Abstract
Seasonal variations in day length and light intensity can affect the circadian rhythm as well as some characteristics of temperature regulation. We investigated characteristics of autonomic (ATR), behavioural (BTR) and nocturnal (NTR) temperature regulation during spring and autumn. Eleven participants underwent experiments in both seasons. To assess ATR, participants performed a 30-min bout of submaximal upright exercise on a cycle ergometer, followed by 100 min of water immersion (28 °C). Thresholds for the onset of shivering and sweating and vasomotor response were measured. BTR was assessed using a water-perfused suit, with participants regulating the water-perfused suit temperature (Twps) within a range, considered as thermally comfortable. The Twps changed in a saw-tooth manner from 10 to 50 °C; by depressing a switch, the direction of the Twps changed, and this limit defined the thermal comfort zone (TCZ) for each participant. A 24-h proximal (calf)–distal (toe) skin temperature gradient (∆Tc-t) was measured to assess NTR. Initiation of vasomotor tone, shivering and sweating was similar between trials. Width of the TCZ was 8.1 °C in spring and 8.6 °C in autumn (p = 0.1), with similar upper and lower regulated temperatures. ∆Tc-t exhibited a typical circadian rhythm with no difference between seasons. Minor changes in skin temperature and oxygen consumption (p ˂ 0.05) between the seasons may indicate a degree of seasonal adaptation over the course of winter and summer, which persisted in spring and autumn. Other factors, such as country, race, sex and age could however modify the outcome of the study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies investigating the thermoregulatory responses of participants during repeated trials need to ensure that the interval between trials is sufficient to prevent any adaptation to the thermal environment, unless such adaptation is the aim of the study. The choice of the time interval between experimental trials is therefore crucial, if adaptation is to be avoided. When experiments are conducted over a longer time scale, and the time interval between experimental trials is several months, then the potential influence of seasonal variation on a given thermoregulatory response must also be considered. Any seasonal influence on thermoregulatory responses would need to be taken into account when comparisons are made between studies conducted in different seasons, or conversely, only the results of studies conducted within the same season should be compared.
The seasonal variation in day length and light intensity has been shown to affect the human circadian rhythm as well as the core interthreshold zone (Honma et al. 1992; Kakitsuba et al. 2011; Kakitsuba et al. 2013). The regulation of circadian rhythm is influenced by melanopsin cells—photoreceptors in the retinal ganglion cells—which are sensitive to changes in ambient light (Berson et al. 2002; Guler et al. 2008; Hattar et al. 2002), patently affected by the seasonal variation in day length and light intensity (Honma et al. 1992). The temperature zone between the core temperature thresholds for onset of shivering and cessation of sweating, termed the core interthreshold zone (CIZ), was reported to be significantly wider in summer than in winter, as well as significantly wider in the presence of a greater level of light intensity (Kakitsuba et al. 2011). Seasonally related greater natural light exposure accelerates growth height in children (Schell et al. 2012), and improves mood (Mcintosh et al. 2010) and cognitive responses (Meyer et al. 2016). Seasonally varying ambient temperatures also affect brown adipose tissue activity (Au-Yong et al. 2009) and metabolic responses to cold exposure (van Ooijen et al. 2004), which are improved in winter, compared to summer. For example, the passive heating of the human body in different seasons has been shown to induce differences in physiological responses, including greater sweat loss and lower increase in rectal temperature in summer, compared to winter (Ihzuka et al. 1986). Conversely, exposure to passive cooling results in greater metabolic responses in winter, compared to summer (van Ooijen et al. 2004). Irrespective of these findings, the modern predominantly indoor lifestyle may diminish some seasonally related changes in circadian rhythm and physiological responses due to behavioural actions, which include air conditioning with constant temperatures (Bain and Jay 2011) and exposure to artificial lighting (lights and electronic devices) throughout the day (Stevens 2006; Van Dongen et al. 1998; Wehr et al. 1995).
Seasonally related differences in physiological as well as psychological and cognitive responses are usually reported between summer and winter, which display great differences in light exposure and ambient temperature. Whether these differences also exist between the spring and autumn has not been investigated. The present study was designed as a repeated measures study. The same trials were conducted during different seasons in the Northern Hemisphere—spring (April) and autumn (October) of the year 2013—which enabled the study of any seasonal effects on physiological responses. Our reasoning was that any evidence of seasonal adaptation that may have been incurred over the course of the winter and summer months may still persist in spring and autumn, respectively.
Methods
The present study was a part of a larger study investigating the separate and combined effects of inactivity/unloading and hypoxia on the cardiovascular, musculoskeletal, neurohumoral and thermoregulatory systems. For this purpose, the participants were confined for a period of 21 days, during which they were (i) inactive in a normoxic ambient condition, (ii) inactive in a hypoxic ambient condition and (iii) active in a hypoxic ambient condition. All participants underwent all three interventions in a randomised order, so that during each intervention the participants were divided in three groups (hypoxic bedrest, normoxic bedrest and hypoxic ambulatory group). The interventions were separated by 5 months, conducted in October 2012, April 2013 and October 2013. Before commencing each intervention, baseline measurements were collected, and we took the opportunity to investigate the autonomic, behavioural and nocturnal responses of participants in different seasons, including spring (April 2013) and autumn (October 2013). Due to logistical reasons, participants entered and exited the study gradually, with two participants at the time on each day, meaning within a week, all participants started or completed each intervention.
The study protocol comprised of a structured daily schedule; individual activities were performed at the same time of the day, including waking up and going to sleep, meal serving and experimental schedule for each participant. During each intervention, participants entered the study in the same order (the same two participants on individual days) and performed each individual experiment on the same consecutive day and time. They were served with an identical daily meal order in each intervention. During the study, participants were accommodated two per room and had full access to a common area, dedicated to socialising and also to the dining area. The experiments were performed in dedicated laboratories or personal rooms and common area. During the baseline measurements (before confinement), participants were allowed an outdoor walk for 1 h after lunch, accompanied by one of the researchers to make sure no exercise other than walking was performed during this period. This was the only time participants experienced direct natural day light, whereas within the facility, they were exposed to indirect day light, combined with artificial light throughout the day. With a busy schedule throughout the day, participants were mostly free to use different electronic devices (smart phone, tablet computer, personal computer, television) in the evening. Based on this, primarily, indoor life during interventions, any influence from the environment was minimised. In each intervention, participants were exposed to similar indoor ambient temperature (24.1 ± 1.1 °C) and relative humidity (52 ± 7%) throughout the day.
The study protocol was approved by the National Committee for Medical Ethics at the Ministry of Health of the Republic of Slovenia, and conformed to the guidelines of the Declaration of Helsinki. Eleven healthy male participants (Table 1) provided the baseline data that were collected before each confinement. Prior to the onset of the study, they had a medical examination, and their participation was subject to physician’s approval. All participants were non-smokers, with no history of heat- or cold-induced illness or chronic cardiovascular and respiratory disease. They were familiarised with the protocol of the study and gave their written consent for participation in the study. The study was conducted at the Olympic Sport Centre Planica (Rateče, Slovenia) situated at an altitude of 940 m.
Autonomic temperature regulation assessment
The assessment of ATR was adapted from the methodology reported previously (Mekjavic et al. 2005a; Mekjavic et al. 1991). Briefly, participants were instrumented and then seated on a cycle ergometer, wearing only swim shorts. After a 5-min-rest period, they performed a 30-min bout of submaximal upright exercise. The workload corresponded to their pre-determined exercising heart rate, and was observed at 50% of maximal workload. Immediately after the exercise, participants were transferred to a tank of well-stirred water, maintained at 28 °C and remained immersed in a semi-reclining position for 100 min. The experiment was performed in a room with no windows, meaning the participants were exposed to artificial lighting during the assessment. The ambient temperature (Ta) and relative humidity (RH) were maintained at 25.0 ± 0.9 °C and 40 ± 10% in both interventions.
Measurements
During the exercise and immersion periods, oxygen uptake (VO2, ml min−1) was monitored with a COSMED K4b2 system (COSMED Srl, Pavona di Albano, Rome, Italy). Weighted mean skin temperature (Tsk, °C), derived from four skin sites (calf, thigh, chest, arm) (Ramanathan 1964) was sampled and stored with a data logger (Almemo Model 5990-2 data-acquisition system, Ahlborn GmbH, Holzkirchen, Germany). Core temperature (Tre, °C) was assessed with a rectal thermistor (MSR Electronics GmbH, Henggart, Switzerland), inserted 12 cm beyond the anal sphincter. Peripheral vasomotor tone was assessed from the difference in skin temperature between the forearm and fingertip of the third digit (ΔTf-f, °C) (House and Tipton 2002; Rubinstein and Sessler 1990), measured with two thermistors (MSR145, Henggart, Switzerland). This hand was consequently not submerged during the immersion period, but was maintained at the level of the heart. The forehead sweating rate (Esw, g.m−2 min−1) was measured with a ventilated capsule (467 mm2 surface area covered by capsule). Constant flow of air at a rate of 1.0 l min−1 through the capsule was ensured and monitored with flow meters at the inlet and outlet of the capsule (Perflow Instruments Ltd., London, UK). The temperature and humidity of the air entering and exiting the capsule was measured with thermistors and resistance hygrometers. The rate of sweating was determined from the difference in water vapour content of the outflowing and inflowing air, adjusting for the surface area of the ventilated capsule. Heart rate was measured with a Polar heart rate monitor (Kempele, Finland). All data were sampled and stored at minute intervals.
Participants provided ratings of temperature perception (7-point scale: 1, cold; 2, cool; 3, slightly cool; 4, neutral; 5, slightly warm; 6, warm; 7, hot) and thermal comfort (4-point scale: 1, comfortable; 2, slightly uncomfortable; 3, uncomfortable; 4, very uncomfortable) (ANSI/ASHRAE 2013) at 5-min intervals.
Data analysis
Two-way repeated measures Anova (intervention (spring, autumn) × time (min)) was used to compare Tre, Tsk, ΔTf-f, VO2 and Esw. If a significant F value was found (p < 0.05), critical differences were analysed by Tukey’s procedure to locate the significant mean differences. Ratings of temperature perception and thermal comfort were analysed with a Wilcoxon non-parametric test. Data are presented as means ± standard deviation for parametric statistics or as medians (range) for non-parametric statistics with the limit of statistical significance set at 0.05. Statistical analysis was performed using Statistica 8.0 (Statsoft Inc., Tulsa, OK).
Behavioural temperature regulation assessment
The assessment of BTR was achieved with a water-perfused suit (WPS), adapted from the methodology reported previously (Ciuha et al. 2015; Ciuha and Mekjavic 2016; Ciuha and Mekjavic 2017). Briefly, the participants were instructed to regulate the WPS temperature (Twps) within a range they considered thermally comfortable throughout a 1-h trial, ranging between heating and cooling, by depressing a button on a remote switch. The WPS covered the entire body, excluding the hands, feet and head. The Twps regulation was uniform in all areas covered by the suit.
During the experiment, the blinds were closed, with participants exposed to artificial lighting throughout the assessment. Ta and RH were maintained at 22.0 ± 1.0 °C and 50 ± 7% in both interventions.
Measurements
During the assessment of BTR, participants’ core temperature (Tre, °C) was measured with a rectal thermistor (MSR Electronics GmbH, Henggart, Switzerland), inserted 12 cm beyond the anal sphincter. Skin temperature was assessed with thermistors (MSR Electronics GmbH, Henggart, Switzerland), secured on the calf, thigh, chest and arm by thin air-permeable tape (Tegaderm, 3M, USA), enabling the calculation of weighted mean skin temperature (Ramanathan 1964). All skin temperature sites were on the right side of the body. All of the data were sampled and stored with a MSR12 (Modular Signal Recorder, MSR Electronics GmbH, Henggart, Switzerland) data logger at 30-s intervals. The average water temperature at the inlet and outlet of the WPS was measured using T-type thermocouples, and defined as Twps. The saw-tooth pattern with its low and high peaks revealed a thermal comfort zone (TCZ) for each participant. Ta and RH were measured with transducers (MSR145, MSR Electronics GmbH, Henggart, Switzerland), situated in close proximity to the participant.
Throughout the experiment, participants provided ratings of their thermal comfort and temperature perception (see “Autonomic temperature regulation assessment”) every 10 min.
Data analysis
Two-way repeated measures Anova (intervention (spring, autumn) × time (min)) was used to compare Tre, Tsk and environmental conditions (Ta and RH). If a significant F value was found (p < 0.05), critical differences were analysed by Tukey’s procedure to locate the significant mean differences. WPS parameters (upper and lower limits of the TCZ and a magnitude of the TCZ) and subjective ratings of temperature perception and thermal comfort were analysed with a Wilcoxon non-parametric test. Data are presented as means ± standard deviation for parametric statistics or as medians (range) for non-parametric statistics with the limit of statistical significance set at 0.05. Statistical analysis was performed using Statistica 8.0 (Statsoft Inc., Tulsa, OK).
Nocturnal temperature regulation
As the study primarily investigated the effect of inactivity/unloading in either normoxic or hypoxic conditions, the proximal (calf) to distal (toe) skin temperature gradient (∆Tc-t) as an index of peripheral vasomotor tone (Rubinstein and Sessler 1990) was chosen as a measurement of potential changes in perfusion in the lower extremities, caused by inactivity. It has previously been suggested the ∆Tc-t is a valid indicator of peripheral perfusion (Ibsen 1967; Joly and Weil 1969; Lima and Bakker 2006; Mekjavic et al. 2005b; Stoner et al. 1991) and plays an important role in the sleep onset (Kräuchi et al. 1999). For this purpose, the ∆Tc-t was measured for 24 h from 9 am until the following morning, during which the participants performed their normal daily activities. The activities were pre-scheduled and identical in all interventions on specific days. Throughout the study, the participants’ daily schedule was regulated and standardised so that they were requested to go to sleep at 11 pm and woken at 7 am. Additionally, the participants were not allowed to nap during the day. Due to a busy daily schedule, participants did not experience any problems following the sleep-wake cycles, but this was however not directly monitored and measured. During the NTR assessment and throughout the study, participants were exposed to similar indoor ambient temperature (24.1 ± 1.1 °C) and relative humidity (52 ± 7%) in their rooms and common area. Apart from a 1-h outdoor walk, participants were exposed to indirect natural day light, combined with artificial light throughout the day. The lights were switched off at 11 pm and on again at 7 am. Participants wore casual clothing throughout the day and pyjamas or underwear during night-time. A standardised white sheet cotton bedding was used throughout the study.
Measurements
∆Tc-t was recorded using two thermistors (MSR145, Henggart, Switzerland). MSR data loggers were secured to two sites, using a breathable transparent film (Tegaderm, 3M, Minnesota, USA) and micropore tape (3M, Minnesota, USA): (a) proximal site: the medial aspect of soleus mid-way between the medial malleolus and the medial condyle of the tibia and (b) distal site: the toe pad of the great toe. The measurement sites were on the non-dominant side. The MSR data logger recorded the calf and toe Tsk at 1-min intervals. The data were then averaged into 60-min bins.
Data analysis
Two-way repeated measures Anova (intervention (spring, autumn) × time (hourly data epochs)) was utilised to assess the data for significance. When a main effect or an interaction was noted, Tukey’s post hoc test was used to define the specific differences. Values are reported as means ± standard deviation. The alpha level of significance was defined a priori at 5%. All statistical analyses were performed using Statistica 10.1 (StatSoft, Inc., Tulsa, Oklahoma, USA).
Results
Meteorological data
The average meteorological conditions for each month in 2013 are presented in Table 2 (ARSO 2013). Summer and winter months are also included, since they could potentially affect the responses observed in early spring and autumn, when the study interventions took place. As expected, differences in meteorological conditions are more prominent between summer and winter, but they also vary between spring and autumn in terms of higher ambient temperature, greater sunshine duration and longer day length in summer and spring, compared to winter and autumn, respectively (p ˂ 0.01).
As seen in Table 2, the winter months, including February and March, were cooler whereas summer months, including July and August, were warmer in relation to the means of the period between 1961 and 1990. There were 3 documented heat waves in the summer—in June, July and August, with maximum daily temperatures exceeding 30 °C, 35 °C and 40 °C in some parts of Slovenia, respectively. The average absolute maximum and minimum daily air temperature ranged from 38.5 to − 10.4 °C between summer and winter.
Autonomic temperature regulation
Exercise
The exercise-induced elevation in Tre was similar between seasons (p = 0.4). Mean Tre increased from resting values of 37.4 ± 0.3 °C in spring and 37.4 ± 0.4 °C in autumn, to asymptotic values of 38.0 ± 0.3 °C in both trials. HR increased from 95 ± 13 to 147 ± 14 bpm in spring and from 89 ± 14 to 140 ± 14 bpm in autumn, with no difference between seasons reported (p = 0.6).
Mean Tsk increased from resting values of 32.4 ± 0.9 °C in spring and 32.3 ± 0.5 °C in autumn, to 33.8 ± 0.6 °C and 33.4 ± 1.0 °C after 30 min of exercise, with a similar increase observed in both seasons (p = 0.5). ΔTf-f was similar between seasons (p = 0.9) with mean values of − 1.6 ± 0.9 °C in spring and − 1.5 ± 0.6 °C in autumn—indicating vasodilatation (House and Tipton 2002)—throughout a 30-min exercise. This vasomotor response (Fig. 1a) was initiated at a similar Tre (spring: 37.5 ± 0.3 °C, autumn: 37.4 ± 0.4 °C; p = 0.4) and time (spring: 6.2 ± 4.2 min−1, autumn: 4.2 ± 2.0 min−1; p = 0.3).
Upper figures: the difference in skin temperature between the forearm and fingertip, an index of skin blood flow (ΔTf-f), plotted as a function of relative change in rectal temperature (ΔTre); middle figures: the relative sweating response (Esw) as a function of ΔTre; bottom figures: the oxygen uptake response (VO2) as a function of ΔTre. Figures on the left present the data obtained during the exercise and on the right during the immersion
An increase in Esw throughout the 30-min exercise was similar between seasons (p = 0.1), with mean values of 8.7 ± 3.5 g.m2 min−1 in spring and 7.8 ± 3.1 g.m2 min−1 in autumn. The Tre threshold for the onset of Esw (Fig. 1b) was similar between the seasons (spring: 37.5 ± 0.3 °C, autumn: 37.4 ± 0.4 °C; p = 0.6) and initiated at similar time (spring: 3.5 ± 2.4 min−1, autumn: 4.4 ± 2.0 min−1; p = 0.4).
The increase in VO2 throughout the exercise was greater in spring (1858 ± 313 ml.min−1), compared to autumn (1627 ± 243 ml min−1; F1,5 = 13.5, p = 0.001).
Immersion
During 100 min of immersion, Tre did not differ significantly between the seasons (p = 0.7). Mean Tre dropped from 38.0 ± 0.3 °C to 36.7 ± 0.3 °C in spring and from 38.0 ± 0.3 to 36.7 ± 0.2 °C in autumn. HR decreased from 101 ± 14 to 72 ± 15 bpm in spring and from 94 ± 10 to 68 ± 12 bpm in autumn, with no difference between seasons reported (p = 0.3). Mean Tsk dropped from 32.7 ± 1.5 to 28.5 ± 0.3 °C in spring and from 32.5 ± 1.3 to 28.9 ± 0.2 °C in autumn, with mean differences between the seasons (spring: 28.7 ± 0.3 °C, autumn: 29.2 ± 0.3 °C) reported as significant (F1,19 = 15.8, p = 0.01). ΔTf-f changed from 0.0 ± 0.9 in spring and − 0.7 ± 0.9 °C in autumn, indicating vasodilatation (House and Tipton 2002), to 5.4 ± 0.9 °C and 6.1 ± 0.9 °C, respectively, indicating vasoconstriction (House and Tipton 2002; Rubinstein and Sessler 1990). No difference in the vasomotor tone was reported between the seasons (p = 0.1). The vasomotor response (Fig. 1d) was initiated at similar Tre (37.8 ± 0.3 °C in spring and 37.7 ± 0.4 °C in autumn; p = 0.9) and time (16.3 ± 10.8 min−1 in spring and 11.9 ± 8.9 min−1 in autumn; p = 0.6).
During the first minute of immersion, the mean Esw was 13.0 ± 3.2 g.m2 min−1 in spring and 9.8 ± 4.4 g.m2 min−1 in autumn, which decreased rapidly with no difference observed between the seasons (p = 0.4). Shivering thermogenesis, reflected in the oxygen uptake response (Fig. 1f), was initiated at a similar Tre (spring: 37.0 ± 0.3 °C, autumn: 36.9 ± 0.3 °C; p = 0.6) and time (spring: 68 ± 29 min−1, autumn: 75 ± 31 min−1; p = 0.5). VO2 values dropped from 1530 ± 391 to 514 ± 145 ml min−1 in spring and from 1035 ± 423 to 438 ± 119 ml min−1 in autumn, with differences between the seasons reported as significant (F1,19 = 24.7, p = 0.002).
Median (range) ratings of thermal comfort and temperature perception indicated that participant perceived the water temperature as slightly cool (spring: 3 ± 3, autumn: 3 ± 2; p = 0.07) and slightly uncomfortable (2 ± 3 in both seasons; p = 0.6).
Core interthreshold zone
The core temperature thresholds for the onset of shivering and cessation of sweating were between 37.0 ± 0.3 and 37.5 ± 0.3 °C during spring, and between 36.9 ± 0.3 and 37.4 ± 0.4 °C during autumn. As such, there was no difference in CIZ observed between the seasons (p = 0.6).
Behavioural temperature regulation
Water-perfused suit temperature and subjective ratings (thermal comfort, temperature perception)
The regulated Twps indicated no difference in the lower and upper limits or in the magnitude (width) of the TCZ (Table 3) between the seasons. According to the thermal comfort and temperature perception ratings, participants remained comfortable and neutral to warm during both interventions (p = 0.1).
Rectal and skin temperature
Throughout the 1-h trial, Tre remained stable with a slight decrease of 0.1 ± 0.1 °C observed in both seasons. Mean Tre was 37.2 ± 0.2 °C in spring and 37.3 ± 0.3 °C in autumn (p = 0.9).
An increase of ~ 1.5 °C in Tsk was observed in the first half of the trial. Mean Tsk was lower in autumn (32.3 ± 1.9 °C), compared to spring (33.6 ± 1.0 °C; F1,69 = 9.3, p = 0.01). However, the relative changes in Tsk from the starting values were insignificant between the seasons, meaning Tsk on average increased by 1.4 ± 0.9 °C in spring and 1.7 ± 1.3 °C in autumn throughout the BTR assessment.
Nocturnal temperature regulation
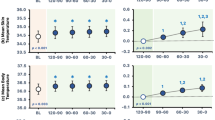
The ∆Tc-t exhibited a typical circadian rhythm whereby the participants were vasoconstricted in the toe during the daytime and vasodilatated in the evening and throughout the night-time (Fig. 2). It is also evident that the toe perfusion is decreased in the early hours of the morning in preparation for the participants to wake. The statistical analysis did not reveal any differences at any time point between the two seasonal measures (p ˃ 0.05).
24-h proximal (calf) to distal (toe) skin temperature gradient (∆Tc-t) as an index of skin blood flow (Rubinstein and Sessler 1990)
Discussion
The aim of the study was to observe the effect of seasonal variation on temperature regulation. Two approaches were considered for such an assessment. One approach was the often-preferred method of comparing the thermoregulatory responses observed at the height of summer and winter, and the other to compare the responses during spring and autumn. The latter approach was adopted in the present study, as it was reasoned that the difference in the day length between the two trials would not be as great as the difference between winter and summer. It was anticipated that any adaptation to the climate of winter and summer months should most likely persist, to some degree, in spring and autumn, respectively.
Autonomic temperature regulation
Heat exposure during summer is known to improve sweating response (Inoue et al. 1995), whereas cold exposure in winter increases metabolic response (van Ooijen et al. 2004), resulting in seasonal differences in sweating and shivering responses between summer and winter. The observed greater oxygen consumption during spring compared to autumn in the case of the present study could be an indicator of winter cold–induced metabolic adaptation, persisting in the spring. The sweating response however remained unchanged, meaning no summer heat adaptation persisting in the autumn was observed. However, a lower oxygen consumption in autumn could be attributed to greater activity level and improved economy (Shephard and Aoyagi 2009) rather than overall VO2max (Keramidas et al. 2016) following summer.
The well-known seasonally related changes in the sweating-to-shivering interthreshold zone have previously been demonstrated (Bruck et al. 1970; Kakitsuba et al. 2011), but were however not evident in the present study. These changes are closely related to light exposure (Aoki et al. 2005; Kakitsuba et al. 2011; Kakitsuba et al. 2013), with greater light intensity widening the CIZ. Since the difference in daylight is much greater between summer and winter than between spring and autumn (ARSO 2013), it is plausible that these minor differences between the latter two seasons were not sufficient enough to induce any changes in the CIZ in the present study.
Behavioural temperature regulation
Participants regulated the Twps in similar ranges in both seasons. A slightly, but not significantly, wider range of regulated temperatures in autumn resulted in lower Tsk, compared to spring. The changes in Tsk from the baseline values were however not different between the seasons, meaning that participants maintained similar relative difference in Tsk in both seasons. The seasonal difference in Tsk could potentially be attributed to a higher preferred Tsk in spring, following the cooler winter months, and a lower preferred Tsk in autumn, following the warmer summer months. A study conducted in Poland (Lindner-Cendrowska and Błażejczyk 2018) reported that active outdoorsy-type individuals perceived their thermal comfort to be between “slightly cool” and “slightly warm” for temperature sensation, depending on the season. In winter and autumn, the “slightly cool” sensation prevailed, whereas in spring, the most common response was “neutral” sensation, and in summer “slightly warm” sensation. Interestingly, in transitional seasons (spring and autumn), the respondents provided different thermal sensation votes when experiencing identical thermal balance states under the same PET (physiological equivalent temperature) values, partly associated with changing clothing insulation (Andrade et al. 2011; Lindner-Cendrowska and Błażejczyk 2013). This seasonally related difference in the thermal sensation could potentially explain the difference in preferred skin temperatures between spring and autumn observed in the present study.
Nocturnal temperature regulation
∆Tc-t, an indicator of vasomotor tone, was not affected by season. Following a typical circadian rhythm pattern, ∆Tc-t was reduced during daytime and increased during night-time (McDonnell et al. 2014). It has been suggested that this nocturnal peripheral vasodilatation is essential for the sleep onset (Van Someren 2004) during which the autonomic control of body temperature is reduced by widening of the CIZ (Tayefeh et al. 1998). Interestingly, the variability in measured ∆Tc-t between participants was much greater during daytime, but decreased during night-time, suggesting that different daily activities, to some extent, affect these individual differences in perfusion, whereas during sleep the perfusion of distal regions is much more uniform.
The measurements were collected during the baseline, before participants were confined. While the environmental conditions were carefully controlled and activities pre-scheduled, the behavioural aspect of participants was not closely monitored (Krauchi and Wirz-Justice 1994), explaining the difference in perfusion throughout the day. However, the idea was to observe the nocturnal temperature regulation, and for this reason, the participants followed the same sleep-wake cycle every day. The results indicate a similar pattern in nocturnal temperature regulation with small variability, presuming the participants were asleep during night-time, with temperature unaffected by daily activities.
Limitations and study considerations
The experimental conditions in the study were carefully controlled, including structured schedule with identical activity time frame each day, and controlled indoor temperature and lighting in the experiments. In both interventions, the same schedule was used, including also the same order of meals, served to participants. Nevertheless, some aspects of the study were difficult to control and maintain identical within interventions, such as sleep quality and participants’ behavioural actions. Therefore, it cannot be excluded that these aspects could have affected some results, including differences in Tsk and VO2 in the ATR and BTR assessments, and daily variation in measured ∆Tc-t between spring and autumn intervention.
The participants came from different regions of Slovenia and were therefore exposed to different ambient temperatures prior to each intervention. They may also have experienced different levels of heat and cold acclimatisation as a result. However, no information regarding the level of thermal adaptation was available.
During this study, we observed high inter-individual variability in the characteristics of the TCZ. Such variability has been reported previously by Jacquot et al. (Jacquot et al. 2014), suggesting that participants could be categorised according to their preference for a warmer or cooler environment. It may therefore be warranted, to categorise participants in such a manner, particularly, within studies involving exposure to more extreme thermal environmental conditions.
Importantly, seasonal effects depend greatly on the climate zone, including the annual air temperature amplitude and amount of solar radiation. Also, there are other factors, such as sex, age and race, which could potentially modify the outcomes of the seasonal temperature regulation. Furthermore, in modern societies, a predominantly indoor lifestyle in stable ambient temperatures with similar lighting levels throughout the day may reduce or diminish any level of a seasonal effect on temperature regulation.
Conclusions
The present study observed some changes in skin temperature and oxygen consumption, indicating some level of seasonal adaptation over the course of the winter and summer, persisting into spring and autumn, respectively. These minor differences were however not sufficient enough to induce any changes in the CIZ in the present study.
References
Andrade H, Alcoforado M-J, Oliveira S (2011) Perception of temperature and wind by users of public outdoor spaces: relationships with weather parameters and personal characteristics. Int J Biometeorol 55:665–680
ANSI/ASHRAE (2013) Standard 55. Thermal Environmental Conditions for Human Occupancy, American Society Of Heating, Ventilating and Air-conditioning Engineers, Atlanta:
Aoki K, Yokoi M, Masago R, Iwanaga K, Kondo N, Katsuura T (2005) Modification of internal temperature regulation for cutaneous vasodilation and sweating by bright light exposure at night. Eur J Appl Physiol 95:57–64. https://doi.org/10.1007/s00421-005-1392-6
ARSO (2013) Our environment. Slovenian Environment Agency, Ljubljana
Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME (2009) Brown adipose tissue and seasonal variation in humans. Diabetes 58:2583–2587. https://doi.org/10.2337/db09-0833
Bain AR, Jay O (2011) Does summer in a humid continental climate elicit an acclimatization of human thermoregulatory responses? Eur J Appl Physiol 111:1197–1205
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. https://doi.org/10.1126/science.1067262
Bruck K, Wunnenberg W, Gallmeier H, Ziehm B (1970) Shift of threshold temperature for shivering and heat polypnea as a mode of thermal adaptation. Eur J Phys 321:159–172
Ciuha U, Eiken O, Mekjavic IB (2015) Effects of normobaric hypoxic bedrest on the thermal comfort zone. J Therm Biol 49-50: 39-46. doi:https://doi.org/10.1016/j.jtherbio.2015.02.001
Ciuha U, Mekjavic IB (2016) Regional thermal comfort zone in males and females. Physiol Behav 161:123–129. https://doi.org/10.1016/j.physbeh.2016.04.008
Ciuha U, Mekjavic IB (2017) Thermal comfort zone of the hands, feet and head in males and females. Physiol Behav 179:427–433. https://doi.org/10.1016/j.physbeh.2017.07.020
Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao H-W, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau K-W, Hattar S (2008) Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453:102–105. https://doi.org/10.1038/nature06829
Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295:1065–1070. https://doi.org/10.1126/science.1069609
Honma K, Honma S, Kohsaka M, Fukuda N (1992) Seasonal variation in the human circadian rhythm: dissociation between sleep and temperature rhythm. Am J Phys 262:885–891. https://doi.org/10.1152/ajpregu.1992.262.5.R885
House JR, Tipton MJ (2002) Using skin temperature gradients or skin heat flux measurements to determine thresholds of vasoconstriction and vasodilatation. Eur J Appl Physiol 88:141–145. https://doi.org/10.1007/s00421-002-0692-3
Ibsen B (1967) Treatment of shock with vasodilators measuring skin temperature on the big toe: ten years’ experience in 150 cases. Diseases of the Chest 52:425–429
Ihzuka H, Hori S, Akamatsu T (1986) Seasonal variations of physiological responses to heat of subtropical and temperate natives. Int J Biometeorol 30:107–113
Inoue Y, Nakao M, Okudaira S, Ueda H, Araki T (1995) Seasonal variation in sweating responses of older and younger men. Eur J Appl Physiol Occup Physiol 70:6–12
Jacquot CM, Schellen L, Kingma BR, van Baak MA, van Marken Lichtenbelt WD (2014) Influence of thermophysiology on thermal behavior: the essentials of categorization. Physiol Behav 128:180–187. https://doi.org/10.1016/j.physbeh.2014.01.025
Joly HR, Weil MH (1969) Temperature of the great toe as an indication of the severity of shock. Circulation 39:131–138
Kakitsuba N, Mekjavic IB, Katsuura T (2011) The effect of season and light intensity on the core interthreshold zone. J Physiol Anthropol 30:161–167. https://doi.org/10.2114/jpa2.30.161
Kakitsuba N, Mekjavic IB, Katsuura T (2013) The core interthreshold zone during exposure to red and blue light. J Physiol Anthropol 32:6. https://doi.org/10.1186/1880-6805-32-6
Keramidas ME, Kölegård R, Mekjavic IB, Eiken O (2016) PlanHab: hypoxia exaggerates the bed-rest-induced reduction in peak oxygen uptake during upright cycle ergometry. Am J Phys Heart Circ Phys 311:H453–H464
Kräuchi K, Cajochen C, Werth E, Wirz-Justice A (1999) Warm feet promote the rapid onset of sleep. Nature 401:36–37
Krauchi K, Wirz-Justice A (1994) Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Phys Regul Integr Comp Phys 267:R819–R829
Lima A, Bakker J (2006) Noninvasive monitoring of peripheral perfusion. Applied Physiology in Intensive Care Medicine. In: (ed.) Applied Physiology in Intensive Care Medicine. Springer, 131-141
Lindner-Cendrowska K, Błażejczyk K (2013) Clothing habits during tourism and leisure activities in urban areas. The case study of Warsaw (Poland). Editors: James D Cotter, Samuel JE Lucas and Toby Mündel: 288
Lindner-Cendrowska K, Błażejczyk K (2018) Impact of selected personal factors on seasonal variability of recreationist weather perceptions and preferences in Warsaw (Poland). Int J Biometeorol 62:113–125
McDonnell AC, Eiken O, Mekjavic PJ, Mekjavic IB (2014) Circadian rhythm of peripheral perfusion during 10-day hypoxic confinement and bed rest. Eur J Appl Physiol 114:2093–2104. https://doi.org/10.1007/s00421-014-2923-9
Mcintosh AM, Sussmann J, Goodwin G (2010) Mood disorder. Companion to psychiatric studies In: Johnstone EC, Cunninghan Owens D, Lawrie SM, Mcintosh AM, Sharpe M (ed.) Companion to psychiatric studies Elsevier, 427-452
Mekjavic IB, Golja P, Tipton MJ, Eiken O (2005a) Human thermoregulatory function during exercise and immersion after 35 days of horizontal bed-rest and recovery. Eur J Appl Physiol 95:163–171. https://doi.org/10.1007/s00421-005-1348-x
Mekjavic IB, Kocjan N, Vrhovec M, Golja P, House C, Eiken O, 2005b: Foot temperatures and toe blood flow during a 12 km winter hike and guard duty. LJUBLJANA UNIV (YUGOSLAVIA) JOZEF STEFAN INST
Mekjavic IB, Sundberg CJ, Linnarsson D (1991) Core temperature “null zone”. J Appl Physiol 71:1289–1295. https://doi.org/10.1152/jappl.1991.71.4.1289
Meyer C, Muto V, Jaspar M, Kusse C, Lambot E, Chellappa SL, Degueldre C, Balteau E, Luxen A, Middleton B, Archer SN, Collette F, Dijk DJ, Phillips C, Maquet P, Vandewalle G (2016) Seasonality in human cognitive brain responses. Proc Natl Acad Sci U S A 113:3066–3071. https://doi.org/10.1073/pnas.1518129113
Ramanathan NL (1964) A new weighting system for mean skin temperature of the body. J Appl Physiol 19:531–533. https://doi.org/10.1152/jappl.1964.19.3.531
Rubinstein EH, Sessler DI (1990) Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology 73:541–545
Schell LM, Knutson KL, Bailey S (2012) Environmental effects on growth human growth and development (second edition). In: Bogin B (ed.) Human growth and development (second edition). Academic Press, 245-286
Shephard RJ, Aoyagi Y (2009) Seasonal variations in physical activity and implications for human health. Eur J Appl Physiol 107:251–271
Stevens RG (2006) Artificial lighting in the industrialized world: circadian disruption and breast cancer. Cancer Causes Control 17:501–507
Stoner H, Barker P, Riding G, Hazlehurst D, Taylor L, Marcuson R (1991) Relationships between skin temperature and perfusion in the arm and leg. Clin Physiol 11:27–40
Tayefeh F, Plattner O, Sessler DI, Ikeda T, Marder D (1998) Circadian changes in the sweating-to-vasoconstriction interthreshold range. Pflugers Arch 435:402–406. https://doi.org/10.1007/s004240050530
Van Dongen HP, Kerkhof GA, Souverijn JH (1998) Absence of seasonal variation in the phase of the endogenous circadian rhythm in humans. Chronobiol Int 15:623–632
van Ooijen AMJ, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR (2004) Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav 82:545–553. https://doi.org/10.1016/j.physbeh.2004.05.001
Van Someren EJW (2004) Sleep propensity is modulated by circadian and behavior-induced changes in cutaneous temperature. J Therm Biol 29:437–444. https://doi.org/10.1016/j.jtherbio.2004.08.003
Wehr TA, Giesen HA, Moul DE, Turner EH, Schwartz PJ (1995) Suppression of men’s responses to seasonal changes in day length by modern artificial lighting. Am J Phys Regul Integr Comp Phys 269:R173–R178
Funding
The research leading to these results has received funding from the European Union’s Framework Programme (2007–2013) under grant agreement no. 284438.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ciuha, U., Kounalakis, S., McDonnell, A.C. et al. Seasonal variation of temperature regulation: do thermoregulatory responses “spring” forward and “fall” back?. Int J Biometeorol 64, 1221–1231 (2020). https://doi.org/10.1007/s00484-020-01898-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-020-01898-w