a). Se supplementation alone was also reported to improve the immune functions in broilers (Niu et al. 2009a) and quails (Sahin et al. 2008) when exposed to HS. Another study by Liao et al. (2012) in broiler chicks reported that dietary supplementation of Se (0.30 mg/kg) improved immune responses and lowered mortality compared to chicks fed diets with low Se (0.027 mg of Se/kg). Research in the last two decades has clearly elucidated the importance and beneficial effects of Se supplementation for immunomodulation (Finch and Turner 1996; Spears and Weiss 2008) and immune functions (Politis et al. 1995; Rooke et al. 2004; Spears and Weiss 2008). For example, supplementation of Se in sheep increased blood and colostrum Se concentrations and resulted in greater Se levels in lamb blood and liver along with enhanced absorption of IgG. Similarly, feeding forage enriched with Se in beef cattle improved the antibody titers of mature cows, and growth rates of calves along with blood Se. Hall and Gow (2013) fed recently weaned beef calves an alfalfa hay, harvested from sodium selenate (0,22.5, 45, or 89.9 g Se/ha) fertilized fields, based diet for 7 weeks and found that the calves fed highest vs the lowest level of alfalfa (enriched with Se) had higher antibody titters following immunization with J-5 Escherichia coli bacterin. Additionally, calves previously fed highest Se-enriched alfalfa hay showed higher slaughter weights and reported less mortality in calves. These results are quite encouraging and suggest that immune responses and growth performance of ruminants fed fodder crops grown in Se deficit soils can be improved by Se enrichment of such fodder crops. The concentration of Se used in these protective studies has been often higher than allowed in some jurisdictions (e.g., EU) and so efforts have been directed at finding plant-derived antioxidants that may provide protection against HS. In this context, we have recently shown that a sugarcane molasses–derived extract that is rich in polyphenols can alleviate OS and increase growth rate of broiler chickens suffering OS (Shakeri et al. 2019). Another plant-derived compound that has among antioxidant properties, as well as other actions, is betaine derived from sugar beet.
Betaine supplementation and farm animal health
Betaine is a glycine derivative and protects cells against osmotic and temperature stresses. Betaine functions as an osmolyte and methyl donor and has been used as a feed additive in livestock to improve the performance (Eklund et al. 2005). Betaine lowers the maintenance requirements and metabolic heat production and recently has been used as an effective nutritional strategy to mitigate HS as Cottrell et al. (2015). In a very recent study, we (Dunshea et al. 2019) found that feeding 15 g/day natural betaine in the concentrate ration for approximately 3 weeks improved the milk production in dairy cattle grazing summer pastures in Australia. Similarly, another very recent study in dairy cattle in China demonstrated that betaine supplementation (15g/day per cow) improved the production performance, rumen health, and antioxidant profile of dairy cows exposed to HS (Shah et al. 2020). An earlier study from our group in feedlot cattle showed energy-sparing effects of betaine feeding especially in animals without access to shade during summer (DiGiacomo et al. 2014). Similarly, dietary betaine supplementation at 2 g betaine/day improves physiological responses of ewes exposed to HS (DiGiacomo et al. 2016). A study in pigs also demonstrated that betaine supplementation (0.125%) mitigated the increase in intestinal permeability following HS as demonstrated by a reduced permeability in the ileum (Gabler et al. 2013). More recently, our group investigated the impacts of betaine supplementation in finishing Ross-308 broilers and demonstrated that supplementation of betaine (0.1%) mitigated some of the negative effects of HS and improved intestinal barrier function and gut health (Shakeri et al. 2020a). Thus, several studies conducted by our lab and few others have consistently demonstrated the beneficial effects of betaine supplementation in animals exposed to heat stress. While the improved rumen and gut health, and production performance of animals have been demonstrated in most of these studies which indirectly reflects the improved animal health, immune responses have not been the focus of these studies and therefore, further studies investigating the beneficial effects of betaine supplementation on immune response and animal health during HS are needed.
Summary and conclusion
Climate change and global warming are likely to exacerbate the problem of HS as heat waves are getting hotter, longer, and more frequent. A range of challenges have been predicted to occur for the livestock industries that will be affected by these changes in environmental temperature, including the increased incidence of diseases and animal mortality as the immune responses in farm animals are negatively influenced by HS. This may cause serious economic repercussions for the global livestock industries. Both cell-mediated and humoral immune responses are affected during exposure to HS in farm animals. However, there are both species and breed variations for the adverse effects of HS on immune response in farm animals. Recent research has suggested that potential biomarkers such as some TLRs and cytokines can be used to measure HS-associated changes in the immune system in livestock. Furthermore, TLR pathways, NF-κB pathway, interferon signaling, stress induction of HSP regulation, and MAPK signaling pathways have been identified as key pathways associated with compromised immune responses in farm animals during HS. Emerging data from our groups suggest that HS leads to OS in farm animals, which is previously well known to be associated with various metabolic diseases, especially in animals exposed to hot conditions during the transition period. Nutritional interventions, supplementing antioxidants reducing oxidative stress and betaine reducing energy requirements, to manage HS are beneficial. However, additional research is needed to elucidate the impacts of such nutritional interventions on immune responses in farm animals.
References
Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR (2006) Anti-inflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203(8):1883–1889. https://doi.org/10.1084/jem.20060336
Agarwal A, Gupta S, Sharma RK (2005) Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3:28. https://doi.org/10.1186/1477-7827-3-28
Aggarwal A, Upadhyay R (2012) Heat stress and immune function. Heat Stress Anim Prod:113–136. https://doi.org/10.1007/978-81-322-0879-2_5
Ahmed BMS (2017) Elevated in utero temperature: a suppressor of fetal development ruminant fitness?. Ph.D. Dissertation. University of Florida
Al-Qudah KM (2011) Oxidant and antioxidant profile of hyperketonemic ewes affected by pregnancy toxemia. Vet Clin Pathol 40(1):60–65. https://doi.org/10.1111/j.1939-165X.2011.00284.x
Archana PR, Aleena J, Pragna P, Vidya MK, Niyas AP, Bagath M, Krishnan G, Manimaran A, Beena V, Kurien EK, Sejian V (2017) Role of heat shock proteins in livestock adaptation to heat stress. J Dairy Vet Anim Res 5(1):00127. https://doi.org/10.15406/jdvar.2017.05.00127
Armstrong DV (1994) Heat Stress Interaction with Shade and Cooling. J Dairy Sci 77(7):2044–2050. https://doi.org/10.3168/jds.S0022-0302(94)77149-6
Bagath M, Krishnan G, Devaraj C, Rashamol VP, Pragna P, Lees AM, Sejian V (2019) The impact of heat stress on the immune system in dairy cattle: a review. Res Vet Sci 126:94–102. https://doi.org/10.1016/j.rvsc.2019.08.011
Bartlett JR, Smith MO (2003) Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci 82(10):1580–1588. https://doi.org/10.1093/ps/82.10.1580
Baumgard LH, Rhoads JRP (2013) Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci 1(1):311–337. https://doi.org/10.1146/annurev-animal-031412-103644
Beede DK, Collier RJ (1986) Potential nutritional strategies for intensively managed cattle during thermal-stress. J Anim Sci 62(2):543–554
Bell AW (1995) Regulation of organic nutrient metabolism during transition form late pregnancy to early lactation. J Anim Sci 73(9):2804–2819
Berman A (2005) Estimates of heat stress relief needs for Holstein dairy cows. J Anim Sci 83(6):1377–1384
Berman A (2011) Invited review: Are adaptations present to support dairy cattle productivity in warm climates? J Dairy Sci 94(5):2147–2158. https://doi.org/10.3168/jds.2010-3962
Bernabucci U, Ronchi B, Lacetera N, Nardone A (2002) Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci 85(9):2173–2179
Bernabucci U, Ronchi B, Lacetera N, Nardone A (2005) Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J Dairy Sci 88(6):2017–2026
Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A (2010) Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 4(7):1167–1183. https://doi.org/10.1017/s175173111000090x
Bernabucci U, Biffani S, Buggiotti L, Vitali A, Lacetera N, Nardone A (2014) The effects of heat stress in Italian Holstein dairy cattle. J Dairy Sci 97(1):471–486. https://doi.org/10.3168/jds.2013-6611
Bharati J, Dangi SS, Mishra SR, Chouhan VS, Verma V, Shankar O, Bharti MK, Paul A, Mahato DK, Rajesh G, Singh G (2017) Expression analysis of toll like receptors and interleukins in Tharparkar cattle during acclimation to heat stress exposure. J Therm Biol 65:48–56. https://doi.org/10.1016/j.jtherbio.2017.02.002
Blackshaw JK, Blackshaw AW (1994) Heat-stress in cattle and the effect of shade on production and behavior. Aus J Exp Agricul 34(2):285–295
Brozos CN, Kiossis E, Georgiadis MP, Piperelis S, Boscos C (2009) The effect of chloride ammonium, vitamin E and Se supplementation throughout the dry period on the prevention of retained fetal membranes, reproductive performance and milk yield of dairy cows. Livest Sci 124(1-3):210–215. https://doi.org/10.1016/j.livsci.2009.01.018
Buffington DE, Collier RJ, Canton GH (1983) Shade management-systems to reduce heat-stress for dairy-cows in hot, humid climates. T ASABE 26(6):1798–1802
Calif. Dep. Food Agric. Hot topics affecting California Agriculture (2006) . An update from Sec. Kawamura. Sacramento: Calif Dep Food Agric (2006). http://www.cdfa.ca.gov/exec/Public_Affairs/pdf/AGOnAg080306.pdf.
Caroprese M, Ciliberti MG, Annicchiarico G, Albenzio M, Muscio A, Sevi A (2014) Hypothalamic-pituitary-adrenal axis activation and immune regulation in heat-stressed sheep after supplementation with polyunsaturated fatty acids. J Dairy Sci 97(7):4247–4258. https://doi.org/10.3168/jds.2013-7696
Caroprese M, Ciliberti MG, De Palo P, Santillo A, Sevi A, Albenzio M (2018) Glucocorticoid effects on sheep peripheral blood mononuclear cell proliferation and cytokine production under in vitro hyperthermia. J Dairy Sci 101(9):8544–8551. https://doi.org/10.3168/jds.2018-14471
Castillo C, Hernandez J, Lopez-Alonso M, Miranda M, Benedito JL (2001) A different point of view of glutathione peroxidase: its relationship to the metabolic changes associate with nutritional management is Assaf ovine breed. Arch Tierzucht 44(3):305–312
Castillo C, Hernandez J, Bravo A, Lopez-Alonso M, Pereira V, Benedito JL (2005) Oxidative status during late pregnancy and early lactation in dairy cows. Vet J 169(2):286–292. https://doi.org/10.1016/j.tvjl.2004.02.001
Castillo C, Hernandez J, Valverde I, Pereira V, Sotillo J, Alonso ML (2006) Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res Vet Sci 80(2):133–139. https://doi.org/10.1016/j.rvsc.2005.06.003
Celi P, Di Trana A, Claps S (2010) Effects of plane of nutrition on oxidative stress in goats during the peripartum period. Vet J 184:95–99. https://doi.org/10.1016/j.tvjl.2009.01.014
Chauhan SS, Celi P, Fahri FT, Leury BJ, Dunshea FR (2014a) Dietary antioxidants at supranutritional doses modulate skeletal muscle heat shock protein and inflammatory gene expression in sheep exposed to heat stress. J Anim Sci 92(11):4897–4908. https://doi.org/10.2527/jas.2014-8047
Chauhan SS, Celi P, Leury BJ, Clarke IJ, Dunshea FR (2014b) Dietary antioxidants at supranutritional doses improve oxidative status and reduce the negative effects of heat stress in sheep. J Anim Sci 92(8):3364–3374. https://doi.org/10.2527/jas.2014-7714
Chauhan SS, Celi P, Ponnampalam EN, Leury BJ, Liu F, Dunshea FR (2014c) Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: role of vitamin E and selenium. Anim Prod Sci 54(10):1525–1536. https://doi.org/10.1071/AN14334
Chauhan SS, Ponnampalam EN, Celi P, Hopkins DL, Leury BJ, Dunshea FR (2016) High dietary vitamin E and selenium improves feed intake and weight gain of finisher lambs and maintains redox homeostasis under hot conditions. Small Rumin Res 137:17–23. https://doi.org/10.1016/j.smallrumres.2016.02.011
Chen H, Wu Y, Zhang Y, Jin L, Luo L, Xue B, Lu C, Zhang X, Yin Z (2006) Hsp70 inhibits lipopolysaccharide-induced NF-κB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett 580(13):3145–3152. https://doi.org/10.1016/j.febslet.2006.04.066
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2018a) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204–7218. https://doi.org/10.18632/oncotarget.23208
Chen S, Wang J, Peng D, Li G, Chen J, Gu X (2018b) Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci Rep 8(1):1–11
Chu GM, Song YM (2018) Growth performance, blood characteristics and immune responses of fattening pigs in different seasons. Asian J Anim Vet Adv 8(5):691–702. https://doi.org/10.3923/ajava.2013.691.702
Colaco CA, Bailey CR, Walker KB, Keeble J (2013) Heat shock proteins: stimulators of innate and acquired immunity. Biomed Res Int 2013:1–11. https://doi.org/10.1155/2013/461230
Collier RJ, Dahl GE, VanBaale MJ (2006) Major advances associated with environmental effects on dairy cattle. J Dairy Sci 89(4):1244–1253
Collier RJ, Collier JL, Rhoads RP, Baumgard LH (2008) Invited review: genes involved in the bovine heat stress response. J Dairy Sci 91(2):445–454. https://doi.org/10.3168/jds.2007-0540
Collier RJ, Eley RM, Sharma AK, Pereira RM, Buffington DE (1981) Smanagement in sub-tropical environment for milk-yield and composition in holstein and jersey cows. J Dairy Sci 64(5):844–849
Correa-Calderon A, Armstrong D, Ray D, DeNise S, Enns M, Howison C (2004) Thermoregulatory responses of Holstein and Brown Swiss Heat-Stressed dairy cows to two different cooling systems. Int J Biometeorol 48(3):142–148. https://doi.org/10.1007/s00484-003-0194-y
Cottrell JJ, Liu F, Hung AT, DiGiacomo K, Chauhan SS, Leury BJ, Furness JB, Celi P, Dunshea FR (2015) Nutritional strategies to alleviate heat stress in pigs. Anim Prod Sci 55(12):1391–1402. https://doi.org/10.1071/an15255
Cronje P (2005) Heat stress in livestock - the role of the gut in its aetiology and a potential role for betaine in its alleviation. Recent Adv Anim Nutrin Aust 15:107–122
Cui Y, Hao Y, Li J, Bao W, Li G, Gao Y, Gu X (2016) Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: a proteomic approach. Int J Mol Sci 17(5):393. https://doi.org/10.3390/ijms17050393
Dado-Senn B, Skibiel AL, Fabris TF, Zhang Y, Dahl GE, Peñagaricano F, Laporta J (2018) RNA-Seq reveals novel genes and pathways involved in bovine mammary involution during the dry period and under environmental heat stress. Sci Rep 8(1):11096. https://doi.org/10.1038/s41598-018-29420-8
Dahl GE, Collier RJ (2017) Heat stress effect on immune function in dairy cattle. Cornell Nutrition Conference for Feed Manufacturers. DOI: http://ansci.cals.cornell.edu/extension-outreach/adult-extension/dairy-management/order-proceedings-resources
Dahl GE, Tao S, Laporta J (2020) Heat stress impacts immune status in cows across the life cycle. Front Vet Sci 7:116. https://doi.org/10.3389/fvets.2020.00116
Davies KJA (2000) Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 50(4-5):279–289
Di Trana A, Celi P, Claps S, Fedele V, Rubino R (2006) The effect of hot season and nutrition on the oxidative status and metabolic profile in dairy goats during mid lactation. Anim Sci 82(5):717–722. https://doi.org/10.1079/as200672
DiGiacomo K, Warner RD, Leury BJ, Gaughan JB, Dunshea FR (2014) Dietary betaine supplementation has energy- sparing effects in feedlot cattle during summer, particularly in those without access to shade. Anim Prod Sci 54(4):450–458. https://doi.org/10.1071/an13418
DiGiacomo K, Simpson S, Leury BJ, Dunshea FR (2016) Dietary betaine impacts the physiological responses to moderate heat conditions in a dose dependent manner in sheep. Anim 6(9):51. https://doi.org/10.3390/ani6090051
Do Amaral BC, Connor EE, Tao S, Hayen J, Bubolz J, Dahl GE (2009) Heat-stress abatement during the dry period: does cooling improve transition into lactation? J Dairy Sci 92(12):5988–5999. https://doi.org/10.3168/jds.2009-234
Drovers Cattle Netw. Heat wave kills as many as 4,000 cattle last week in Iowa. (2011). http://www.cattlenetwork.com/cattle-resources/hot-topics/Heat-wave-kills-as-many-as-4000-cattle-last-week-inIowa-126763608.html
Dunshea FR, Leury BJ, Fahri F, DiGiacomo K, Hung A, Chauhan S, Clarke IJ, Collier R, Little S, Baumgard L, Gaughan JB (2013) Amelioration of thermal stress impacts in dairy cows. Anim Prod Sci 53(9):965–975. https://doi.org/10.1071/an12384
Dunshea FR, Oluboyede K, DiGiacomo K, Leury BJ, Cottrell JJ (2019) Betaine improves milk yield in grazing dairy cows supplemented with concentrates at high temperatures. Anim 9(2):57
Eklund M, Bauer E, Wamatu J, Mosenthin R (2005) Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev 18(1):31–48. https://doi.org/10.1079/NRR200493
Elvinger F, Natzke RP, Hansen PJ (1992) Interactions of heat stress and bovine somatotropin affecting physiology and immunology of lactating cows. J Dairy Sci 75(2):449–462. https://doi.org/10.3168/jds.S0022-0302(92)77781-9
Finch JM, Turner RJ (1996) Effects of selenium and vitamin E on the immune responses of domestic animals. Res Vet Sci 60(2):97–106
Gabler NK, Frouel S, Awati A, Owusu-Asiedu A, Amerah AM, Patridge GG, Dunshea FR (2013) Betaine mitigates intestinal permeability in growing pigs induced by heat stress. In ‘Manipulating pig production XIV’. In: Pluske JR, Pluske JM (eds) . (Australasian Pig Science Association (Inc.), Melbourne, p 85
Gabler NK, Koltes D, Schaumberger S, Murugesan GR, Reisinger N (2018) Diurnal heat stress reduces pig intestinal integrity and increases endotoxin translocation. Translat Anim Sci 2:1–0. https://doi.org/10.1093/tas/txx003
Ganesan S, Reynolds C, Hollinger K, Pearce SC, Gabler NK, Baumgard LH, Rhoads RP, Selsby JT (2018) Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am J Phys Regul Integr Comp Phys 310(11):R1288–R1296. https://doi.org/10.1152/ajpregu.00494.2015
Gomes da Silva R, Paranhos da Costa MJR, Silva Sobrinho AG (1992) Influence of hot environments on some blood variables of sheep. Int J Biometeorol 36(4):223–225. https://doi.org/10.1007/BF02726402
Gonzalez-Rivas PA, Chauhan SS, Ha M, Fegan N, Dunshea FR, Warner RD (2020) Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci 162:108025. https://doi.org/10.1016/j.meatsci.2019.108025
Gross WB (1992) Effect of short-term exposure of chickens to corticosterone on resistance to challenge exposure with Escherichia coli and antibody response to sheep erythrocytes. Am J Vet Res 53(3):291–293
Gupta S, Gupta HK, Soni J (2005) Effect of Vitamin E and selenium supplementation on concentrations of plasma cortisol and erythrocyte lipid peroxides and the incidence of retained fetal membranes in crossbred dairy cattle. Theriogenology 64(6):1273–1286. https://doi.org/10.1016/j.theriogenology.2005.03.008
Hall RA, Gow NA (2013) Mannosylation in C andida albicans: role in cell wall function and immune recognition. Mol Microbial 90(6):1147–1161
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150
Hefnawy AE, Tortora-Perez JL (2010) The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res 89(2-3):185–192. https://doi.org/10.1016/j.smallrumres.2009.12.042
Hemsworth PH, Barnett JL, Beveridge L, Matthews LR (1995) The welfare of extensively managed dairy-cattle - A Review. Appl Anim Behav Sci 42(3):161–182
Hirakawa R, Nurjanah S, Furukawa K, Murai A, Kikusato M, Nochi T, Toyomizu M (2020) Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front Vet Sci 7:46. https://doi.org/10.3389/fvets.2020.00046
Hogan JS, Weiss WP, Smith KL (1993) Role of vitamin-e and selenium in host-defense against mastitis. J Dairy Sci 76(9):2795–2803
Huo C, Xiao C, She R, Liu T, Tian J, Dong H, Tian H, Hu Y (2019) Chronic heat stress negatively affects the immune functions of both spleens and intestinal mucosal system in pigs through the inhibition of apoptosis. Microb Pathog 136:103672. https://doi.org/10.1016/j.micpath.2019.103672
IPCC (2014) Summary for policymakers. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schlömer S, von Stechow C, Zwickel T, Minx JC (eds) Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, New York
Jamme I, Petit E, Divoux D, Gerbi A, Maixent JM, Nouvelot A (1995) Modulation of mouse cerebral Na+,K+-ATPase activity by oxygen free radicals. Neuroreport 7(1):333–337
Joy A, Dunshea FR, Leury BJ, Clarke IJ, DiGiacomo K, Chauhan SS (2020) Resilience of small ruminants to climate change and increased environmental temperature: a review. Anim 10:867
Ju X-H, Yong Y-H, Xu H-J, An L-L, Xu Y (2011) Impacts of heat stress on baseline immune measures and a subset of T cells in Bama miniature pigs. Livest Sci 135(2):289–292. https://doi.org/10.1016/j.livsci.2010.07.009
Ju XH, Xu HJ, Yong YH, An LL, Jiao PR, Liao M (2014) Heat stress upregulation of Toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: an in vivo and in vitro study. Anim 8(9):1462–1468. https://doi.org/10.1017/S1751731114001268
Kapila N, Sharma A, Kishore A, Sodhi M, Tripathi PK, Mohanty AK, Mukesh M (2016) Impact of heat stress on cellular and transcriptional adaptation of mammary epithelial cells in riverine buffalo (Bubalus bubalis). PLoS One 11:e0157237. https://doi.org/10.1371/journal.pone.0157237
Kendall PE, Nielsen PP, Webster JR, Verkerk GA, Littlejohn RP, Matthews LR (2006) The effects of providing shade to lactating dairy cows in a temperate climate. Livest Sci 103(1–2):148–157. https://doi.org/10.1016/j.livsci.2006.02.004
Key N, Sneeringer S (2014) Potential effects of climate change on productivity of U.S. dairies. Am J Agric Econ 96:1136–1156
Khongdee S, Sripoon S, Chousawai S, Hinch G, Chaiyabutr N, Markvichitr K, Vajrabukka C (2010) The effect of modified roofing on the milk yield and reproductive performance of heat-stressed dairy cows under hot-humid conditions. Anim Sci J 81(5):606–611. https://doi.org/10.1111/j.1740-0929.2010.00771.x
Knapp DM, Grummer RR (1991) Response of lactating dairy cows to fat supplementation during heat stress. J Dairy Sci 74(8):2573–2579. https://doi.org/10.3168/jds.S0022-0302(91)78435-X
Koch F, Thom U, Albrecht E, Weikard R, Nolte W, Kuhla B, Kuehn C (2019) Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc Natl Acad Sci U S A 116(21):10333–10338. https://doi.org/10.1073/pnas.1820130116
Lacetera N (2018) Impact of climate change on animal health and welfare. Anim Front 9(1):26–31. https://doi.org/10.1093/af/vfy030
Lacetera N, Bernabucci U, Ronchi B, Nardone A (1996) Effects of selenium and vitamin E administration during a late stage of pregnancy on colostrum and milk production in dairy cows, and on passive immunity and growth of their offspring. Am J Vet Res 57:1776–1780
Lacetera N, Bernabucci U, Scalia D, Basiricò L, Morera P, Nardone A (2006) Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J Dairy Sci 89(12):4606–4612. https://doi.org/10.3168/jds.S0022-0302(06)72510-3
Lambert GP (2009) Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 87(14):E101–E108. https://doi.org/10.2527/jas.2008-1339
Lean IJ, Van Saun R, DeGaris PJ (2013) Mineral and antioxidant management of transition dairy cows. Vet Clin North Am Food Anim Pract 29(2):367–36+. https://doi.org/10.1016/j.cvfa.2013.03.004
Liao X, Lu L, Li S, Liu S, Zhang L, Wang G, Li A, Luo X (2012) Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biol Trace Elem Res 150(1-3):158–165. https://doi.org/10.1007/s12011-012-9517-3
Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U (2016) Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp Physiol 101(7):801–810. https://doi.org/10.1113/EP085746
Lu Z, Chu M, Li Q, Jin M, Fei X, Ma L, Zhang L, Wei C (2019) Transcriptomic analysis provides novel insights into heat stress responses in sheep. Anim 9:387
Lykkesfeldt J, Svendsen O (2007) Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J 173(3):502–511. https://doi.org/10.1016/j.tvjl.2006.06.005
Madhusoodan AP, Sejian V, Afsal A, Bagath M, Krishnan G, Savitha ST, Rashamol VP, Devaraj C, Bhatta R (2019) Differential expression patterns of candidate genes pertaining to productive and immune functions in hepatic tissue of heat-stressed Salem Black goats. Biol Rhythm Res 2:1–2. https://doi.org/10.1080/09291016.2019.1607213
Mashaly MM, Hendricks GL 3rd, Kalama MA, Gehad AE, Abbas AO, Patterson PH (2004) Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 83(6):889–894. https://doi.org/10.1093/ps/83.6.889.10.3390/ani9060387
Mates JM, Perez-Gomez C, De Castro IN (1999) Antioxidant enzymes and human diseases. Clin Biochem 32(8):595–603
Mayorga EJ, Ross JW, Keating AF, Rhoads RP, Baumgard LH (2020) Biology of heat stress; the nexus between intestinal hyperpermeability and swine reproduction. Theriogenology 154:73–83. https://doi.org/10.1016/j.theriogenology.2020.05.023
McDaniel CD, Hood JE, Parker HM (2004) An attempt at alleviating heat stress infertility in male broiler breeder chickens with dietary ascorbic acid. Int J Poult Sci 3(9):593–602. https://doi.org/10.3923/ijps.2004.593.602
McDowell LR, Williams SN, Hidiroglou N, Njeru CA, Hill GM, Ochoa L, Wilkinson NS (1996) Vitamin E supplementation for the ruminant. Anim Feed Sci Technol 60(3-4):273–296
Miller JK, Brzezinska-Slebodzinska E, Madsen FC (1993) Oxidative stress, antioxidants, and animal function. J Dairy Sci 76(9):2812–2823. https://doi.org/10.3168/jds.S0022-0302(93)77620-1
Mishra A, Hooda O, Singh G, Meur S (2011) Influence of induced heat stress on HSP70 in buffalo lymphocytes. J Anim Physiol Anim Nutr 95(4):540–544
Mitchell JB, Russo A (1983) Thiols, thiol depletion, and thermosensitivity. Radiat Res 95(3):471–485
MLA (Meat and Livestock Australia). Animal health survey of the Australian feedlot industry. (2010). file:///C:/Users/LENOVO/Downloads/P.PSH.0547_Final_Report.pdf
Moeini M, Karami H, Mikaeili E (2009) Effect of selenium and vitamin E supplementation during the late pregnancy on reproductive indices and milk production in heifers. Anim Reprod Sci 114(1-3):109–114
Morgante M, Beghelli D, Pauselli M, Dall’Ara P, Capuccella M, Ranucci S (1999) Effect of administration of vitamin E and selenium during the dry period on mammary health and milk cell counts in dairy ewes. J Dairy Sci 82(3):623–631
Morrow-Tesch JL, McGlone JJ, Salak-Johnson JL (1994) Heat and social stress effects on pig immune measures. J Anim Sci 72(10):2599–2609
Mujahid A, Yoshiki Y, Akiba Y, Toyomizu M (2005) Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult Sci 84(2):307–314
Mukherjee J, Pandita S, Huozha R, Ashutosh M (2011) In vitro immune competence of buffaloes (Bubalus bubalis) of different production potential: effect of heat stress and cortisol. Vet Med Inter DOI 2011:1–5. https://doi.org/10.4061/2011/860252
Niu Z, Liu F, Yan Q, Li L (2009a) Effects of different levels of selenium on growth performance and immunocompetence of broilers under heat stress. Arch Anim Nutr 63(1):56–65
Niu Z, Liu F, Yan Q, Li W (2009b) Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci 88(10):2101–2107
Ohtsu H, Yamazaki M, Abe H, Murakami H, Toyomizu M (2015) Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J Poult Sci 52:282–287
Osei-Amponsah R, Chauhan SS, Leury BJ, Cheng L, Cullen B, Clarke IJ, Dunshea FR (2019) Genetic selection for thermotolerance in ruminants. Animals (Basel) 9(11):948
Paul A, Dangi S, Gupta M, Singh J, Thakur N, Naskar S, Nanda P, Mohanty N, Das A, Bandopadhayay S (2015) Expression of TLR genes in Black Bengal goat (Capra hircus) during different seasons. Small Rumin Res 124:17–23
Pavlata L, Prasek J, Filipek J, Pechova A (2004) Influence of parenteral administration of selenium and vitamin E during pregnancy on selected metabolic parameters and colostrum quality in dairy cows at parturition. Vet Med 49:149–155
Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK (2013) Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8(8):e70215
Politis I, Hidiroglou M, Batra T, Gilmore J, Gorewit R, Scherf H (1995) Effects of vitamin E on immune function of dairy cows. Am J Vet Res 56(2):179–184
Quinteiro-Filho WM, Calefi AS, Cruz D, Aloia TPA, Zager A, Astolfi-Ferreira CS, Ferreira JP, Sharif S, Palermo-Neto J (2017) Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet Immunol Immunopathol 186:19–28
Rashamol V, Sejian V, Bagath M, Krishnan G, Beena V, Bhatta R (2019) Effect of heat stress on the quantitative expression patterns of different cytokine genes in Malabari goats. Int J Biometeorol 63(8):1005–1013
Renaudeau D, Collin A, Yahav S, De Basilio V, Gourdine J, Collier RJ (2012) Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Anim 6(5):707–728
Rezapour A, Taghinejad-Roudbaneh M (2011) Effects of food restriction on oxidative stress indices in Ghezel ewes. J Anim Vet Adv 10(8):980–986
Rhoads M, Rhoads R, VanBaale M, Collier RJ, Sanders S, Weber WJ, Crooker BA, Baumgard L (2009) Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J Dairy Sci 92(5):1986–1997
Rhoads RP, Baumgard LH, Suagee JK, Sanders SR (2013) Nutritional interventions to alleviate the negative consequences of heat stress. Adv Nutr 4(3):267–276
Rimbach G, Minihane AM, Majewicz J, Fischer A, Pallauf J, Virgli F, Weinberg PD (2002) Regulation of cell signalling by vitamin E. Proc Nutr Soc 61(4):415–425
Rooke J, Robinson J, Arthur J (2004) Effects of vitamin E and selenium on the performance and immune status of ewes and lambs. J Agric Sci 142(3):253–262
Rostagno MH (2020) Effects of heat stress on the gut health of poultry. J Anim Sci 98(4):skaa090
Rotruck JT, Pope AL, Ganther HE, Swanson A, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590
Safa S, Kargar S, Moghaddam GA, Ciliberti MG, Caroprese M (2019) Heat stress abatement during the postpartum period: effects on whole lactation milk yield, indicators of metabolic status, inflammatory cytokines, and biomarkers of the oxidative stress. J Anim Sci 97(1):122–132
Sahin N, Onderci M, Sahin K, Kucuk O (2008) Supplementation with organic or inorganic selenium in heat-distressed quail. Biol Trace Elem Res 122(3):229–237
Sathya A, Prabhakar S, Sangha S, Ghuman S (2007) Vitamin E and selenium supplementation reduces plasma cortisol and oxidative stress in dystocia-affected buffaloes. Vet Res Commun 31(7):809–818
Savitha S, Girish Kumar V, Amitha J, Sejian V, Bagath M, Krishnan G, Devaraj C (2019) Bhatta R (2019) Comparative assessment of thermo-tolerance between indigenous Osmanabadi and Salem black goat breeds based on expression patterns of different intracellular toll-like receptor genes during exposure to summer heat stress. Biol Rhythm Res 22:1–9. https://doi.org/10.1080/09291016.2019.1592350
Schütz K, Rogers A, Cox N, Webster J, Tucker C (2011) Dairy cattle prefer shade over sprinklers: Effects on behavior and physiology. J Dairy Sci 94(1):273–283
Schütz K, Cox N, Tucker C (2014) A field study of the behavioral and physiological effects of varying amounts of shade for lactating cows at pasture. J Dairy Sci 97(6):3599–3605
Shah AM, Ma J, Wang Z, Zou H, Hu R, Peng Q (2020) Betaine supplementation improves the production performance, rumen fermentation, and antioxidant profile of dairy cows in heat stress. Anim 10(4):634
Shakeri M, Cottrell JJ, Wilkinson S, Le HH, Suleria HA, Warner RD, Dunshea FR (2019) Growth performance and characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants 8(9):336
Shakeri M, Cottrell JJ, Wilkinson S, Zhao W, Le HH, McQuade R, Furness JB, Dunshea FR (2020a) Dietary betaine improves intestinal barrier function and ameliorates the impact of heat stress in multiple vital organs as measured by Evans blue dye in broiler chickens. Anim 10(1):38
Sharma N, Singh N, Singh O, Pandey V, Verma P (2011) Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Australas J Anim Sci 24(4):479–484
Sheikh AA, Aggarwal A, Aarif O (2016) Effect of in vitro zinc supplementation on HSPs expression and Interleukin 10 production in heat treated peripheral blood mononuclear cells of transition Sahiwal and Karan Fries cows. J Therm Biol 56:68–76
Sivakumar AVN, Singh G, Varshney VP (2010) Antioxidants Supplementation on Acid Base Balance during Heat Stress in Goats. Asian-Australasian J Anim Sci 23(11):1462–1468
Smith KL, Hogan J, Weiss W (1997) Dietary vitamin E and selenium affect mastitis and milk quality. J Anim Sci 75(6):1659–1665
Smith TR, Chapa A, Willard S, Herndon C, Williams RJ, Crouch J, Riley T, Pogue D (2006) Evaporative tunnel cooling of dairy cows in the southeast. II: Impact on lactation performance. J Dairy Sci 89(10):3915–3923
Sophia I, Sejian V, Bagath M, Bhatta R (2016) Quantitative expression of hepatic toll-like receptors 1–10 mRNA in Osmanabadi goats during different climatic stresses. Small Rumin Res 141:11–16
Sophia I, Sejian V, Bagath M, Bhatta R (2017) Influence of different environmental stresses on various spleen toll like receptor genes expression in Osmanabadi goats. Asian J Biol Sci 10(1):9–16. https://doi.org/10.3923/ajbs.2017.9.16
Sordillo LM, Aitken SL (2009) Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol 128(1-3):104–109
Sordillo L, Mavangira V (2014) The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim Prod Sci 54(9):1204–1214
Sordillo JE, Alwis UK, Hoffman E, Gold DR, Milton DK (2011) Home characteristics as predictors of bacterial and fungal microbial biomarkers in house dust. Environ Health Perspect 119(2):189–195
Spears JW, Weiss WP (2008) Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J 176(1):70–76
Srikanth K, Kwon A, Lee E, Chung H (2017) Characterization of genes and pathways that respond to heat stress in Holstein calves through transcriptome analysis. Cell Stress Chaperones 22(1):29–42
Steele M (2016) Does heat stress affect immune function in dairy cows? Vet Evidence 1(3). https://doi.org/10.18849/ve.v1i3.39
St-Pierre N, Cobanov B, Schnitkey G (2003) Economic losses from heat stress by US livestock industries. J Dairy Sci 86:E52–E77
Sun Y, Liu J, Ye G, Gan F, Hamid M, Liao S, Huang K (2018) Protective effects of zymosan on heat stress-induced immunosuppression and apoptosis in dairy cows and peripheral blood mononuclear cells. Cell Stress Chaperones 23(5):1069–1078
Tao S, Connor E, Bubolz J, Thompson I, Do Amaral B, Hayen M, Dahl G (2013) Effect of heat stress during the dry period on gene expression in mammary tissue and peripheral blood mononuclear cells. J Dairy Sci 96(1):378–383
Thaxton P, Sadler C, Glick B (1968) Immune response of chickens following heat exposure or injections with ACTH. Poult Sci 47(1):264–266
Thompson I, Tao S, Monteiro A, Jeong K, Dahl G (2014) Effect of cooling during the dry period on immune response after Streptococcus uberis intramammary infection challenge of dairy cows. J Dairy Sci 97(12):7426–7436
Tirawattanawanich C, Chantakru S, Nimitsantiwong W, Tongyai S (2011) The effects of tropical environmental conditions on the stress and immune responses of commercial broilers, Thai indigenous chickens, and crossbred chickens. J Appl Poult Res 20(4):409–420
Tirumurugaan K, Dhanasekaran S, Raj GD, Raja A, Kumanan K, Ramaswamy V (2010) Differential expression of toll-like receptor mRNA in selected tissues of goat (Capra hircus). Vet Immunol Immunopathol 133(2-4):296–301
Tucker CB, Rogers AR, Schutz KE (2008) Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasturebased system. Appl Anim Behav Sci 109(2–4):141–154. https://doi.org/10.1016/j.applanim.2007.03.015
Vandana G, Bagath M, Sejian V, Krishnan G, Beena V, Bhatta R (2019) Summer season induced heat stress impact on the expression patterns of different toll-like receptor genes in Malabari goats. Biol Rhythm Res 50(3):466–482
Waller KP, Sandgren CH, Emanuelson U, Jensen SK (2007) Supplementation of RRR-α-tocopheryl acetate to periparturient dairy cows in commercial herds with high mastitis incidence. J Dairy Sci 90(8):3640–3646
Watson RR (1998) Vitamin E and the immune system. In: Peter JD (ed) Encyclopedia of ommunology, 2nd edn. Elsevier, Oxford, pp 2500–2501
Williams CM (2000) Dietary fatty acids and human health. Ann Zootech 49:165–180
Wojtas K, Cwynar P, Kołacz R (2014) Effect of thermal stress on physiological and blood parameters in merino sheep. Bull Vet Inst Pulawy 58(2):283–288
Zhang F, Weng X, Wang J, Zhou D, Zhang W, Zhai C, Hou Y, Zhu Y (2014) Effects of temperature–humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows. J Anim Sci 92(7):3026–3034
Zhang M, Dunshea FR, Warner RD, DiGiacomo K, Osei-Amponsah R, Chauhan SS (2020) Impacts of heat stress on meat quality and strategies for amelioration: a review. Int J Biometeorol 64:1613–1628. https://doi.org/10.1007/s00484-020-01929-6
Zininga T, Ramatsui L, Shonhai A (2018) Heat shock proteins as immunomodulants. Molecules 23(11):2846
Author information
Authors and Affiliations
Contributions
All authors SSC, VPR, VS, MB, and FRD have contributed equally and made substantial, direct, and intellectual contributions to the work, and approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauhan, S.S., Rashamol, V.P., Bagath, M. et al. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int J Biometeorol 65, 1231–1244 (2021). https://doi.org/10.1007/s00484-021-02083-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-021-02083-3