Abstract
The aim of this study was to better understand the inflection point of RT and BSTs and measure different body surface temperatures (BSTs) under different temperature-humidity index (THI) conditions. A total of 488 Holstein dairy cows were chosen to manually measure rectal temperature (RT) and BSTs including left side of eye, ear, cheek, forehead, flank, rump, fore udder, and rear udder by infrared thermography for 14 times. Those measurements included six times under high THI (THI > 78), three times under moderate THI (72 ≤ THI ≤ 78), and five times under low THI (THI < 72). Results showed that BSTs were affected by THI conditions (P < 0.01). The THI conditions where mean and maximum forehead temperatures started to increase rapidly (71.4 and 66.8) were lower than that where RT started to increase rapidly (74.1). The correlation coefficients of mean and maximum forehead temperatures to THI were 0.808 and 0.740, and were 0.557 and 0.504 to RT, all showing the highest as compared to other region temperatures with THI and RT, respectively. Thus, we conclude that BSTs are more sensitive to thermal environment than RT, suggesting the variability of BST to reflect body core temperature. In addition, the forehead is a relatively reliable region to assess the heat stress reflecting RT compared to the eye, ear, cheek, flank, rump, fore udder, and rear udder regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With particular references to tropical and subtropical zones, heat stress is one of most recognized factors influencing livestock production and welfare. Large economic losses to the dairy industry (St-Pierre et al. 2003; Wang et al. 2010) are related to negative effects of heat stress on production (Joksimović-Todorović et al. 2011; Haqiya et al. 2017), reproductive performance (García-Ispierto et al. 2007), and immunity (Lacetera et al. 2005).
The temperature-heat index (THI), a widely used measure to assess heat stress in cattle (Gaughan et al. 2008), advises that exceeding a value of 72 may suggest that cows are suffering heat stress (Ingraham et al. 1976). However, genetic pressure for increased production performance has decreased heat tolerance in cattle due to the metabolic cost of increased milk yield and growth (West et al. 2003; Bryant et al. 2007; Oltenacu et al. 2010). Thus, it makes possible that considering 72 as a cutting-edge point to declare heat stress may underestimate the threshold of heat stress in cows. Consequently, inflection point of body temperatures under different THI conditions may reflect threshold of heat stress and help to ensure the accuracy of heat stress evaluation as well as improved management strategies for cows.
Rectal temperature (RT) increases whenever lactating cows are exposed to high environmental temperatures (Bouraoui et al. 2002) causing concomitant changes to the homeostasis status of animals (Younes et al. 2011). Thus, RT is regarded as a common and practical tool to determine heat stress (Hicks et al. 2001). However, RT has limitations that include being time and labor consuming, requires direct contact with animals, and could cause stressful experiences to cows (Suthar et al. 2012; Ammer et al. 2016). On the other hand, due to its convenience, speediness, non-contact, and non-stress source, infrared thermography has been widely used in human medicine (Ng et al. 2004; Chiang et al. 2008) and animal science (Stewart et al. 2005; Ludwig et al. 2010; Do et al. 2013).
Due to the variation of skin vasomotor activity (Montanholi et al. 2008), variations of skin surface temperature can be assessed by using infrared thermography. Therefore, measuring BSTs has arisen as a stress indicator (Martello et al. 2016) and is of increasing importance (Adams et al. 2013). For instance, Collier et al. (2006) found that environmental temperature influenced the BST by heat exchange among the organism and environment. Nevertheless, despite many studies have reported the evaluation of body surface temperature (BST) on heat stress (Firk et al. 2002; Alzahal et al. 2011), there is a paucity of scientific information exploring which body region temperature reflects better the RT under differing THI conditions. Hence, the aim of this study was to better understanding of inflection point of RT and BSTs and depict the best body region reflecting RT to assess heat stress and under different THI conditions.
Materials and methods
Location
This study was carried out at a private dairy farm of breed for dairy (Zhongdi breeder farm for dairy) in Shunyi, Beijing, from July to October 2016. The zone of the farm is located at latitude between 40° 00′ and 40° 18′ and east longitude between 116° 28′and 116° 58′ and belongs to warm temperate semi-humid continental monsoon climate. Chinese Holstein cows in one barn were used.
Animals, feeding, and handling
Inclusion-exclusion criteria to select experimental cows were parity (two to four calving, at the beginning of the experiment), milk yield above 30 kg/day (the average of June, July), and days in milk (DIM; at the beginning of the experiment) below 200 days (Table 1). A total of 488 cows were used. The barn (15 m × 260 m) having the capacity of 280 cows was concrete-floored without outdoor area, where every cow had more than one bed (1.45 m × 1.8 m) with litter of rice husk. Cows were fed a total mixed ration three times daily at 7:00 am, 14:00 pm, and 19:00 pm with free access to water and diet, and were milked three times daily at 8:00–8:40 am, 15:10–15:50 pm, and 21:40–22:20 pm, respectively. The barn used automatic manure scraper to remove feces. Besides, five auto-controlled axial fans and the spray of cooling system were installed, which were used after testing in the afternoon.
Experimental design and procedures
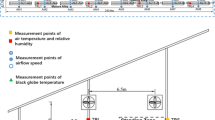
The dry bulb temperature (T) and relative humidity (RH) of air inside the cowshed were obtained with handheld hygrothermograph (Testo-625, Germany), at intervals of 30 min during testing at sampling days from 11:00 am to 12:30 am, while recorded by automatic air detection machine at other days from 8:00 am to 20:00 pm (Fig. 1). The THI was calculated according to the below model: THI = (1.8 × T + 32) − [(0.55 − 0.0055 × RH) × (1.8 × T − 26)] (Rowsell 1972) using 20 times values of T and TH during the daytime. As previously described (Ingraham et al. 1976), THI values were ranked as > 78, 72–78, and < 72 to define high, moderate, and low THI, respectively. Six times with high THI in July and August, three times with moderate THI in September, and five times with low THI in September and October, totaling 14 days were chosen to measure RT and BSTs of cows (Table 2).
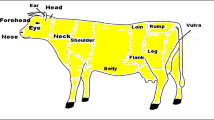
On the sampling days, cows were fixed by neck clip (MZX-001, Muzhixing livestock co, Itd., Cangzhou, China), and tested at 11:00–12:30 am (neither the feeding nor the milking time). A portable infrared camera (IRC) VarioCAM® (InfraTec, Germany) was used to take infrared at least three images with temperature field of 640 × 480 pixels as referring the previous studies used in pigs (Loughmiller et al. 2001) and dairy as well (Berry et al. 2003). Eight different regions were measured, which were eye (orbita plus surrounding), ear (inner side at the base of the ear), cheek, forehead, flank, rump, fore udder, and rear udder in the left side of the body (Fig. 2). These regions were prevented from direct sunlight and dirt spot during the measurement. The camera was parallel to the height of determined regions and with 1.5-m distance to cow according to Montanholi et al. (2008). Cows kept standing and quiet during the measurements.
The infrared images were interpreted using IRBIS 3 Standard software (Yashihengyuan, Beijing, China). Using the eye for example, 1134 pixels were applied to calculate mean and maximum temperatures of the specific area (Fig. 3). The minimum temperature is the easiest to be affected by external environment and smudge. The mean temperature represents the mean value of the specific area and reflects the overall situation, which however may be also affected by external environment and smudge. The maximum temperature represents the highest temperature in the specific area, which is hardly affected by smudge. Thus, the mean and maximum temperature are usually used in the studies (Montanholi et al. 2008; Salles et al. 2016; Martello et al. 2016). Meanwhile, RT was manually measured with a rectal thermometer (Kelibo Co., Ltd., Wuhan, China) to a depth of 10 cm to minimize bias according to Burfeind et al. (2010) between individuals. After an acoustic signal emitted from the thermometer, each measurement was finished in 15 s.
Data analysis and statistics
Date were presented as mean ± standard deviation (SD) and analyzed by SAS 9.3 (SAS Institute Inc., Cary, NC, USA). One-way ANOVA was used to analyze differences of THI values under high, moderate, and low THI conditions.
To analyze effects of THI on RT and BSTs, the following model of PROC GLM was used:
where Yij is the dependent variable (RT and BSTs) of cow j at THI i, μ is the overall mean, Ti is the fixed effect of THI (i: 1, 2, and 3 represent high, moderate, and low THIs, respectively), b1Xj is the covariant factor of DIM, b2Xj is the covariant factor of milk yield, Cj is the fixed effect of cow (j: 1, ..., 488), and εij is the random residual error. When significant interactions (P < 0.05) were detected, adjustments to the level of significance of the P value were used to account for the comparisons among different THI conditions using Tukey-Kramer adjustments. Pearson’s partial correlation by counting the DIM, milk yield, and cow into considerations was used to calculate the correlation between BSTs and THI, and RT, respectively.
The segment regression analysis, which integrates the inflection point into the regression and obtains the most optimum value when the residual sum of squares showing lowest, was used to calculate the inflection point of RT and the mean and maximum forehead temperatures under different THI conditions.
The inflection points on RT, mean, and maximum forehead temperatures were calculated by the following formula, respectively:
where yRT, ymean, and ymax are the RT, mean, and maximum forehead temperature, respectively (°C), and x is the THI.
Significance and great significance were declared as P < 0.05 and P < 0.01, respectively.
Results
Rectal temperature
As shown in Table 1, THI ranged from 58 to 84 with significant differences (P < 0.01) among high (81.8 + 1.339), moderate (76 ± 0.901), and low (67.6 ± 1.234).
In relation to the inflection point, RT changed rapidly when THI was above 74.1 than below (Fig. 4).
Body surface temperatures
Table 2 shows the BSTs under high, moderate, and low THI conditions. Both the mean and maximum temperatures of cheek and forehead as well as mean ear and rump temperatures were higher under high THI, followed by moderate THI, than low THI condition (all, P < 0.05). Both the mean and maximum temperatures of eye, flank, and rear udder, as well as maximum temperatures of ear and rump, were higher under high and moderate THI conditions, than low THI condition (all, P < 0.05). Referring to fore udder, the mean temperature was higher under high and moderate THI conditions, than low THI condition (P < 0.01), while the maximum temperature was higher under moderate THI than high and low THI conditions (P < 0.05).
Under high THI condition, the mean temperatures of eye, fore, and rear udder showed relatively high value with 36.30 ± 0.665, 36.08 ± 0.967, and 35.81 ± 0.900 °C, respectively, while the forehead showed a lower value (32.80 °C), whereas the maximum temperatures were also seen high values in eye, fore, and rear udder (37.30 ± 0.718, 36.80 ± 0.976, 36.44 ± 0.890 °C) and a lower value in forehead (33.66 ± 1.024 °C). These results are consistent with the region temperatures under moderate and low THI conditions.
The correlation coefficients of BSTs with THI were shown in Table 3. Both the mean and maximum temperatures of forehead showed the highest correlation coefficients (0.808 and 0.740) with THI. The mean and maximum forehead temperatures started to increase rapidly when THI was 71.4 (Fig. 5) and 66.8 (Fig. 6), respectively.
Correlations between BSTs and RT
As compared to averaged RT (38.86 ± 0.255 °C; seen in Table 1), the BSTs showed relatively low temperature (31.65–36.11 °C; seen in Table 4). Body temperatures were significantly correlated with RT (P < 0.01; Table 3). In relation to the mean temperatures, the forehead showed the highest correlation coefficient with RT (0.557), while the rear udder showed the lowest (0.343). And on the maximum temperatures, still the forehead had the highest correlation coefficient with RT (0.504), while the eye showed the lowest (0.259). Both the mean and maximum ear, cheek, flank, and rump temperatures showed relatively high coefficients with RT, while relatively low in both the mean and maximum eye, fore udder, and rear udder temperatures.
Discussion
Rectal temperature is a major physiological indicator to evaluate the impact of environment on growth and reproduction in dairy cows (West 1999). Our study also revealed that RT was higher under high THI than moderate and low THI conditions, which was consistent with a previous study (Bouraoui et al. 2002). The RT, however, varied when the environment temperature was beyond the regulation range of dairy cows (Bouraoui et al. 2002; Brown-Brandl et al. 2003), and the measurement of RT is time- and labor-consuming, and requires direct contact with cows, which may generate stressful experiences in animals (Suthar et al. 2012). Thus, the application of infrared thermography to measure BSTs is of increasing importance (Schaefer et al. 2007), especially referring to detect disease in dairy cows (Schutz and Bewley 2009; Adams et al. 2013). However, BST is influenced by various external and internal factors such as breed, milk production, parity, season, and climatic conditions (Bewley et al. 2008; Suthar et al. 2012; Adams et al. 2013; Liang et al. 2013). Despite the limitation of BST to reflect body core temperature, it is still practical for the early assessment of heat stress because of easy to use and requires minimal manpower as compared to the measure of RT and ruminal temperature as well.
In this study, all BSTs positively correlated with the THI; that is to say, the higher the THI is, the higher the BSTs are. Further, the mean BSTs were lower (2.75–7.21 °C) than RT, which was similar with the study (2–7 °C) of Martello et al. (2016). The inflection point of RT (THI = 74.1), at which the temperature began to surge, was higher than that of the mean and maximum temperatures of forehead (THI = 71.4 and 66.8, respectively). Since RT is still regarded as the most common temperature to reflect body core temperature (Sarkar et al. 2013). Thus, BSTs were more sensitive to thermal environment than RT, suggesting the variability of BST to ambient environment.
Eye temperature as a stress indicator has been investigated in both human (Pavlidis et al. 2000) and animal studies (Stewart et al. 2008) and was suitable for indicating core temperature as not affected by environment (Gloster et al. 2011. In our study, the variation of eye temperature was smaller compared with other regions under high, moderate, and low THI conditions. The correlation coefficient between the mean temperature of eye and RT was 0.379, which was similar with a previous study (0.430) (Martello et al. 2016). The coefficient of the maximum temperature of eye with RT was much lower (0.259) than other studies (0.520 and 0.300) (Martello et al. 2016; Salles et al. 2016). This inconsistent result may be caused by two main reasons: (1) different selected eye areas in different studies. In this experiment, we used the specific circles to analyze the infrared thermal images of the eye and selected the mean and maximum temperature for analysis; (2) different environment in different studies. In this experiment, THI ranged widely (58.4–84.0) covering from non- to moderate heat stress conditions (Ingraham et al. 1976), since eye temperature hardly changed under a non-stress condition, which may be the reason resulting in a lower value of correlation coefficient. Furthermore, cows move their eyes frequently, which made it difficult to photograph, and accurate eye temperature data could only be obtained when IRC was vertically aligned with the eye. Therefore, we consider that the eye area may be not an ideal region to assess heat stress to reflect RT.
Compared with other regions, the mean temperature of ear (0.613), cheek (0.582), and rump (0.516) had relatively higher correlation coefficients with THI, which suggested that these three regions could reflect the changes of BSTs. Our finding agreed with the study of Hoffmann et al. (2013), showing that the back of the ear would be the preferable region for applying infrared thermography to detect the changes of BST. The correlation coefficients between the mean temperature of BSTs and RT were varied from 0.343 to 0.557, among which the correlation between the ear, cheek temperatures, and RT were not at higher values (r = 0.484, 0.472, respectively). These results were consistent with the study of Martello et al. (2016). The correlation coefficients of mean and maximum rump temperatures with RT also presented an intermediate value (0.476 and 0.430). Therefore, due to the low correlation coefficients, we consider that these regions are not the ideal regions for evaluation of heat stress to reflect RT by infrared thermography.
The head region (i.e., brain), which is near the central nervous system regulating body temperature (Weschenfelder et al. 2013), was a suitable indicator of core body temperature because of its proximity to the brain (Mccafferty 2007; Kessel et al. 2010). Our study revealed that the forehead temperature started to increase rapidly when THI was at 71.4 (mean) and 66.8 (maximum). Given that THI reaching at 72 indicated no stress or relatively slight heat stress in cows (Ingraham et al. 1976), the inflection points of forehead occurred during a non- or slight heat stress condition. Further, the non-susceptibility to smudge of forehead (Hoffmann et al. 2013) made it easy to photograph. More importantly, the correlation coefficient between mean forehead temperature and THI was up to 0.808, which was consistent with a recent result (0.810) (Salles et al. 2016). Whereas that between the mean forehead temperature and RT was also the highest (0.557), which was similar with the previous study (0.600) (Martello et al. 2016). This above suggests that the forehead was sensitive in the thermal environment and suitable for monitoring early heat stress by infrared thermography, and showed a relatively high correlation coefficient between forehead temperature and RT.
The fore and rear udder showed relatively higher temperatures than other regions (except for the eye), which was consistent with Montanholi et al. (2008). This finding may be due to the thinner skin thickness and lower hair density (Arkin et al. 1991). The fore udder temperatures were higher than the rear udder temperatures, which we assumed that fore udder was shielded by the side legs leading to less heat emission. Nevertheless, it is not clear why the maximum temperature of fore udder was higher under moderate THI than high THI condition. Further, the measurement of both fore and rear seemed difficult in placing the infrared thermography to photograph and needed additional labor to lift the tail when measured rear udder temperature. Besides, the tail easily contacts feces especially when cows were laying, which resulted in an inaccurate measurement, as proven in our previous study that the degree of smudge had a great influence on udder surface temperature (Peng et al. 2016). Moreover, the coefficients of fore and rear udder with THI and RT were low. Therefore, we consider that the fore and rear udder are not the ideal regions for evaluation of heat stress to reflect RT by infrared thermography.
The correlation coefficient of the mean flank temperatures to THI (r = 0.543) was inconsistent with the study of Salles et al. (2016) (r = 0.810). The infrared images of the flank were easy to obtain, which was the advantage for using infrared thermography to monitor. However, due to its close distance to rumen, the flank temperature was considered as a good indicator to reflect the rumen temperature (Getty 1975; Montanholi et al. 2008), and thus not reliable to reflect RT measured by IRT. However, since rumen generated heat in ruminant animals (Czerkawski 1980; Beatty et al. 2008), the flank temperature reflecting rumen temperature may probably be useful to evaluate heat stress, however, a low correlation coefficient (0.430) with RT.
As aforementioned, measurements of reflecting body core temperature have various limitations. Such as, the BST is easily influenced by external and internal factors (Bewley et al. 2008; Suthar et al. 2012; Adams et al. 2013; Liang et al. 2013), the ruminal temperature is affected by water and feed intake (Adams et al. 2013), while the measurement of RT is time-consuming and requires manpower (Suthar et al. 2012; Ammer et al. 2016). Despite the limitation, our study would provide more specific information of BST to measure body core temperature.
Conclusions
The averaged BST was lower than RT under the same THI conditions. The THI conditions where mean and maximum forehead temperatures started to increase rapidly (71.4 and 66.8) were lower than that where RT started to increase quickly (74.1), suggesting the variability of BST to reflect body core temperature. The correlation coefficients of mean and maximum forehead temperatures to THI were 0.808 and 0.740, and were 0.557 and 0.504 to RT, all showing higher values than other coefficients of region temperatures with THI and RT, respectively. Thus, we conclude that BSTs were more sensitive to thermal environment than RT, suggesting the potential of using IRT-measured BSTs to early detect heat stress in cows. In addition, the forehead is a relatively reliable region to early assess the heat stress reflecting RT using IRT compared to the eye, ear, cheek, flank, rump, fore udder, and rear udder regions.
References
Adams AE, Olea-Popelka FJ, Roman-Muniz IN (2013) Using temperature-sensing reticular boluses to aid in the detection of production diseases in dairy cows. J Dairy Sci 96:1549–1555. https://doi.org/10.3168/jds.2012-5822
Alzahal O, Alzahal H, Steele MA, Van Schaik M, Kyriazakis I, Duffield TF, McBride BW (2011) The use of a radiotelemetric ruminal bolus to detect body temperature changes in lactating dairy cattle. J Dairy Sci 94:3568–3574. https://doi.org/10.3168/jds.2010-3944
Ammer S, Lambertz C, Gauly M (2016) Comparison of different measuring methods for body temperature in lactating cows under different climatic conditions. J Dairy Res 83:165–172. https://doi.org/10.1017/S0022029916000182
Arkin H, Kimmel E, Herman A, Broday D (1991) Heat transfer properties of dry and wet furs of dairy cows. T Asae 34:2550–2558. https://doi.org/10.13031/2013.31905
Beatty DT, Barnes A, Taylor E, Maloney SK (2008) Do changes in feed intake or ambient temperature cause changes in cattle rumen temperature relative to core temperature?[J]. J Therm Biol 33(1):12–19. https://doi.org/10.1016/j.jtherbio.2007.09.002
Berry RJ, Kennedy AD, Scott SL, Kyle BL, Schaefer AL (2003) Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: potential for mastitis detection. Canadian J Anim Sci 83(4):687–693. https://doi.org/10.4141/A03-012
Bewley JM, Einstein ME, Grott MW, Schutz MM (2008) Comparison of reticular and rectal core body temperatures in lactating dairy cows. J Dairy Sci 91:4661–4672. https://doi.org/10.3168/jds.2007-0835
Bouraoui R, Lahmar M, Majdoub A, Djemali M, Belyea R (2002) The relationship of temperature-humidity index with milk production of dairy cows in a mediterranean climate. Anim Res 51:479–491. https://doi.org/10.1051/animres:2002036
Brown-Brandl T, Nienaber MJA, Eigenberg RA, Hahn GL, Freetly H (2003) Thermoregulatory responses of feeder cattle. J Therm Biol 28:149–157. https://doi.org/10.1016/S0306-4565(02)00052-9
Bryant J, López-Villalobos RN, Pryce JE, Holmes CW, Johnson DJ (2007) Quantifying the effect of thermal environment on production traits in three breeds of dairy cattle in New Zealand. New Zealand J Agric Res 50:327–338. https://doi.org/10.1080/00288230709510301
Burfeind O, Keyserlingk MAGV, Weary DW, Veira DM, Heuwieser W (2010) Repeatability of measures of rectal temperature in dairy cows. J Dairy Sci 93:624–627. https://doi.org/10.3168/jds.2009-2689
Chiang M, Lin FPW, Lin LF, Chiou HY, Chien CW, Chu SF (2008) Mass screening of suspected febrile patients with remote-sensing infrared thermography: alarm temperature and optimal distance. J Formos Med Assoc 107:937–944. https://doi.org/10.1016/S0929-6646(09)60017-6
Collier R, Dahl JGE, Vanbaale MJ (2006) Major advances associated with environmental effects on dairy cattle. J Dairy Sci 89:1244–1253. https://doi.org/10.3168/jds.S0022-0302(06)72193-2
Czerkawski JW (1980) A novel estimate of the magnitude of heat produced in the rumen. Br J Nutr 42:239–243
Do P, Borges PTBO, De MTLP, Gomes EF, Dallago BS, Fadel R (2013) Thermographic evaluation of climatic conditions on lambs from different genetic groups. Int J Biometeorol 57:59–66. https://doi.org/10.1007/s00484-012-0533-y
Firk R, Stamer E, Junge W, Krieter J (2002) Automation of oestrus detection in dairy cows: a review. Lives Prod Sci 75:219–232. https://doi.org/10.1016/S0301-6226(01)00323-2
García-Ispierto I, López-Gatius F, Bech-Sabat G, Santolaria P, Yániz JL, Nogareda C, De Rensis F, López-Béjar M (2007) Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 67:1379–1385. https://doi.org/10.1016/j.theriogenology.2007.02.009
Gaughan JB, Mader TL, Holt SM, Lisle A (2008) A new heat load index for feedlot cattle1. J Anim Sci 86:226–234. https://doi.org/10.2527/jas.2007-0305
Getty R (1975) Sisson and Grossman’s the anatomy of the domestic animals. Prog Electromagn Res 16:61–68
Gloster J, Ebert K, Gubbins S, Bashiruddin J, Paton DJ (2011) Normal variation in thermal radiated temperature in cattle: implications for foot-and-mouth disease detection. BMC Vet Res 7:73. https://doi.org/10.1186/1746-6148-7-73
Haqiya K, Hayasaka K, Yamazaki T, Shirai T, Osawa T, Terawaki Y, Nagamine Y, Masuda Y, Suzuki M (2017) Effects of heat stress on production, somatic cell score and conception rate in Holsteins. Anim Sci J 88:3–10. https://doi.org/10.1111/asj.12617
Hicks LC, Hicks WS, Bucklin RA, Shearer JK, Bray DR, Soto P, Carvalho V (2001) Comparison of methods of measuring deep body temperatures of dairy cows. In Livestock environment VI: Proc 6th Int. Symp. ASAE, pp. 432–438, Louisville, Kentucky, USA
Hoffmann G, Schmidt M, Ammon C, Rose-Meierhöfer S, Burfeind O, Heuwieser W, Berg W (2013) Monitoring the body temperature of cows and calves using video recordings from an infrared thermography camera. Vet Res Commun 37:91–99. https://doi.org/10.1007/s11259-012-9549-3
Ingraham RH, Stanley RW, Wagner WC (1976) Relationship of temperature and humidity to conception rate of Holstein cows in Hawaii. J Dairy Sci 59:2086–2090. https://doi.org/10.3168/jds.S0022-0302(76)84491-8
Joksimović-Todorović M, Davidović V, Hristov S, Stanković B (2011) Effect of heat stress on milk production in dairy cows. Biotech Anim Husbandry 27:1017–1023. https://doi.org/10.2298/BAH1103017J
Kessel L, Johnson L, Arvidsson H, Larsen M (2010) The relationship between body and ambient temperature and corneal temperature. Invest Ophth Vis Sci 51:6593–6597. https://doi.org/10.1167/iovs.10-5659
Lacetera N, Bernabucci U, Scalia D, Ronchi B, Kuzminsky G, Nardone A (2005) Lymphocyte functions in dairy cows in hot environment. Int J Biometeorol 50:105–110. https://doi.org/10.1007/s00484-005-0273-3
Liang D, Wood CL, McQuerry KJ, Ray DL, Clark JD, Bewley JM (2013) Influence of breed, milk production, season, and ambient temperature on dairy cow reticulorumen temperature. J Dairy Sci 96:5072–5081. https://doi.org/10.3168/jds.2012-6537
Loughmiller JA, Spire MF, Dritz SS, Fenwick BW, Hosni MH, Hogge SB (2001) Relationship between mean body surface temperature measured by use of infrared thermography and ambient temperature in clinically normal pigs and pigs inoculated with Actinobacillus pleuropneumoniae. Am J Vet Res 62(5):676–681. https://doi.org/10.2460/ajvr.2001.62.676
Ludwig N, Gargano M, Luzi F, Carenzi C, Verga M (2010) Applicability of infrared thermography as a non invasive measurements of stress in rabbit. World Rabbit Sci 15:199–206
Martello LS, Silva SDL, da Costa GR, da Silva CRRP, Leme PR (2016) Infrared thermography as a tool to evaluate body surface temperature and its relationship with feed efficiency in Bos indicus cattle in tropical conditions. Int J Biometeorol 60:173–181. https://doi.org/10.1007/s00484-015-1015-9
Mccafferty DJ (2007) The value of infrared thermography for research on mammals: previous applications and future directions. Mammal Rev 37:207–223. https://doi.org/10.1111/j.1365-2907.2007.00111.x
Montanholi YR, Odongo NE, Swanson KC, Schenkel FS, Mcbride BW, Miller SP (2008) Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J Therm Biol 33:468–475. https://doi.org/10.1016/j.jtherbio.2008.09.001
Ng E, Kawb G, Chang WM (2004) Analysis of IR thermal imager for mass blind fever screening. Microvasc Res 68:104–109. https://doi.org/10.1016/j.mvr.2004.05.003
Oltenacu PA, Broom DM, Kirkwood JK, Weddell S, Hubrecht RC, Wickens SM (2010) The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim Welf 19:39–49
Pavlidis I, Levine JA, Baukol P (2000) Thermal imaging for anxiety detection. IEEE 2:315–318
Peng D, Chen J, Zhao Y, Han L, Li G, Yang C, Gu X (2016) Effect of smudgy degree on temperature distribution of the udder surface in dairy cow. J Anim Vet Sci 47:844–851
Rowsell HC (1972) A guide to environmental research on animals. Tr Inst Im Pastera 56:32–44
Salles MSV, da Silva SC, Salles FA, Roma LC, El Faro L, Mac Lean PAB, de Oliveira CEL, Martello LS (2016) Mapping the body surface temperature of cattle by infrared thermography. J Therm Biol 62:63–69. https://doi.org/10.1016/j.jtherbio.2016.10.003
Sarkar S, Donn SM, Bhagat I, Dechert RE, Barks JD (2013) Esophageal and rectal temperatures as estimates of Core temperature during therapeutic whole-body hypothermia. J Pediatrics 162:208–210. https://doi.org/10.1016/j.jpeds.2012.08.039
Schaefer AL, Cook NJ, Church JS, Basarab J, Perry B, Miller C (2007) The use of infrared thermography as an early indicator of bovine respiratory disease complex in calves. Res Vet Sci 83:376–384. https://doi.org/10.1016/j.rvsc.2007.01.008
Schutz MM, Bewley JM (2009) Implications in changes in core body temperature. Pages 39–54 in Tri-State Dairy Nutr. Conf. Proc., Columbus, OH
Stewart M, Wwbster J, Schaefer A, Cook N, Scott S (2005) Infrared thermography as a non-invasive tool to study animal welfare. Anim Welf 14:319–325
Stewart M, Stafford KJ, Dowling SK, Schaefer AL, Webster JR (2008) Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol Behav 93:789–797. https://doi.org/10.1016/j.physbeh.2007.11.044
St-Pierre NR, Cobanov B, Schnitkey G (2003) Economic losses from heat stress by US livestock industries. J Dairy Sci 86:E52–E77. https://doi.org/10.3168/jds.S0022-0302(03)74040-5
Suthar V, Burfeind O, Bonk S, Voigtsberger R, Keane C, Heuwieser W (2012) Factors associated with body temperature of healthy Holstein dairy cows during the first 10 days in milk. J Dairy Res 79:135–142. https://doi.org/10.1017/S0022029911000896
Wang JP, Bu DP, Wang JQ, Luo XK, Guo TJ, Wei HY, Zhou LY, Rastani RR, Baumgard LH, Li FD (2010) Effect of saturated fatty acid supplementation on production and metabolism indices in heat-stressed mid-lactation dairy cows. J Dairy Sci 93:4121–4127 https://doi.org/10.3168/jds.2009-2635
Weschenfelder AV, Saucier L, Maldague X, Rocha LM, Schaefer AL, Faucitano L (2013) Use of infrared ocular thermography to assess physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci 95:616–620. https://doi.org/10.1016/j.meatsci.2013.06.003
West JW (1999) Nutritional strategies for managing the heat-stressed dairy cow. J Anim Sci 77:21
West JW, Mullinix BG, Bernard JK (2003) Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J Dairy Sci 86:232–242. https://doi.org/10.3168/jds.S0022-0302(03)73602-9
Younes RB, Ayadi M, Najar T, Caccamo M, Schadt I, M’Rad MB (2011) Hormonal (thyroxin, cortisol) and immunological (leucocytes) responses to cistern size and heat stress in Tunisia. Life Sci 11:332–338
Acknowledgements
We express sincere thanks to Mr. Dengsheng Sun for the helpful comments and Mr. Chuang Zhang and Mr. Guangju Wang from Chinese Academy of Agricultural Sciences for their assistants for this experiment. And many thanks to all members from the dairy farm for their support and kind help.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFD0500507, 2017YFD0502003), the Beijing Dairy Industry Innovation Team Project (BAIC06-2017), and The Agricultural Science and Technology Innovation Program (ASTIP-IAS07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The experimental protocols were approved by the Experimental Animal Care and Committee of Institute of Animal Science, Chinese Academy of Agricultural Sciences (approval number 2016IAS018).
Rights and permissions
About this article
Cite this article
Peng, D., Chen, S., Li, G. et al. Infrared thermography measured body surface temperature and its relationship with rectal temperature in dairy cows under different temperature-humidity indexes. Int J Biometeorol 63, 327–336 (2019). https://doi.org/10.1007/s00484-018-01666-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-01666-x