Abstract
It is widely accepted that substantial nighttime sap flux (J s,n) or transpiration (E) occurs in most plants, but the physiological implications are poorly known. It has been hypothesized that J s,n or E serves to enhance nitrogen uptake or deliver oxygen; however, no clear evidence is currently available. In this study, sap flux (J s) in Eucalyptus grandis × urophylla with apparent stem photosynthesis was measured, including control trees which were covered by aluminum foil (approximately 1/3 of tree height) to block stem photosynthesis. We hypothesized that the nighttime water flux would be suppressed in trees with lower stem photosynthesis. The results showed that the green tissue degraded after 3 months, demonstrating a decrease in stem photosynthesis. The daytime J s decreased by 21.47 %, while J s,n decreased by 12.03 % in covered trees as compared to that of control, and the difference was statistically significant (P < 0.01). The linear quantile regression model showed that J s,n decreased for a given daytime transpiration water loss, indicating that J s,n was suppressed by lower stem photosynthesis in covered trees. Predawn (ψ pd) of covered trees was marginally higher than that of control while lower at predawn stomatal conductance (g s), indicating a suppressed water flux in covered trees. There was no difference in leaf carbon content and δ13C between the two groups, while leaf nitrogen content and δ15N were significantly higher in covered trees than that of the control (P < 0.05), indicating that J s,n was not used for nitrogen uptake. These results suggest that J s,n may act as an oxygen pathway since green tissue has a higher respiration or oxygen demand than non-green tissue. Thus, this study demonstrated the physiological implications of J s,n and the possible benefits of nighttime water use or E by the tree.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants do not completely close their stomata during the night and inevitably lose water, which is counterproductive, because they minimize water loss during daytime carbon assimilation. It is reported that a substantial amount of nighttime transpiration (E) occurs in most plant species within different biomes or environments (Forster 2014). Nighttime E is considered to enhance nitrogen uptake (hypothesis I), for example, desert shrubs uptake more 15N as compared to those with blocked nighttime E (Snyder et al. 2008). Nighttime E or sap flux (J s) keeps the stomata open and was previously considered to enhance CO2 diffusion into the leaves to optimize photosynthesis of the following morning (Scholz et al. 2007; Barbeta et al. 2012) (hypothesis II). However, a recent study on the sunflower plant indicates that predawn stomatal conductance (g s) for less than 15 min has a minimal influence on photosynthesis the next day and thus refutes this hypothesis (Auchincloss et al. 2014). Results from studies on Arabidopsis thaliana and two Eucalyptus trees suggest that nighttime water flux is a result of the circadian rhythm (hypothesis III) (Dodd et al. 2005; de Dios et al. 2013; Resco de Dios et al. 2013). Active nighttime J s could probably be helpful for oxygen delivery to the xylem as a mechanism to increase rate of growth (Daley and Phillips 2006); however, no experimental evidence is currently available (hypothesis IV). Nighttime E, i.e., stomata remaining open, was also considered as a stress response to drought by several groups (hypothesis V) (e.g., Cavender-Bares et al. 2007; Phillips et al. 2010; Zeppel et al. 2012).
Large amounts of water transpired during the day could result in a substantial deficit of water in the stem, necessitating increased water uptake during the night, especially at the onset of dusk (Fuentes et al. 2013). Therefore, nighttime water flux is affected by daytime physiological process (Ludwig et al. 2006); for example, studies on nighttime open stomata in Vicia faba showed that nighttime g s is significantly affected by photosynthesis (Easlon and Richards 2009). Another study showed that midnight g s positively correlated with midday g s in Quercus rubra (Barbour et al. 2005). Cavender-Bares et al. (2007) found that midnight g s has a positive relationship with daytime leaf hydraulic conductance. Biological traits, such as non-foliar, stem photosynthesis, could exert some influence on water use characteristics similar to that by leaves. Species showing stem photosynthesis are expected to have higher whole-shoot water use efficiency (WUE) due to re-fix of respired CO2 (Nilsen et al. 1993; Vick and Young 2009). In contrast, an increase in WUE was observed when stem photosynthesis in Eucalyptus miniata was blocked, suggesting that stem photosynthesis has a strong influence on carbon-water balance and boosts biomass (Cernusak and Hutley 2011). Despite the improvement of WUE in mangrove saplings, hydraulic conductance and E are lowered in the absence of stem photosynthesis, indicating the important role of stem photosynthesis in regulating water flux (Schmitz et al. 2012).
The influence of stem photosynthesis is not limited to carbon fixation or radial growth but includes canopy physiology. For example, an enhancement in leaf photosynthetic capacity and the leaf nitrogen content following defoliation was observed in Eucalyptus globulus seedlings due to more light shed on the stems, considered as a compensation for loss of carbon fixation by leaves (Eyles et al. 2009). The leaf photosynthetic capacity increased by 10.3 % in mangrove saplings after stem photosynthesis was blocked, indicated a possible increase in leaf nitrogen content (Schmitz et al. 2012). However, the hydraulic conductance or E was lowered in the covered saplings, suggesting an important hydraulic function of chloroplasts. A similar study was conducted in Prunus ilicifolia, Umbellularia californica, and Arctostaphylos manzanita, where the authors reported that chlorophyll in the stem is degraded and radial growth is slowed down when stems were covered with aluminum foil and concluded a positive contribution of stem photosynthesis to budding and early carbon intake (Saveyn et al. 2010). The increase in leaf photosynthesis and nitrogen content is a compensation strategy for maintaining carbon balance when stem photosynthesis decreases; therefore, enhancement of WUE is more likely due to the increase in photosynthetic capacity.
This study was performed to quantify the nighttime water flux of a Eucalyptus tree species with apparent stem photosynthesis and to ascertain the effect of stem photosynthesis on nighttime water flux and the possible physiological implication. We hypothesize that the nighttime J s would be reduced in trees with decreased stem photosynthesis. If J s helps plants to acquire nitrogen, the nitrogen content will be decreased in trees when stem photosynthesis is blocked (Snyder et al. 2008) (hypothesis I); on the contrary, if an increase in photosynthetic capacity results from the decrease in J s or E, the nitrogen content will be increased (Eyles et al. 2009). Due to the decrease in water transport efficiency (Schmitz et al. 2012), degradation of chlorophyll and therefore the decrease in oxygen demand, the nighttime J s for a given transpiration water loss could be lowered in trees with lower stem photosynthesis. Linking stem photosynthesis and nighttime J s could help us understand the eco-physiological significance or the possible metabolic benefits of nighttime water loss.
Materials and methods
Site description and experimental design
This study was conducted in the Huangmian state forest farm (109° 54′ E, 24° 46′ N), which has been used since the last 6 years, mainly for the plantation of Eucalyptus grandis × urophylla, a hybrid with a high growth rate. The farm is located in a mid-subtropical and lower subtropical transition zone and is predominantly considered as a low mountainous region (Zhang et al. 2004). The research site was at an elevation of 219 m. This region receives abundant radiation and rainfall, with an annual pan evaporation of 1426–1650 mm, annual precipitation of 1750–2000 mm, and annual average temperature of 19 °C (Zhang et al. 2004). Forest soils are mainly red and yellow, derived from sandstone and sand. For this timber forest, phosphorus fertilization was used. The LAI during the experiment is 1.6 m2 m−2 and stem density is 1375 NO ha−1.
Sap flux sensors were deployed on the 12 trees selected for this study, on 21 April 2013, and the corresponding environmental variables were measured. A total of 6 trees of the selected 12 trees were covered by aluminum foil while another 6 trees with similar biometric parameters were considered as control. The cover length was approximately 4 to 4.5 m from the ground, which accounted for approximately one third of the tree height. The aluminum foil would reflect sunlight and thus blocked photosynthesis in the stem. After 3 months of shade conditions, leaf water potential and stomatal conductance were measured between 16 and 20 July 2013. In order to quantify the effect of lowered stem photosynthesis on the physiology of the tree canopy, we measured the carbon (C) and nitrogen (N) content as well as the isotopic signatures for carbon and nitrogen (δ13C, δ15N) in the leaves at the end of the experiment. We also compared the tree diameter in July with that in April to assess tree growth response to shade conditions. In total, we obtained data for daytime sap flux for 66 days, nighttime sap flux for 69 days, and environmental variables data for 82 days. The experiments were completed on 31 July 2013. The characteristics of trees studied are summarized in Table 1.
Sap flux measurements
Estimates of sap flux using the standard equation provided by Granier (1987) probably significantly underestimate tree water use since the parameters in the original equation are species-specific; therefore, we used the calibrated equation by Hubbard et al. (2010) who calibrated the E. grandis × urophylla hybrid using similar age and size as this study. Granier thermal dissipation probes (TDP) were directly inserted into the xylem of the selected trees to measure sap flux (Granier 1987). Each TDP sensor consisted of a pair of 20-mm long, 2-mm thick stainless steel probes installed approximately 10 to 15 cm apart along the axis of the hydroactive xylem. The upper probe was heated using constant 0.2-W power and 120-mA DC supply, whereas the lower probe remained unheated. Instantaneous temperature difference between the probes was converted into a voltage value, recorded every 30 s, and stored as 10-min averages using a Delta-T logger (DL2e, UK) (Zhao et al. 2005). Finally, the sap flux (J s) density (g H2O m−2 s−1) was calculated as previously described by Hubbard et al. (2010):
where ΔT m is the temperature difference obtained under zero flux conditions and ΔT is the instantaneous temperature (Granier 1987). ΔT m was determined separately for each tree over 7 days to avoid the underestimation of nocturnal sap flux (Lu et al. 2004; Moore et al. 2008). Nighttime sap flux (J s,n) is defined as the sum of sap flux occurring at PAR = 0. After the deployment of TDP, it is preferred to allow for discrepancies in sapwood dynamics until a homeostasis is achieved; therefore, sap flux data obtained for the month of April 2013 were discarded and not considered for analysis.
Environmental variables measurements
Data on photosynthetically active radiation (PAR), air temperature (T), and air humidity (RH), wind speed (m s−1) were obtained from the observation tower (25 m) in the forest. Radiation (LI-COR, Lincoln, USA), temperature, and humidity (Delta-T Devices Ltd., Cambridge, UK) sensors were deployed on the top of the tower. We calculated vapor pressure deficit (VPD, kPa) by combining the air temperature and RH:
where a, b, and c are fixed parameters, namely 0.611 kPa, 17.502 (without units), and 240.97 °C, respectively.
Three volumetric soil water content sensors (ML2x, Delta-T Devices, UK) were randomly placed at 20- to 30-cm depth at the research site in order to measure volumetric soil water content in the soil. The volumetric soil water content (cm3 cm−3) was taken as the average of three measurements.
Sapwood width, tree height, and diameter increment
To avoid destruction of sample trees in the experimental plot, 25 trees with varying diameters were cut down around the research site and wood discs samples were collected. Sapwood width was determined visually from color changes between heartwood and sapwood (Table 1). Diameter at breast height (DBH) was directly measured at 1.3 m above the ground using a DBH ruler. Tree height was measured using a Tandem-360R/PC type altimeter (Suunto, Finland) and a trigonometric formula.
DBH increment (%) is defined as the percentage difference in tree diameter at the end of the experiment compared to the initial diameter:
where DBHApril is the diameter measured on 20 April 2013 and DBHJuly is that measured on 20 July 2013.
Measurement of leaf water potential and stomatal conductance (g s)
Leaf water potential of the 12 studied trees was measured with a Scholander-type pressure chamber (PMS 1000, PMS Instrument Company, Albany, OR, USA), where 3 to 5 branches were collected from sunny locations on each studied tree using an 8-m length lopper and a ladder. Leaf water potential (ψ) measurements were performed in situ immediately after cutting. Predawn leaf water potential (ψ pd) was measured between 5:00 and 6:00 a.m.
Leaf stomatal conductance (g s) was measured by SC-1 Leaf Porometer (Decagon Devices, Inc., Pullman, Washington), where 3 to 5 branches from each tree and 5 leaves per branch were chosen for the measurement between 9:00 and 12:00 a.m. The same branches were used for leaf water potential measurements as well. When the transpiration or g s was high, the leaf water potential would be very low, thus leading to a highly negative relationship between the two variables (g s vs. ψ). The measurement of g s took approximately 10 min per branch, which could have led to large errors during comparison of the predawn g s between the two groups; so we firstly measured ψ and g s of 3 studied trees in each group to establish a predictive relationship and extrapolated the result to assess predawn g s of the 12 studied trees.
Biochemical parameters
Approximately 25 leaves from each studied tree were fixed at 105 °C to obtain a constant weight and dried at 80 °C, crushed using a mortar and pestle, and then sieved through a 250 mesh prior to testing. The C and N contents (mg g−1) were analyzed using a VARIO EL3 precision elemental analyzer (Elementar, Germany). Approximately 3- to 5-mg samples were analyzed on a Delta XP isotope ratio mass spectrometer (Finnigan MAT253) following pyrolysis in a high-temperature furnace (Thermoquest TC/EA, Finnigan MAT). The C and N isotopic signatures (δ13C and δ15N, respectively, measured in parts per thousand) were determined using the dried leaves via a standard procedure to Pee Dee Belemnite (PDB) limestone and with N2 as the standard.
where, R sample and R standard are 13C/12C or 15N/14 N ratios of samples and the PDB and N2 standard for carbon and nitrogen, respectively.
Statistical analysis
Statistical analyses of leaf water potential and g s, leaf carbon and nitrogen contents, and carbon and nitrogen isotope ratios were performed using an independent-sample t test and the predictive analysis software (PASW, IBM, USA). The least significant difference at P = 0.05 denoted significance. To quantify the relationship of daily total J s,n and VPD, we used an exponential saturation model [y = a × (1 – e −bx)] or linear model (y = ax + b) to predict J s,n from VPD, where a and b were fitting parameters (Ewers et al. 2002; 2005). The best model was that with the lowest Akaike information criterion (AIC) value (Burnham and Anderson 2002).
Nighttime sap flux mainly contributed to transpiration or stem water recharge and regression analysis would show the extent of this contribution by J s,n. Previous studies showed that J s,n or g s was affected by daytime water flux and environmental variables (Barbour et al. 2005; Easlon and Richards 2009); thus, quantification of the effects of daytime sap flux (J s,d) is crucial to understand the mechanism of nighttime water use. In this study, to test the extent to which the J s,n was influenced by J s,d, we fitted linear models using both OLS and quantile regressions.
Quantile regression seeks to complement classical linear regression analysis to estimate all parts of the response distribution conditional to the predictor variable, thus providing a more comprehensive characterization of the effects than those provided by estimates of the conditional mean made with OLS regression (Cade et al. 2005). Quantile regression is a non-parametric test that makes no assumptions regarding normality of distribution or variance homogeneity. Thus, quantile regression overcomes various problems that OLS regression is confronted with. For instance, by focusing on the mean, information about the tails of a distribution is lost. Additionally, OLS regression is rather sensitive to extreme outliers, which can distort the results significantly. By contrast, being based on absolute values rather than on squared deviations, quantile regression reduces outlier effects (Cade and Noon 2003; Ricotta et al. 2010). We estimated the quantile regression functions of 0.25, 0.5, and 0.75 quantiles using the R package “quantreg” (Koenker 2013).
Results
Variations of J s,n and environmental variables
The J s,n varied with VPD in both covered and control trees, J s,n decreased under shade conditions. However, the J s,n did not show monthly or seasonal variation (Fig. 1). Air temperature increased during the experiment while PAR showed relatively limited increase (Fig. 1). After plotting J s,n against nighttime VPD, we found that both were strongly controlled by VPD. The J s,n and VPD of control was well described by exponential saturation model while was a linear model for covered trees (Fig. 2). The average volumetric soil water content was 37.2 % (data not shown), indicating there was no drought during the experiment which was performed during the wet season.
Nighttime sap flux (J s,n) of Eucalyptus grandis × urophylla covered trees (filled circles) and those without aluminum foil (opened circles). The environmental variables, photosynthetic active radiation (PAR, blue dots), daytime air temperature (red dots), and nighttime vapor pressure deficit (VPD) (solid line) during the experiments were shown. Sap flux data are expressed as the mean of six individual trees (n = 6), bars indicating standard deviation (SD) are omitted for clarity. ‡ P < 0.1; *P < 0.05
The relationship of nighttime sap flux (J s,n) and VPD of E. grandis × urophylla trees without (a) and with aluminum foil (b). The best fitted lines are y = 5.4 × (1 − e −5.59) (r 2 = 0.362) and y = 4.14x + 2.1 (r 2 = 0.288) for the control and covered trees, respectively. Bars indicating SD are omitted for clarity. ***P < 0.001
Suppressed J s,n in covered trees
The J s of covered trees was plotted against control and the results showed that J s,d and J s,n of covered trees was 78.53 and 87.97 % of the control trees, respectively, and these were highly significantly different from the slope of 1 (P < 0.001, P = 0.007 for daytime and nighttime J s, respectively) (Fig. 3), indicating that J s,n was suppressed in Eucalyptus trees with reduced stem photosynthesis. In total, there were 9 days that J s,n-control was higher than J s,n-covered trees at 0.1 level (P < 0.1) (Fig. 1, lower panel). Green tissue was degraded after 3 months of shade conditions, indicating lowered stem photosynthesis in covered trees (Fig. 4). When J s,n was plotted against J s,d by quantile regression model, it showed that in any quantiles (τ = 0.25, 0.5 and 0.75) or OLS could well describe the relationship between the two variables in control trees. In contrast, the two variables correlated well only at τ = 0.25 in covered trees (Fig. 5, Table 2). OLS regression showed that J s,d could explain 17.6 % of variance of J s,n in the control, while 3.7 % variance of J s,n was explained by J s,d in covered trees (Table 2). The slopes of each quantile were higher in the control than covered trees, indicating a more active J s,n (Table 2).
Scatter plot and quantile regression showing the relationship between nighttime sap flux (J s,n) and daytime sap flux (J s,d) in the control (a) and covered (b) trees. The gray lines correspond to the quantiles (0.25, 0.5, and 0.75), and the blue dotted line is the least-square estimate of the conditional mean function (see Table 2 for further information)
Leaf water potential, g s, and biochemical parameters
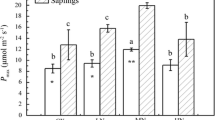
The highly negative correlation between leaf water potential and g s denoted an isohydric control of stomatal function (Fig. 6). The predawn g s was assessed based on the relationship of leaf water potential and g s (Fig. 6). Our prior verification showed that the leaf g s of detached and intact leaves could be alternative on the early morning (data not shown). There were no differences in ψ pd and predawn g s between the two groups; however, the ψ pd of covered trees was marginally higher than that of control while lower at predawn g s (Fig. 7), indicating a suppressed water flux in covered trees. The leaf carbon content and δ13C did not differ between the two groups, to the contrary, leaf nitrogen content and δ15N were higher in covered trees than in the control (P < 0.05) (Fig. 8). The δ13C in covered trees was more positive than that of control, indicating an enhancement of WUE, but not significantly (P = 0.21; Fig. 8c). Leaf nitrogen content and δ15N were positively correlated (r = 0.478, P = 0.116, data not shown), indicating δ15N could be used as a measure of nitrogen availability. Figure 9 showed links of possible physiological variables and nighttime water flux.
Conceptual diagram of the experimental study results, illustrating the suppressed nighttime sap flux in covered trees (control, left; covered trees, right). After being covered for 3 months, the chlorophyll in the stem of E. grandis × urophylla trees was degraded and thus the stem could not carry out photosynthesis. The aluminum foil had a strong effect on the stem not only in terms of reflecting sunlight, but in degradation of green tissue (approximately 2-mm thickness) (Fig. 4). When the root takes up water from the soil, the oxygen dissolved in the water is also absorbed, which could relieve anoxia. The xylem or sapwood parenchyma respires (release of CO2 in the internal parenchymatous tissues of wood) and consumes O2, and at the same time, sap flux may act as an oxygen delivery pathway. In the current study, the daytime sap flux was also decreased in covered trees, indicating that the daytime canopy transpiration was lowered. The circle indicating O2 generation and CO2 depletion suggests that simplified photosynthesis occurred in the stem, implicating re-fix of respired CO2 by green tissue
Discussion
We quantified the relationship between J s,n of E. grandis × urophylla and VPD, and the source of variance. J s,n was driven by VPD and also affected by daytime transpiration water loss. The physiological implication was assessed by analyzing the source of variance. Table 3 summarizes the hypotheses explaining J s,n, stomatal conductance or transpiration water loss occurring in plants and the related benefits. The inconsistency of studies that tested the same hypothesis, which is probably due to variations in the species selected and the experimental design, thus no confirmatory conclusion could be drawn. A conceptual model of this study is presented in Fig. 9.
Relationship between stem photosynthesis and J s,n
The relationship of J s,n and VPD in the control was non-linear but linear for the covered trees after partially blocking stem photosynthesis, indicating that nighttime E was more influenced by stomatal regulation in the covered trees while affected by stomatal and leaf energy balance or non-hydraulic signals, e.g., respiration of green tissue in control (Rosado et al. 2012). The J s,d was decreased by 21.47 % and J s,n was decreased by 12.03 %, demonstrating that J s,d was lowered further as compared to J s,n, thus daytime water use was influenced to a greater extent by covering the stem. Linear quantile regression model showed that J s,n of covered trees was not active for a given daytime transpiration water loss as compared to the control. Some studies have shown that chlorophyll content in green tissue is positively correlated with respiration and net photosynthesis. For example, in the study on branch chlorophyll content and photosynthesis, the authors found that stem photosynthesis and respiration are higher in species with more chlorophyll (Ren et al. 2009). In another study on stem photosynthesis of nine tree species, the authors concluded that photosynthesis is positively correlated with chlorophyll content and dark respiration (Berveiller et al. 2007). In this study, chlorophyll in covered trees was degraded which could have led to a reduction in respiration and demand for O2 (Fig. 4). The internal xylem would respire a larger quantity of CO2, and since most of the CO2 remains in the stem, CO2 concentration in the stem has been reported 500 times or even higher than the ambient CO2 (Teskey et al. 2008). The J s is considered as an oxygen delivery pathway (Gansert 2003); thus, it is suggested that the suppressed J s,n was due to lower O2 demand in internal xylem or sapwood parenchyma. The Eucalyptus trees with stem photosynthesis do not show an increase in internal stem CO2 content during the day; to the contrary, stem photosynthesis would generate additional O2 (Pfanz et al. 2002; Wittmann and Pfanz 2014). However, stem green tissue is transformed from “oxygen source” to “oxygen sink” during the night (when there is no light available), while O2 delivered by J s would relieve the anoxia (Gansert 2003; Sorz and Hietz 2008; cf. Fig. 9). Some studies have shown that oxygen radially transported through lenticels is probably consumed before it reaches the internal living tissue, thus the transpiration stream plays an important role in the gas balance of the internal stem (Mancuso and Marras 2003).
Physiological significance of suppressed J s,n
We found that J s,n was suppressed in covered trees, thus confirming our hypothesis. The decrease in J s,n is more likely due to the reduction of whole tree hydraulic conductance (Schmitz et al. 2012). However, we did not find a decrease in leaf nitrogen content; to the contrary, the leaf nitrogen content was higher in covered trees than in the controls. This is verified by δ15N value, i.e., higher in covered trees than the control, indicating that the covered trees have stronger nitrogen availability or uptake when stem photosynthesis is blocked (Craine et al. 2009). This is contrary to results from the study on phreatophytic shrubs (Snyder et al. 2008), in which the authors concluded that the nighttime water flux increases nitrogen uptake. Although there are some reports illustrating a positive relationship between nighttime g s and leaf nitrogen content (cf. Table 3), in studies on Helianthus species (Howard and Donovan 2007), A. thaliana (Christman et al. 2009a), and two Populus trees (Howard and Donovan 2010), the authors concluded that nighttime g s or E is affected to a greater extent by soil water content and not soil fertility. Since all the trees in this study were grown in the same environment and there was no drought conditions during the experiment, the results of this study do not support the hypothesis that nitrogen uptake is benefited by nighttime water use. Due to a positive correlation between nitrogen content and ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) activation, regeneration, and ultimately photosynthesis (Evans 1989), the enhancement of nitrogen content in covered trees may lead to an increase in photosynthesis, i.e., a “compensatory effect” when a loss of stem photosynthesis occurs (Eyles et al. 2009).
Nighttime water use accounts for 5 to 15 % total water use, sometimes even up to 30 % (Daley and Phillips 2006; Scholz et al. 2007; Caird et al. 2007b), which leads to a disequilibrium between predawn plant and soil water potential (Donovan et al. 2003). For example, Zeppel et al. (2010) attributed the disequilibrium to nighttime E in the study of water use characteristics of Eucalyptus parramattensis and Angophora bakeri. The leaf water potential is not equilibrated with soil and an apparent predawn g s exists, indicating an intensive water deficit in the stem due to E, which is consistent with more active J s,n in the control (Figs. 1 and 3). For instance, VPD could explain 28.8 % variance in covered trees as compared to 36.2 % seen in the control (Fig. 2). The suppressed E or J s in covered trees would inevitably lead to a decrease in carbon gain. At the end of the experiment, we found that the DBH increment was 3.5 % in the control and 2.5 % in covered trees (data not shown), suggesting a decrease in carbon income when stem photosynthesis was blocked. The reduction in radial stem growth or growth rate denotes a lowered oxygen demand, or in other words, water flux (Poorter et al. 1991); however, this logical analysis is rather speculative, thus needs further study to verify.
Implications for nighttime water use
The following hypotheses were formulated to explain the observation of nighttime water use by plants in the absence of available light, which seemed to be wasteful behavior because of no carbon gain (Table 3). Hypothesis (I) suggests a nutritional benefit through nighttime water flux, where nighttime E is a complemented method of nutrition acquisition (Robert E. Ricklefs pers. comm.). However, our results do not support this hypothesis; in contrast, the leaf nitrogen in covered trees was higher than that in control. To date, there is no clear evidence for a necessary association between nitrogen, inorganic nutrients, amino acid, plant hormones, and the transpiration stream (Tanner and Beevers 2001; Lambers et al. 2008; Christman et al. 2009a). Hypothesis (II) suggests that the stomata staying open during nighttime would decrease the CO2 diffusional limitations and thus increase carbon fixation. In a study on three tree species, Scholz et al. (2007) found that the proportion of nighttime water use is positively correlated with predawn g s and concluded that stomata staying open during predawn would enhance photosynthesis. However, in the study of predawn g s and morning photosynthesis in Helianthus annuus, the authors did not find a positive relationship between the two variables and morning photosynthesis was affected to a greater extent by biochemical limitations, thus hypothesis (II) was rejected (Auchincloss et al. 2014). In this study, active J s,n was proportional with predawn g s, and active nighttime water flux could be considered as a prerequisite for stomata staying open. Due to the difficulty in accessing the canopy, we did not measure leaf-level photosynthesis in situ; however, the lower nitrogen content in control trees suggested that biochemical limitations rather than stomatal barriers are more likely to occur. The positive δ13C in covered trees, i.e., higher WUE, is more likely attributable to higher photosynthetic capacity. So, we were inclined to conclude that the phenomenon of stomata staying open was not a guarantee of higher photosynthesis the following morning. Hypothesis (III) suggests that J s,n or E was controlled by a biorhythm and this was confirmed in A. thaliana and two Eucalyptus trees (Table 3). For example, Resco de Dios et al. (2013) used whole-tree chambers to characterize the degree to which biorhythm determines J s,n and reported that 23 to 56 % variance is explained by the circadian rhythm, which matched the influence of VPD. Hypothesis (IV) suggests that J s,n may act as an oxygen delivery pathway; for example, Daley and Phillips (2006) found that the J s,n of paper birch is substantial when compared to the other two co-occurring tree species and concluded that active J s,n is a mechanism for higher growth rate. It is plausible then, to link J s,n and oxygen delivery as J s is positively associated with O2 concentration (Gansert 2003). To our knowledge, there is no clear experimental evidence to support these hypotheses and no rebuttal either. Hypothesis (V) suggests J s,n or g s is the inevitable response to evaporative demand and this has been previously confirmed by many groups (cf. Forster 2014), including this study. However, hypothesis (V) is not an explanation for the benefit of nighttime water use, which considers that the phenomenon of stomata staying open is a physiological adaptation, especially in dry environments (Ogle et al. 2012; Rosado et al. 2012). Therefore, we conclude that trees with greater J s,n are fast-growing, which can probably explain previous contradicting results.
Conclusion
In this study, Granier thermal dissipation probes were deployed to determine J s of E. grandis × urophylla and quantify the extent to which J s was suppressed after blocking stem photosynthesis. Sap flux, biochemical parameters, and water balance were substantially altered after 3 months of shade conditions, showing that nitrogen acquisition was enhanced in covered trees as expected when J s,n was suppressed, which rejected hypothesis (I). Based on the strong correlation between J s and oxygen concentration, we conclude that J s,n may function as a pathway for oxygen delivery, which is consistent to hypothesis (IV). Finally, we propose that the quantification of oxygen concentration in the internal xylem should be investigated in the future.
References
Auchincloss L, Easlon HM, Levine D, Donovan L, Richards JH (2014) Pre-dawn stomatal opening does not substantially enhance early-morning photosynthesis in Helianthus annuus. Plant Cell Environ 37:1364–1370
Barbeta A, Ogaya R, Peñuelas J (2012) Comparative study of diurnal and nocturnal sap flow of Quercus ilex and Phillyrea latifolia in a Mediterranean holm oak forest in Prades (Catalonia, NE Spain). Trees 26:1651–1659
Barbour MM, Buckley TN (2007) The stomatal response to evaporative demand persists at night in Ricinus communis plants with high nocturnal conductance. Plant Cell Environ 30:711–721
Barbour MM, Cernusak LA, Whitehead D, Griffen KL, Turnbull MH, Tissue DT, Farquhar GD (2005) Nocturnal stomatal conductance and implications for modeling δ18O of leaf-respired CO2 in temperate tree species. Funct Plant Biol 32:1107–1121
Berveiller D, Kierzkowski D, Damesin C (2007) Interspecific variability of stem photosynthesis among tree species. Tree Physiol 27:53–61
Buckley TN, Turnbull TL, Pfautsch S, Adams MA (2011) Nocturnal water loss in mature subalpine Eucalyptus delegatensis tall open forests and adjacent E. pauciflora woodlands. Ecol Evol 1:435–450
Burnham KP, Anderson DR (2002) Model selection and multimodel inference, 2nd edn. Springer, New York
Cade BS, Noon BR (2003) A gentle introduction to quantile regression for ecologists. Front Ecol Environ 1:412–420
Cade BS, Noon BR, Flather CH (2005) Quantile regression reveals hidden bias and uncertainty in habitat models. Ecology 86:786–800
Caird MA, Richards JH, Hsiao TC (2007a) Significant transpirational water loss occurs throughout the night in field-grown tomato. Funct Plant Biol 34:172–177
Caird MA, Richards JH, Donovan LA (2007b) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143:4–10
Cavender-Bares J, Sack L, Savage J (2007) Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiol 27:611–620
Cernusak LA, Hutley LB (2011) Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiol 155:515–523
Christman MA, Richards JH, McKay JK, Stahl EA, Juenger TE, Donovan LA (2008) Genetic variation in Arabidopsis thaliana for night-time leaf conductance. Plant Cell Environ 31:1170–1178
Christman MA, Donovan LA, Richards JH (2009a) Magnitude of nighttime transpiration does not affect plant growth or nutrition in well-watered Arabidopsis. Physiol Plant 136:264–273
Christman MA, James JJ, Drenovsky RE, Richards JH (2009b) Environmental stress and genetics influence night-time leaf conductance in the C4 grass Distichlis spicata. Funct Plant Biol 36:50–55
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992
Daley MJ, Phillips NG (2006) Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol 26:411–419
Dawson TE, Burgess S, Tu KP, Oliveira RS, Santiago LS, Fisher JB, Simonin KA, Ambrose AR (2007) Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol 27:561–575
de Dios VR, Turnbull MH, Barbour MM, Ontedhu J, Ghannoum O, Tissue DT (2013) Soil phosphorous and endogenous rhythms exert a larger impact than CO2 or temperature on nocturnal stomatal conductance in Eucalyptus tereticornis. Tree Physiol 33:1206–1215
Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630–633
Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84:463–470
Easlon HM, Richards JH (2009) Photosynthesis affects following night leaf conductance in Vicia faba. Plant Cell Environ 32:58–63
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Ewers BE, Mackay DS, Gower ST, Ahl DE, Burrows SN, Samanta SS (2002) Tree species effects on stand transpiration in northern Wisconsin. Water Resour Res 38:1103. doi:10.1029/2001WR000830
Ewers BE, Gower ST, Bond-Lamberty B, Wang CK (2005) Effects of stand age and tree species on canopy transpiration and average stomatal conductance of boreal forests. Plant Cell Environ 28:660–678
Eyles A, Pinkard EA, O’Grady AP, Worledge D, Warren CR (2009) Role of corticular photosynthesis following defoliation in Eucalyptus globulus. Plant Cell Environ 32:1004–1014
Forster MA (2014) How significant is nocturnal sap flow? Tree Physiol 34:757–765
Fuentes S, Mahadevan M, Bonada M, Skewes MA, Cox JW (2013) Night-time sap flow is parabolically linked to midday water potential for field-grown almond trees. Irrig Sci 31:1265–1276
Gansert D (2003) Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Ehr.). Plant Cell Environ 26:1803–1814
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol 3:309–320
Howard AR, Donovan LA (2007) Helianthus nighttime conductance and transpiration respond to soil water but not nutrient availability. Plant Physiol 143:145–155
Howard AR, Donovan LA (2010) Soil nitrogen limitation does not impact nighttime water loss in Populus. Tree Physiol 30:23–31
Hubbard RM, Stape J, Ryan MG, Almeida AC, Rojas J (2010) Effects of irrigation on water use and water use efficiency in two fast growing Eucalyptus plantations. For Ecol Manag 259:1714–1721
Koenker R (2013) Quantreg: quantile regression. R package version 5.05. [WWW document] URL http://cran.r-project.org/web/packages/quantreg/quantreg.pdf. Accessed 8 Jun 2014
Kupper P, Rohula G, Saksing L, Sellin A, Lõhmus K, Ostonena I, Helmisaari HS, Sõber A (2012) Does soil nutrient availability influence night-time water flux of aspen saplings? Environ Exp Bot 82:37–42
Lambers H, Chapin FS III, Pons JL (2008) Plant physiological ecology, 2nd edn. Springer-Verlag, Inc., New York
Lu P, Urban L, Zhao P (2004) Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: theory and practice. Acta Bot Sin 46:631–646
Ludwig F, Jewitt RA, Donovan LA (2006) Nutrient and water addition effects on day- and night-time conductance and transpiration in a C3 desert annual. Oecologia 148:219–225
Mancuso S, Marras AM (2003) Different pathways of the oxygen supply in the sapwood of young Olea europaea trees. Planta 216:1028–1033
Marks CO, Lechowicz MJ (2007) The ecological and functional correlates of nocturnal transpiration. Tree Physiol 27:577–584
Moore GW, Cleverly JR, Owens MK (2008) Nocturnal transpiration in riparian Tamarix thickets authenticated by sap flux, eddy covariance and leaf gas exchange measurements. Tree Physiol 28:521–528
Nilsen ET, Karpa D, Mooney HA, Field C (1993) Patterns of stem photosynthesis in two invasive legumes (Spartium junceum, Cytisus scoparius) of the California coastal region. Am J Bot 80:1126–1136
Ogle K, Lucas RW, Bentley LP, Cable JM, Barron-Gafford GA, Griffith A, Ignace D, Jenerette GD, Tyler A, Huxman TE et al (2012) Differential daytime and night-time stomatal behavior in plants from North American deserts. New Phytol 194:464–476
Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M (2002) Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften 89:147–162
Phillips NG, Lewis JD, Logan BA, Tissue DT (2010) Inter- and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol 30:586–596
Poorter H, Van der Werf A, Atkin OK, Lambers H (1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiol Plant 83:469–475
Ren FF, Sun GY, Hu YB, Fan CH, Cai SY (2009) A preliminary study on photosynthetic characteristics of chlorenchyma in several tree barks. Plant Physiol J 45:249–252
Resco de Dios V, Díaz-Sierra R, Goulden ML, Barton CVM, Boer MM, Gessler A, Ferrio JP, Pfautsch S, Tissue DT (2013) Woody clockworks: circadian regulation of night-time water use in Eucalyptus globulus. New Phytol 200:743–752
Ricotta C, Godefroid S, Rocchini D (2010) Invasiveness of alien plants in Brussels is related to their phylogenetic similarity to native species. Divers Distrib 16:655–662
Rohula G, Kupper P, Räim O, Sellin A, Sõber A (2014) Patterns of night-time water use are interrelated with leaf nitrogen concentration in shoots of 16 deciduous woody species. Environ Exp Bot 99:180–188
Rosado BHP, Oliveira RS, Joly CA, Aidar MPM, Burgess SSO (2012) Diversity in nighttime transpiration behavior of woody species of the Atlantic Rain Forest, Brazil. Agric For Meteorol 158–159:13–20
Saveyn A, Steppe K, Ubierna N, Dawson TE (2010) Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant Cell Environ 33:1949–1958
Schmitz N, Egerton JJG, Lovelock CE, Ball MC (2012) Light-dependent maintenance of hydraulic function in mangrove branches: do xylary chloroplasts play a role in embolism repair? New Phytol 195:40–46
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol 27:551–559
Snyder KA, James JJ, Richards JH, Donovan LA (2008) Does hydraulic lift or nighttime transpiration facilitate nitrogen acquisition? Plant Soil 306:159–166
Sorz J, Hietz P (2008) Is oxygen involved in beech (Fagus sylvatica) red heartwood formation? Trees 22:175–185
Tanner W, Beevers H (2001) Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci U S A 98:9443–9447
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Vick JK, Young DR (2009) Corticular photosynthesis: a mechanism to enhance shrub expansion in coastal environments. Photosynthetica 47:26–32
Wang H, Zhao P, Cai XA, Wang Q, Ma L, Rao XQ, Zeng XP (2007) Partitioning of night sap flow of Acacia mangium and its implication for estimating whole-tree transpiration. Chin J Plant Ecol 31:777–786
Wittmann C, Pfanz H (2014) Bark and woody tissue photosynthesis a means to avoid hypoxia or anoxia in developing stem tissues. Funct Plant Biol 41:940–953
Zeppel M, Tissue D, Taylor D, Macinnis-Ng C, Eamus D (2010) Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiol 30:988–1000
Zeppel MJB, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT (2012) Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol 193:929–938
Zhang L, Luo TX, Deng KM, Dai Q, Huang Y, Jiang ZF, Tao MY, Zeng KY (2004) Biomass and net primary productivity of secondary evergreen broadleaved forest in Huangmian Forest Farm, Guangxi. Chin J Appl Ecol 15:2029–2033
Zhao P, Rao XQ, Ma L, Cai XA, Zeng XP (2005) Application of Granier’s sap flow system in water use of Acacia mangium forest. J Trop Subtrop Bot 13:457–468
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant No. 41030638, 31170673, and 41275169) and the Natural Science Foundation of Guangdong Province (Grant No. S2012020010933). We greatly acknowledge Fei Gao, Xiuhua Zhao, Zhenzhen Zhang, and Liwei Zhu for their assistance in field work. We also thank Professor Ram Oren of Duke University for his comments of the early manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Zhou, J., Sun, Z. et al. Suppression of nighttime sap flux with lower stem photosynthesis in Eucalyptus trees. Int J Biometeorol 60, 545–556 (2016). https://doi.org/10.1007/s00484-015-1050-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-015-1050-6