Abstract
Responses of transpiration (E c) to rain pulses are presented for two semiarid tree species in a stand of Pinus tabulaeformis and Robinia pseudoacacia. Our objectives are to investigate (1) the environmental control over the stand transpiration after rainfall by analyzing the effect of vapor pressure deficit (VPD), soil water condition, and rainfall on the post-rainfall E c development and recovery rate, and (2) the species responses to rain pulses and implications on vegetation coverage under a changing rainfall regime. Results showed that the sensitivity of canopy conductance (G c) to VPD varied under different incident radiation and soil water conditions, and the two species exhibited the same hydraulic control (−dG c/dlnVPD to G cref ratio) over transpiration. Strengthened physiological control and low sapwood area of the stand contributed to low E c. VPD after rainfall significantly influenced the magnitude and time series of post-rainfall stand E c. The fluctuation of post-rainfall VPD in comparison with the pre-rainfall influenced the E c recovery. Further, the stand E c was significantly related to monthly rainfall, but the recovery was independent of the rainfall event size. E c enhanced with cumulative soil moisture change (ΔVWC) within each dry–wet cycle, yet still was limited in large rainfall months. The two species had different response patterns of post-rainfall E c recovery. E c recovery of P. tabulaeformis was influenced by the pre- and post-rainfall VPD differences and the duration of rainless interval. R. pseudoacacia showed a larger immediate post-rainfall E c increase than P. tabulaeformis did. We, therefore, concluded that concentrated rainfall events do not trigger significant increase of transpiration unless large events penetrate the deep soil and the species differences of E c in response to pulses of rain may shape the composition of semiarid woodlands under future rainfall regimes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding tree and stand water use is key for successful ecological restoration in semiarid regions where water availability is the primary limiting factor for tree survival and stand development (Dulamsuren et al. 2010; McVicar et al. 2007; Prieto et al. 2009; Yaseef et al. 2010). For example, in drought-prone regions, intermittent water availability threatens the survival of woodlands (Batisani and Yarnal 2010; Krol and Bronstert 2007; Tietjen et al. 2010) especially in summer due to high evaporative demand, which reduces shallow, short-lived soil moisture resources. Obviously, summer rainfall events, though highly variable, are critical for the survival of plants in some regions. Therefore, rainfall patterns (intensity, amount, interval, and seasonality) have a strong influence on stand development due to their direct influence over water infiltration into deep soil that support tree survival during dry spells (Loik et al. 2004; McVicar et al. 2007; Schwinning et al. 2004).
However, tree and stand transpiration responses to rain pulses are rarely investigated, even though they are of particular importance for understanding and predicting the structure and function of water-limited ecosystems susceptible to the shifts in rainfall regime in semiarid regions (Campbell and Murray 1990; Ivans et al. 2006; Nagler et al. 2007). Our current understanding on the response of transpiration to rain pulses comes from the studies conducted over grassland and shrubs (Fravolini et al. 2005; Ignace et al. 2007; Potts et al. 2006). These studies have examined the influence of soil texture and species on the response of transpiration to rain pulses (Cheng et al. 2006; Fravolini et al. 2005), threshold of pulse size for triggering significant responses in these ecosystems (Cheng et al. 2006), the impact of changing rainfall regimes on vegetation composition (Bendevis et al. 2010; Salve et al. 2011), and how such responses affect the biomass growth (Cheng et al. 2006). Plant life form (grasses or trees) and species influence the response of transpiration to rain pulses, as they may extract water from different soil depths (Zeppel et al. 2008a). Trees with deep and extensive roots exploit substantial soil water for transpiration (Huxman et al. 2005; Lubczynski and Gurwin 2005) and thus are more influential on the regional water balance and water availability (Brown et al. 2005; Farley et al. 2005; van Dijk and Keenan 2007). A recent synthesis study comparing a range of shrub and tree species in southern Australia concluded that four types of transpiration responses to rainfall events are no, delayed, small and rapid, and large and rapid responses (Burgess 2006). A threshold of 20 mm rainfall was observed to trigger significant transpiration response by a Eucalyptus–Callitris forest (Zeppel et al. 2008a). By contrast, it takes only 10 mm rainfall to induce significant response of mesquite shrubs (Fravolini et al. 2005). Despite the pronounced species differences in reacting to rainfall, a wide range of species, on the other hand, observed the same hydraulic control across various habitats and environmental conditions. A 0.6 ratio between stomatal conductance at 1 kPa (G sref) and the stomatal sensitivity to vapor pressure deficit (VPD) (−dG s/dlnVPD) was observed across the various habitats (Ewers et al. 2005; Katul et al. 2009; Oren et al. 1999). With convenient and accurate monitoring of the G cref and VPD, reliable G c can be obtained at any given time. Therefore, such a relationship may facilitate the estimation of canopy conductance of a stand which is composed of different species. Knowledge of specific transpiration response process of mixed-stand to rainfall pulses in a water-limited habitat is needed to understand the local ecohydrology.
Tree and forest transpiration is also largely controlled by environmental factors and thus its responses to rain pulses needs to be explored in the context of those controlling factors (David et al. 2004; Ewers et al. 2008; Hernández-Santana et al. 2008; Komatsu et al. 2006; Oren and Pataki 2001; Tang et al. 2006; Tognetti et al. 2005, 2009; Wullschleger et al. 2000). There have been studies demonstrating how the responses are governed by environmental factors, such as VPD, soil water distribution (Wullschleger and Hanson 2006), rainless interval (Sponseller 2007), and soil properties (Fravolini et al. 2005). VPD influences transpiration through canopy conductance. Stomatal closure is a major physiological process regulating water use (Giorio et al. 1999) and a mechanism to avoid irreversible damage to the plant hydraulic system (Martínez-Vilalta et al. 2002), therefore, is considered the initial response of tree and stand transpiration to water stress (Bréda et al. 2006). Consequently, canopy conductance decreases against increasing VPD (Cunningham 2004; El-Sharkawy et al. 1985; Renninger et al. 2010; Wallace and McJannet 2010). Also, VPD exerts a major influence over the duration of how long the moisture pulse generated by each rain event can last a critical mechanism for plant growth under dry environments. Soil water associated with rainfall regimes influences stand transpiration through the fluxes of water within the root zone. Percolation into the soil profile during large rain events, hydraulic lift (Cermák et al. 1995; Dawson 1993; Schwinning et al. 2002), and deep water reserves (Jackson et al. 1999) provides a water source for deep rooted trees. Therefore, soil water is the final form of rainfall that ultimately enters the transpiration system. Interactions between environmental factors translating rainfall to available water for transpiration (Fravolini et al. 2005) are becoming more important to understand and predict ecological and hydrological processes at multiple temporal and spatial scales (Box 1996; Sellers et al. 1997; Zhao et al. 2006).
A 3-year (2008–2010) study was conducted to measure the tree transpiration, microclimatic conditions, and soil water content in a Chinese pine (Pinus tabulaeformis) and Black Locust (Robinia pseudoacacia) plantation forest in Shanxi Province, China. These two species are widely used in semiarid regions in China for ecological restoration and erosion control campaigns. Moreover, they have contrasting physiological characteristics that enable the comparative study on the species difference in responding to environmental factors. Physiologically, the conduit of gymnosperm is narrower than that of the angiosperm, leading to an increased resistance in conifers to cavitation. Moreover, the root distribution of Chinese pine trees in the region was proved deeper than that of Black Locust trees (Wang et al. 2010). We, therefore, hypothesized that Chinese pine trees would be more responsive to VPD after rainfall as it suffered less from cavitation-related xylem dysfunction. Our specific objectives are to investigate (1) the environmental control over the stand transpiration after rainfall by analyzing the effect of VPD, soil water condition, and rainfall on the post-rainfall E c development and its recovery rate; and (2) the species differences in response to rain pulses and its implication on vegetation coverage under changing rainfall regimes.
Method and materials
Site description
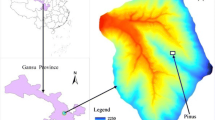
The experiment was conducted in the Caijiachuan catchment (36°14′27″∼36°18′23″N, 110°39′45″∼110°47′45″ E) located in the semiarid Loess Plateau in Ji County, Shanxi Province, China. The catchment that sits within a semiarid region features a typical tableland and gully terrain on the Loess Plateau. The mean annual pan evaporation is 1,723 mm, whereas the mean annual precipitation is 579 mm (Bi et al. 2008), 70 % of which falls between June and September. The annual averaged temperature is 10 °C with the highest being 38.1 °C and the lowest −20.4 °C, giving an annual frost-free period of 172 days. Soil in the studied area is loess-derived cinnamon soil (Bi et al. 2008). The experiment plot is located in a 18° north-facing slope with the elevation of 1,125 m a.s.l. The stand is composed of P. tabulaeformis and R. pseudoacacia, and the demographical survey is shown in Fig. 1. The age of the stand is 16 years. The planting space is 1 m × 3 m with a stand density of 2,450 trees/ha and a canopy coverage of 0.6. The root distribution of P. tabulaeformis and R. pseudoacacia are concentrated within 0–100 cm (Liu et al. 2007; Shan and Liang 2006). In contrast to evergreen P. tabulaeformis species, R. pseudoacacia start to shed leaves by the end of September.
Demographical survey of the stand. Vertical bars represent number of trees in a given DBH class (Frequency), line and dot for the ratio of total sapwood area in given class (DBH i ) to that of all trees of the same species in the stand (SADBHi /SAsum). The DBH and tree height (H) are given as mean ± standard error (S.E.)
Sap flux measurement and calculation
The sap flux measurements were conducted continuously from 11 July to 31 October 2008, 1 July to 31 October 2009, and 1 July to 12 October 2010. Sap flow was measured at a height of 1.3 m of each sampled tree using thermal dissipation probes (TDP) (Granier 1987). Sixteen trees including all diameter at breast height (DBH) classes were instrumented within a 20 × 20 m plot in the stand, representing size classes within the plots (Table 1). A square of 5 × 5 cm bark on the north face of the tree was peeled off and a set of sensors was inserted into holes drilled in the debarked trunk. Ten to 20 cm probes were used in order to avoid the contact with non-conducting wood due to small DBHs. Silicon foam was applied to seal the crevice so that sensors were not influenced by water outside. Finally, the part of the trunk with implanted sensors was covered with aluminum foil to prevent ambient thermal interference. The sensors were connected to CR1000 data logger (Campbell Scientific Inc., Logan, UT, USA) powered by a 12-V solar cell system. The measurement interval of the sap flux density was preprogrammed to be 10 s and a 30-min average recorded. No data were recorded on some days because of power failure.
Sap flux density was calculated according to standard calibration for the TDP method based on temperature differences between the two probes (Granier 1987):
in which J s is sap flow density (in gram per square centimeter per second), ΔT m is the maximum temperature difference between sensors during a day (in degree Celsius), and ΔT the temperature difference between sensors at any given time (in degree Celsius). ΔT m was determined according to Lu et al. (2004)
A test of the radial profile of sap flux indicated that there was no significant relationship between J s and depth following the approach developed by Oishi et al. (2008). Therefore, we assumed an uniform sap flux across the sapwood for the two species and the J s of entire xylem area was represented by respective sensors installed in individual trees. The J s of sampled trees was used as the average J s of their respective DBH classes. The transpiration rate on hourly or daily scale (E, in gram per hour or gram per day) of certain DBH class was calculated by multiplying J s with total sapwood area (A s, in square centimeter) of that DBH class and time conversion coefficient from seconds to hours (3,600). The stand transpiration (in gram per hour or gram per day) was obtained by adding all different classes together. The individual sapwood area was determined by discriminating between sapwood and heartwood of the increment cores based on color difference. Two cores were drilled along the north–south and the east–west radius of each tree, respectively. Cores were not taken from the sampled trees for TDP observation to avoid the possible influence from the wound. The relationship between A s and DBH for the two species are A s = 3.4DBH − 8.7 (n = 8, R 2 = 0.96) for R. pseudoacacia and A s = 10.1DBH − 37.1 (n = 8, R 2 = 0.99) for P. tabulaeformis. The daily canopy transpiration (daily E c, in millimeter per day) was calculated as the total daily sap flow amount of the stand divided by ground area.
Estimation of canopy conductance
First, we plotted J s against VPD and the hysteresis loop between J s and VPD was less significant under the scenario of no time lag than of 1 h time lag between transpiration and water uptake (Komatsu et al. 2006). Therefore, it is valid to assume that the effect of stem water capacitance was negligible. Secondly, we confirmed good aerodynamic coupling between the stand canopy and the ambient air. Under well-coupled conditions, the canopy is surrounded by the boundary layer and the depth of the layer would be not vary with wind speed (Bernier et al. 2006), and thus the canopy conductance response under different wind speeds shows no significant difference. In our study, canopy conductance and VPD did not show significant differences under different wind speed ranges (Fig. 2), this indicated that the plantation canopy was well coupled aerodynamically with the atmosphere (Jarvis and McNaughton 1986). With these two conditions being satisfied, the canopy conductance (G c, in millimeter per second) can be calculated by using a simplified Penman–Monteith equation (Yunusa et al. 2010):
in which λ is the latent heat of vaporization (2.45 MJ kg−1), E c the transpiration rate in seconds (in kilogram per square meter per second), γ the psychrometric constant (0.066 kPa °C−1), ρ the air density (1.29 kg m−3), C p the specific heat capacity of air (0.001 MJ kg−1 °C−1), and VPD the vapor pressure deficit (in kilopascal). G c values on rainy days were not included as J s values were subjected to noise (To et al. 2008). G c at VPD > 0.6 kPa (Ewers and Oren 2000) was used to examine the influence of VPD over physiological control of tree transpiration.
To estimate the average canopy stomatal sensitivity to VPD, we employed the simplified formula developed by Mackay et al. (2003):
where G c is canopy conductance, as the substitute for an estimate of average stomatal conductance over the canopy (in millimeter per second) (Ewers et al. 2001), and −m is the slope of G c versus lnVPD (−dG c/dlnVPD), quantifying the sensitivity of average canopy stomatal conductance to VPD. G cref is reference canopy conductance at VPD = 1 kPa and can be used as surrogate for G cmax (Ewers et al. 2001). The ratio of –m to G cref is 0.6 across a wide range of species (Oren et al. 1999) and environmental conditions (Ewers et al. 2001). The ratio is lower than 0.6 when tree species allow the minimum leaf water potential to drop with increasing VPD and the range of VPD increases. It is higher than 0.6 when the ratio of boundary layer conductance to stomatal conductance is low (Oren et al. 1999). Boundary analysis described by Ewers et al. (2005) and Schäfer et al. (2000) was used to analyze physiological response under measured conditions. The G c data of each soil water and light class were partitioned into 0.2 kPa VPD intervals. We calculated the mean and standard deviation of G c for each interval and removed the outliers using Dixon’s test. G c data above the mean and one standard deviation were averaged for each VPD interval. If there were less than 5 data points, the interval would not be considered to avoid potential lack of representativeness.
Meteorological and soil moisture measurement
Meteorological data were obtained from an automatic weather station at the foot of the plot-located hill established by Beijing Forestry University in 2008 and 2009, and from HOBOU30 (Onset Computer Corp., Bourne, MA, USA) located on the hill top in 2010. All meteorological data were measured every 10 s and automatically recorded as 30 min average. The raw temperature and humidity data were used to calculate the VPD (in kilopascal) following standard equations (Campbell and Norman 1998).
Volumetric soil water content (VWC, θ, in cubic centimeter per cubic centimeter) was measured using EM50 (Decagon Devices Inc., Pullman, WA, USA). VWC of the surface layer (25 cm) was monitored from 2008 to 2010 and additional sensors were installed to measure the VWC of the deep layer (75–100 cm) in 2010. Relative extractable water (REW, unitless) (Gartner et al. 2009; To et al. 2004; Tognetti et al. 2009) was calculated by using averaged θ across layers as:
where θ max and θ min are the saturated water content (maximum measured θ) and minimum soil water content during the observation. REW varies between 0 and 1. Soil moisture conditions were defined as severely stressed when REW ranged between 0 and 0.1, moderately stressed when REW = [0.1, 0.4], and non-stressed when REW >0.4, respectively, according to Bréda et al. (2006).
To examine the response of tree/stand transpiration to rain pulses, we defined Transpiration Recovery to compare daily E c in consecutive days after a rainfall event with E c in the day before the rain event:
where E i stands for transpiration on the ith day after a rainfall event and E lst for transpiration on the last day before the event. Therefore, rain pulses within 24 h were counted as a single rain event in order to study the course of transpiration after rainfall. Only rainfall events exceeding 5 mm were included in the transpiration recovery analysis.
Statistical analysis
All statistical analysis was performed using SPSS (Version 16.0, Chicago, IL). Curve fitting was run through SigmaPlot (version 10.0, Systat Software, California, USA). Datasets that did not observe normal distributions were square root-transformed, and ANOVA analysis was then employed to test the existence of significant differences among groups, and the multiple comparisons of the results were performed by LSD post hoc test (least significant difference t test). This test is based on the premises that the initial ANOVA is significant. It has the least limiting conditions and independent critical t from the number of groups
Results
Environmental variables and transpiration
Environmental factors varied among years, triggering interannual variation of transpiration (Fig. 3). The average daily incident radiation was 14.58, 13.7, and 15.55 MJ day−1 m−2 for 2008, 2009, and 2010, respectively, and there was no significant difference among 3 years (P = 0.17, one-way ANOVA). Daily averaged VPD in 2009 (0.67 kPa) and in 2010 (0.63 kPa) was significantly lower than that in 2008 (0.79 kPa) (P = 0.008, ine-way ANOVA), indicating that year 2008 had drier air and soil than the other years. Averaged daily VWC was 0.09 ± 0.003 (S.E.) cm3 cm−3 in 2008, significantly lower than that in 2010 (0.19 ± 0.006 cm3 cm−3) and 2009 (0.19 ± 0.007 cm3 cm−3). VWC of 0–25 cm reflected the occurrence of rainfall and recovered substantially when daily rainfall was more than 20 mm. VWC of 75–100 cm began a slight increase by the end of August. Soil moisture between layers of 0–25 cm and 75–100 cm in 2010 showed significant differences as summer progressed (P < 0.01, paired t test).
Progression of transpiration of the two species and environmental factors during the study period. Incident radiation (in megajoule per square meter per day), rainfall (in millimeter per day), and VPD (in kilopascal) from 11 July to 19 October 2008 (DOY192-292) (a), 25 June to 19 October 2009 (DOY176-292) (b), and 11 Jun to 12 October 2010 (DOY162-285) (c). Volumetric soil water content data (VWC, in cubic centimeter per cubic centimeter) were collected from 11 July to 12 October in all 3 years. Surface soil layer refers to 0–25 cm for all 3 years while deep layer refers to 75–100 cm only for 2010
In the rainy season, a large percentage of rainfall was not used for stand transpiration. Stand transpiration remained at a stable low range of 10 ∼ 15 mm month−1 despite of the contrasting rainfall amount, leading to varied transpiration to rainfall ratio among months (Table 2).
E c and G c in relation to incident radiation and VPD
Canopy conductance (G c) and transpiration (E c) on sunny days followed the same daily pattern, but varied in magnitude under different REW ranks (Fig. 4a–c). The influence of solar radiation (R) on G c was limited on the morning before R reached 1.8 MJ m−2 h−1 and the fluctuation of R was not reflected in G c patterns after that value. However, G c decrease did not induce an instant decline of E c. In contrast, E c kept increasing before VPD peaked whereas R had dropped for hours. Contrasting to most studies, our stand failed to sustain the maximum transpiration with increasing VPD (Fig. 5), even though logarithmic decrease of G c against the VPD was observed for both species (Fig. 6b).
Daily course of stand canopy conductance (G c), transpiration (E c), vapor pressure deficit (VPD), and incident radiation (R) for the sunny days under a SSP (severely stressed period, REW < 0.1); b MSP (medium stressed period, REW = [0.1, 0.4]); c WP(wet period, REW > 0.4). Values were pooled from 15 days in 2010 and given as averages with error bars representing standard error. Horizontal lines indicate R = 1.8 MJ m−2 h−1 with vertical lines pinpointing the corresponding G c
Canopy conductance (G c) in relation to simultaneous VPD on an hourly basis for P. tabulaeformis and R. pseudoacacia. The relationship between G c and VPD for both species (a) after applying boundary line analysis (b). Relationship between G c sensitivity to VPD (−dG c/dlnVPD) and G cref for both species under different solar radiation (c) and soil water condition (REW) (d) ranks. Dashed lines represent the theoretical proportionality described by Eq. (3). Filled symbols represent R. pseudoacacia and blank ones P. tabulaeformis under respective environmental ranks
G c data were sorted into specific ranks in order to examine the role of R and REW affecting its relationship with VPD. G c was the most sensitive to VPD when R was between 1 and 2 MJ m−2 for both species (Fig. 6c), but the two species differed in the sequencing of sensitivity magnitude under different soil water conditions (Fig. 6d). Although the G c sensitivity and G cref varied among different ranks for both species, no significant deviation from 0.6 was found in the –dG c/dlnVPD to G cref ratios (Fig. 6c, d). In contrast to R. pseudoacacia, G c of P. tabulaeforms failed to show any response pattern to VPD when solar radiation was lower than 1 MJ m−2 (Fig. 6c).
Transpiration recovery and influencing factors
The timing, rate, and course of transpiration recovery were different between species and influenced by VPD, rainfall, and VWC. Statistics showed that the transpiration of R. pseudoacacia reached the maximum recovery rate earlier than that of P. tabulaeformis did for 71 % of rainfall events. The timing for transpiration to reach the after-rainfall maximum was dependent more on VPD (P < 0.032) than VWC of observed layers (P > 0.05). In addition, the pattern of E c after rainfall was more correlated to VPD than to VWC (75–100 cm) (Fig. 7a). The maximum transpiration recovery was species-specific, but the E c recovery of P. tabulaeformis was significantly related to the difference between post- and pre-rainfall VPD values (Fig. 7b). Moreover, E c recovery of P. tabulaeformis and the stand was also significantly influenced by the time interval between the neighboring rain events (Fig. 7c), and the recovery of E c declined as the interval extended.
a Evolution of daily transpiration (mean) of R. pseudoacacia (E cR), P. tabulaeformis (E cP) between two consecutive effective rain events (24-h rainfall exceeded 5 mm) with respect to simultaneous VWC (75–100 cm) (mean and SD)and VPD change (mean) in 2010. SD was not indicated on the plot for E c and VPD for the sake of clarity. The mean SD were 0.07, 0.02, and 0.22 for E cP, E cR, and VPD, respectively. b, c Maximum transpiration recovery of the two species and whole stand in relation to VPD difference and the rainless interval (Note: VPD difference refers to the difference between VPD values on the day of maximum E c after rainfall and on the previous day before the rainfall. Rainless interval refers to the time between two consecutive rainfall events)
Although the rainfall amount did not affect the E c recovery on the event scale, it exerted a significant influence over the total transpiration on the monthly scale (Fig. 8). However, the saturation of stand transpiration increased with respect to large rainfall (∼100 mm month−1) (Fig. 8a) and the substantial margin between the transpiration and monthly rainfall (Table 2) indicated that the rainfall was lost through other hydrological processes such as surface runoff and/or canopy interception during the rainy season. Moreover, daily timing of rainfall occurrence influenced transpiration. Soil water influence over transpiration showed on a scale of dry–wet cycle (Fig. 8b).
Discussion
Low E c
Considering the percentage of transpiration in rainfall, the transpiration by the two species and the stand as a whole were low. Moreover, the range of transpiration response to rainfall was narrow and transpiration did not increase after rainfall larger than 100 mm month−1 (Fig. 8a). Low transpiration was governed by strict physiological regulation (Fig. 6) and daily VPD exceeding 1 kPa (Fig. 5). This indicates that trees adopted a conservative water use strategy and can be attributed to a gradual stomatal closure against VPD increase, which maintains water potential above a critical threshold and protects the xylem from runaway cavitation (Sperry 2000; Tognetti et al. 1998b; Tyree and Ewers 1991). As the soil dries, water conductivity increasingly limits water transport from soil to the roots (Yunusa et al. 2010) and leads to a decreased water supply rate. Because water supply could not match transpiration, as would occur during uninhibited stomatal conductance, plants will rapidly dehydrate or develop xylem cavitation if transpiration is not reduced. As a result, an internal hydraulic limit (Kolb and Sperry 1999; Meinzer et al. 1999; Tognetti et al. 1998a; Williams et al. 2001) in the flow path will be imposed, constituting a mechanism to determine a maximum E affordable by the plant (Hogg et al. 1997; Hogg and Hurdle 1997). The restraint on sustaining maximum E (Fig. 5) indicated the adaptation of trees to water stress in order to prevent cavitation at low soil water availability. Therefore, it is apparent that trees are adopting a conservative water use strategy.
The low E c was also caused by the stand structure, reflecting the dominant influence of tree size over transpiration amount (Cienciala et al. 2000; Meinzer et al. 2004); E c from this mixed stand in our study is comparable with the stand transpiration of a R. pseudoacacia stand on the Loess plateau (Wang et al. 2010) that has a similar DBH distribution despite of the species composition difference. Based on such influence of tree size over E c, the low transpiration of our stand can be partially ascribed to conservative water use strategies and the poor growth rate that led to low DBH and A s/A ground. The demographic survey indicated that stem growth of the stand occurred only in smaller DBH classes, mainly from 4–6 cm in 2008 to 6–8 cm in 2010 other than in larger DBH classes (>8 cm) (data not shown). Therefore, stand structure factors should be taken into account when the species differences in transpiration are tested.
Environmental control over E c and E c recovery after rainfall
By pinpointing the turning point when G c began to decline (indicated by vertical lines in Fig. 4) under all REW conditions, G c showed no further increase with incident radiation above 1.8 MJ m−2 h−1, supporting the role of R in controlling the opening and closure of stomata. G c reached a plateau as R increased, suggesting that stomata had fully opened. The apparent increase of transpiration with R in the morning was attributable to the increasing VPD as well as highly coupling between canopy and the atmosphere.
Among studies of E c recovery after rainfall, few have examined the link between VPD and the recovery of E c. But as demonstrated in temperate Australian woodland (Zeppel et al. 2008a), our study demonstrated the influence of VPD over the pattern and the extent of E c recovery (Fig. 7). Additionally, E c pattern after rainfall followed that of VPD, especially when daily VPD was under 1 kPa (Fig. 7a) which posed very limited constraints over transpiration (Fig. 4). Although not exerting direct influence over E c progression, soil water content also affected the relationship between the timing of the largest E c recovery and VPD.
The influence of soil moisture over transpiration has been established by many studies (Kume et al. 2007; Llorens et al. 2010; Oren and Pataki 2001). Our current study was unable to establish a firm relationship between transpiration and soil water content of the observed layers. Soil moisture variation after rainfall failed to show consistent influence over transpiration (Fig. 7a). However, the prolonged rainless interval lowered E c recovery (Fig. 7c) and cumulative VWC change within respective wet–dry cycle showed significant influence over the stand E c (Fig. 8b). Moreover, averaged daily E c also increased as the soil moisture condition improved (Fig. 4). The buffering effect of deep layer water storage may play a role because transpiration did not show significant decline under the condition of higher deep layer VWC even though the upper layer VWC kept decreasing (Fig. 3c). Similar observations of uncoupling between upper soil water and transpiration have been reported (Lundblad and Lindroth 2002; Zeppel et al. 2008b). Moreover, species differed in extracting soil water, causing the variation of soil water distribution. The influence of soil water on transpiration may also be obscured by atmospheric factors such as high VPD. The dry air causes strengthened stomatal control over transpiration regardless of soil water conditions (Brodribb and Holbrook 2006). Therefore, the influence of episodic drought-spells over this semiarid stand was influenced by multiple variables.
There was no significant relationship between single-event rainfall amount and E c recovery period. It could partially be attributed to the fact that there was a time lag between the rainfall and soil water replenishment. It took about 10 days for deep layer soil water to increase significantly (Fig. 3). However, the insufficient driving force by VPD after rainfall was the main reason. We found that E c reached the maximum as VPD fell between 1 to 1.5 kPa (Fig. 5).The statistics showed that less than 25 % of the days within the months having rainfall larger than 100 mm met this criterion for large E c. Therefore, even with great supplement from rainfall, E c is limited due to low driving force demanded by the air. As a result, monthly E c failed to increase against monthly rainfall larger than ∼100 mm. Although contributing little to increase E c, large rainfall events are necessary to maintain deep water reservoirs. Small rainfall events were not able to replenish the deep soil effectively due to root absorption of water and evaporation during the percolation process; therefore, the deep layer VWCmax within a dry–wet cycle was not significantly enhanced. By contrast, a large rainfall event (83.8 mm) induced the highest VWC during the whole study period and the replenish period was prolonged. Based on the fact that deep layer VWC continued to stay high, it was clear that the replenishment from large rainfall could be sustained even when there was no comparable event to contribute water directly to the deep layers. In addition to the indirect influence of rainfall size on E c through soil water, the rainless interval duration was negatively related to the recovery level of E c of P. tabulaeformis.
Influence of species differences over vegetation covering
Species differences in transpiration were observed, especially in responses to environmental drivers. However, the hydraulic control factor (−dG c/dlnVPD to G cref ratio) cannot be ruled out. Many species observed the same hydraulic control (−dG c/dlnVPD to G cref ratio) across different habitat and environmental conditions as indicated by a fixed ratio of 0.6 between stomatal conductance at 1 kPa (G sref) and the stomatal sensitivity to VPD (−dG s/dlnVPD) (Ewers et al. 2005; Katul et al. 2009; Oren et al. 1999). Our two studied species also exhibited the same extent of hydraulic control (−dG c/dlnVPD to G cref ratio) over transpiration under contrasting solar radiation and soil water conditions (Fig. 6). But species with high G cref has the disadvantage of dramatic change of G c with VPD variation. This accounted for the rapid response of E c by P. tabulaeformis to VPD after rainfall (Fig. 7a) and led to the differed transpiration recovery after rainfall between the two species. Due to more sensitive stomatal response to VPD, P. tabulaeformis, thus the stand, was able to regulate transpiration according to VPD (Fig. 7 C) while R. pseudoacacia not (Fig. 7b). Another factor that contributes to species-specific E c response pattern to rainfall is the root distribution as the depth to which roots can reach and absorb soil water varies greatly among species (Liu et al. 2007). Consequently, shallow-rooted trees respond faster to precipitation than deeply rooted ones (Cienciala et al. 2000; Meinzer et al. 2004). Moreover, the negatively related E c recovery of the two species indicated that the two species react differently to the rainfall which replenished different layers depending upon the size. R. pseudoacacia trees have a more robust shallow root system than P. tabulaeformis at this site (Wang et al. 2010). This accounts for the larger E c increase rate immediate after rainfall in R. pseudoacacia than P. tabulaeformis.
In water-limited environments, the ability of tree species to exploit soil water is crucial for survival. Isotopic study has found that proportions of water contribution from different soil layers for plant uptake vary seasonally and among plant species (Rong et al. 2011). The distribution of soil water among layers will be influenced by changing rainfall regimes in the future. Under the scenario of less intensive, but more frequent rainfall in the future, plants equipped with shallow root systems stand a higher chance of survival than their deep-rooted co-habitants. This is because their drought stress could be quickly alleviated and they may use up the limited rainfall so that less water will access deep layers. Further, the desiccation of the soil upper layer induces an absolute increase of water uptake from the deeper wet layers (Li et al. 2002; Rong et al. 2011). Alternatively, if the duration of the drought becomes longer and rainfall becomes more concentrated, the deep-rooted species that can access to the reserved water are more likely to survive as the upper layer water crucial for shallow-rooted species can be evaporated quickly. Species composition and tree growth dependence on the soil water profile has been observed on mountain slopes. Deep-rooting species showed better diameter growth on deeper soil sites than on shallower ones, although a shallow-rooting species showed little difference between them (Yanagisawa and Fujita 1999). Therefore, the nature of rainfall pattern changes will reshape the species composition in semiarid areas. In order to develop a comprehensive understanding on this issue, future studies examining the relationship between E c and soil water, as well as the redistribution of water within and among soil layers are needed (Caldwell et al. 1998; Ishikawa and Bledsoe 2000).
Conclusions
The two species showed the same hydraulic control (−dG c/dlnVPD to G cref ratio) but varied explicitly in post-rainfall transpiration recovery to environmental drivers and thus in transpiration responses to rain pulses. Compared with soil water, VPD exerted a major influence over E c and the recovery of E c after rainfall in semiarid region. The differences between pre- and post-rainfall VPD contributed to the transpiration recovery potential. Soil water was not replenished sufficiently by the rainfall and failed to maintain transpiration. But the continued duration of certain soil water conditions determine the transpiration intensity. Therefore due to the buffering effect of soil water, the lasting improvement of transpiration will show only after rainfall wets the soil to a stable high level. Rainfall had a significant influence over monthly transpiration, but failed to trigger a proportionate increase in E c in large rain events and large pulses are necessary for the sustainment of transpiration in later growing season. The timing of the rainfall events is another important factor in affecting the total amount of stand transpiration. Species differences were found in E c and E c recovery in response to environmental factors. These findings indicate that species differences in transpiration response to pulses of rain will reshape the vegetation patterns in the future climates. However, the same hydraulic control of the two species over transpiration can be used to estimate the stand transpiration accurately. Our study highlights that it is crucial to understand how the distribution of soil water following pulses of rain supports E c for a better understanding of the maintenance of tree transpiration and survival during drought stress in future climates and more extreme hydrological cycles.
References
Batisani N, Yarnal B (2010) Rainfall variability and trends in semi-arid Botswana: implications for climate change adaptation policy. Appl Geogr 30:483–489
Bendevis MA, Owens MK, Heilman JL, McInnes KJ (2010) Carbon exchange and water loss from two evergreen trees in a semiarid woodland. Ecohydrol 3:107–115
Bernier PY, Bartlett P, Black TA, Barr A, Kljun N, McCaughey JH (2006) Drought constraints on transpiration and canopy conductance in mature aspen and jack pine stands. Agric Forest Meteorol 140:64–78
Bi H, Zhang J, Zhu J, Lin L, Guo C, Ren Y, Yun L et al (2008) Spatial dynamics of soil moisture in a complex terrain in the semi-arid Loess Plateau Region, China. J Am Water Resour Assoc 44:1121–1131
Box EO (1996) Plant functional types and climate at the global scale. J Veg Sci 7:309–320
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644
Brodribb TJ, Holbrook NM (2006) Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell &Environment 29:2205–2215
Brown AE, Zhang L, McMahon TA, Western AW, Vertessy RA (2005) A review of paired catchment studies for determining changes in water yield resulting from alterations in vegetation. J Hydrol 310:28–61
Burgess SSO (2006) Measuring transpiration responses to summer precipitation in a Mediterranean climate: a simple screening tool for identifying plant water-use strategies. Physiol Plantarum 127:404–412
Caldwell MM, Dawson TE, Richards JH (1998) Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113:151–161
Campbell DI, Murray DL (1990) Water balance of snow tussock grassland in New Zealand. J Hydrol 118:229–245
Campbell GS, Norman JM (1998) An introduction to environmental biophysics. Springer, New York
Cermák J, Cienciala E, Kucera J, Lindroth A, Bednárová E (1995) Individual variation of sap-flow rate in large pine and spruce trees and stand transpiration: a pilot study at the central NOPEX site. J Hydrol 168:17–27
Cheng X, An S, Li B, Chen J, Lin G, Liu Y, Luo Y et al (2006) Summer rain pulse size and rainwater uptake by three dominant desert plants in a desertified grassland ecosystem in northwestern China. Plant Ecolog 184:1–12
Cienciala E, Kucera J, Malmer A (2000) Tree sap flow and stand transpiration of two Acacia mangium plantations in Sabah, Borneo. J Hydrol 236:109–120
Cunningham SC (2004) Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Trees-Struct Funct 18:399–407
David TS, Ferreira MI, Cohen S, Pereira JS, David JS (2004) Constraints on transpiration from an evergreen oak tree in southern Portugal. Agric Forest Meteorol 122:193–205
Dawson TE (1993) Hydraulic lift and water use by plants: implications for water balance, performance and plant–plant interactions. Oecologia 95:565–574
Dulamsuren C, Hauck M, Leuschner C (2010) Recent drought stress leads to growth reductions in Larix sibirica in the western Khentey, Mongolia. Global Change Biol 16:3024–3035
El-Sharkawy M, Cock J, Hernandez A (1985) Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosynth Res 7:137–149
Ewers B, Oren R (2000) Analyses of assumptions and errors in the calculation of stomatal conductance from sap flux measurements. Tree Physiol 20:579–589
Ewers BE, Oren R, Johnsen KH, Landsberg JJ (2001) Estimating maximum mean canopy stomatal conductance for use in models. Can J For Res 31:198–207
Ewers BE, Gower ST, Bond-Lamberty B, Wang CK (2005) Effects of stand age and tree species on canopy transpiration and average stomatal conductance of boreal forests. Plant Cell & Environ 28:660–678
Ewers BE, Mackay DS, Tang J, Bolstad PV, Samanta S (2008) Intercomparison of sugar maple (Acer saccharum Marsh.) stand transpiration responses to environmental conditions from the Western Great Lakes Region of the United States. Agric Forest Meteorol 148:231–246
Farley KA, Jobbágy EG, Jackson RB (2005) Effects of afforestation on water yield: a global synthesis with implications for policy. Global Change Biol 11:1565–1576
Fravolini A, Hultine K, Brugnoli E, Gazal R, English N, Williams D (2005) Precipitation pulse use by an invasive woody legume: the role of soil texture and pulse size. Oecologia 144:618–627
Gartner K, Nadezhdina N, Englisch M, Cermak J, Leitgeb E (2009) Sap flow of birch and Norway spruce during the European heat and drought in summer 2003. For Ecol Manage 258:590–599
Giorio P, Sorrentino G, d’Andria R (1999) Stomatal behaviour, leaf water status and photosynthetic response in field-grown olive trees under water deficit. Environ Exp Bot 42:95–104
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol 3:309–320
Hernández-Santana V, David TS, Martínez-Fernández J (2008) Environmental and plant-based controls of water use in a Mediterranean oak stand. For Ecol Manage 255:3707–3715
Hogg EH, Hurdle PA (1997) Sap flow in trembling aspen: implications for stomatal responses to vapor pressure deficit. Tree Physiol 17:501–509
Hogg E, Black T, den Hartog G, Neumann H, Zimmermann R, Hurdle P, Blanken P et al (1997) A comparison of sap flow and eddy fluxes of water vapor from a boreal deciduous forest. J Geophys Res 102:28,929–28,937
Huxman TE, Wilcox BP, Breshears DD, Scott RL, Snyder KA, Small EE, Hultine K et al (2005) Ecohydrological implications of woody plant encroachment. Ecol 86:308–319
Ignace D, Huxman T, Weltzin J, Williams D (2007) Leaf gas exchange and water status responses of a native and non-native grass to precipitation across contrasting soil surfaces in the Sonoran Desert. Oecologia 152:401–413
Ishikawa CM, Bledsoe CS (2000) Seasonal and diurnal patterns of soil water potential in the rhizosphere of blue oaks: evidence for hydraulic lift. Oecologia 125:459–465
Ivans S, Hipps L, Leffler AJ, Ivans CY (2006) Response of water vapor and CO2 fluxes in semiarid lands to seasonal and intermittent precipitation pulses. J Hydrometeorology 7:995–1010
Jackson PC, Meinzer FC, Bustamante M, Goldstein G, Franco A, Rundel PW, Caldas L et al (1999) Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiol 19:717–724
Jarvis P, McNaughton K (1986) Stomatal control of transpiration: scaling up from leaf to region. Advances in ecological research 15:49
Katul GG, Palmroth S, Oren RAM (2009) Leaf stomatal responses to vapour pressure deficit under current and CO2-enriched atmosphere explained by the economics of gas exchange. Plant Cell Environ 32:968–979
Kolb KJ, Sperry JS (1999) Transport constraints on water use by the Great Basin shrub, Artemisia tridentata. Plant Cell Environ 22:925–935
Komatsu H, Kang Y, Kume T, Yoshifuji N, Hotta N (2006) Transpiration from a Cryptomeria japonica plantation, part 1: aerodynamic control of transpiration. Hydrol Processes 20:1309–1320
Krol MS, Bronstert A (2007) Regional integrated modelling of climate change impacts on natural resources and resource usage in semi-arid Northeast Brazil. Environ Model Softw 22:259–268
To K, Saitoh TM, Sato Y, Morooka T, Manfroi OJ, Kuraji K, Suzuki M (2004) Transpiration, canopy conductance and the decoupling coefficient of a lowland mixed dipterocarp forest in Sarawak, Borneo: dry spell effects. J Hydrol 287:237–251
To K, Tateishi M, Shimizu T, Otsuki K (2008) Transpiration and canopy conductance at two slope positions in a Japanese cedar forest watershed. Agric Forest Meteorol 148:1444–1455
Kume T, Takizawa H, Yoshifuji N, Tanaka N, Tanaka K, Tantasirin C, Suzuki M (2007) Severe drought resulting from seasonal and interannual variability in rainfall and its impact on transpiration in a hill evergreen forest in Northern Thailand. In: Sawada H, Araki M, Chappell NA, LaFrankie JV, Shimizu A (eds) Forest environments in the Mekong River Basin. Springer, Japan, pp. 45–55
Li Y, Fuchs M, Cohen S, Cohen Y, Wallach R (2002) Water uptake profile response of corn to soil moisture depletion. Plant Cell Environ 25:491–500
Liu XP, Chen LH, Song WF, Wu YL (2007) Fractal analysis on morphology distribution of the Pinus tabulaeformis Carr. root system. Bulletin Soil Water Conserv 27:47–54 (in Chinese)
Llorens P, Poyatos R, Latron J, Delgado J, Oliveras I, Gallart F (2010) A multi-year study of rainfall and soil water controls on Scots pine transpiration under Mediterranean mountain conditions. Hydrol Processes 24:3053–3064
Loik ME, Breshears DD, Lauenroth WK, Belnap J (2004) A multi-scale perspective of water pulses in dryland ecosystems: climatology and ecohydrology of the western USA. Oecologia 141:269–281
Lu P, Urban L, Zhao P (2004) Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: theory and practice. Acta Bot Sin 46:631–646
Lubczynski MW, Gurwin J (2005) Integration of various data sources for transient groundwater modeling with spatio-temporally variable fluxes—Sardon study case, Spain. J Hydrol 306:71–96
Lundblad M, Lindroth A (2002) Stand transpiration and sap flow density in relation to weather, soil moisture and stand characteristics. Basic Appl Ecol 3:229–243
Mackay DS, Samanta S, Nemani RR, Band LE (2003) Multi-objective parameter estimation for simulating canopy transpiration in forested watersheds. J Hydrol 277:230–247
Martínez-Vilalta J, Prat E, Oliveras I, Piñol J (2002) Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. Oecologia 133:19–29
McVicar TR, Li L, Van Niel TG, Zhang L, Li R, Yang Q, Zhang X et al (2007) Developing a decision support tool for China’s re-vegetation program: simulating regional impacts of afforestation on average annual streamflow in the Loess Plateau. For Ecol Manag 251:65–81
Meinzer FC, Goldstein G, Franco AC, Bustamante M, Igler E, Jackson P, Caldas L et al (1999) Atmospheric and hydraulic limitations on transpiration in Brazilian cerrado woody species. Funct Ecol 13:273–282
Meinzer FC, James SA, Goldstein G (2004) Dynamics of transpiration, sap flow and use of stored water in tropical forest canopy trees. Tree Physiol 24:901–909
Nagler PL, Glenn EP, Kim H, Emmerich W, Scott RL, Huxman TE, Huete AR (2007) Relationship between evapotranspiration and precipitation pulses in a semiarid rangeland estimated by moisture flux towers and MODIS vegetation indices. J Arid Environ 70:443–462
Oishi AC, Oren R, Stoy PC (2008) Estimating components of forest evapotranspiration: a footprint approach for scaling sap flux measurements. Agric For Meteorol 148:1719–1732
Oren R, Pataki DE (2001) Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia 127:549–559
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KVR (1999) Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell & Environ 22:1515–1526
Potts DL, Huxman TE, Cable JM, English NB, Ignace DD, Eilts JA, Mason MJ et al (2006) Antecedent moisture and seasonal precipitation influence the response of canopy-scale carbon and water exchange to rainfall pulses in a semi-arid grassland. New Phytol 170:849–860
Prieto P, Peñuelas J, Lloret F, Llorens L, Estiarte M (2009) Experimental drought and warming decrease diversity and slow down post-fire succession in a Mediterranean shrubland. Ecography 32:623–636
Renninger HJ, Phillips N, Salvucci GD (2010) Wet- vs dry-season transpiration in an Amazonian rain forest palm Iriartea deltoidea. Biotropica 42:470–478
Rong L, Chen X, Chen X, Wang S, Du X (2011) Isotopic analysis of water sources of mountainous plant uptake in a karst plateau of southwest China. Hydrol Processes 25:3666–3675
Salve R, Sudderth EA, Clair SS, Torn MS (2011) Effect of grassland vegetation type on the responses of hydrological processes to seasonal precipitation patterns. J Hydrol. doi:10.1016/j.jhydrol.2011.1009.1003
Schäfer K, Oren R, Tenhunen J (2000) The effect of tree height on crown level stomatal conductance. Plant Cell Environ 23:365–375
Schwinning S, Davis K, Richardson L, Ehleringer J (2002) Deuterium enriched irrigation indicates different forms of rain use in shrub/grass species of the Colorado Plateau. Oecologia 130:345–355
Schwinning S, Sala OE, Loik ME, Ehleringer JR (2004) Thresholds, memory, and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 141:191–193
Sellers PJ, Dickinson RE, Randall DA, Betts AK, Hall FG, Berry JA, Collatz GJ et al (1997) Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Sci 275:502–509
Shan CJ, Liang ZS (2006) Relationship between root distribution of Locust Plantation and soil water in the Loess Plateau (in Chinese). J of Central South Forest 1:19–22
Sperry JS (2000) Hydraulic constraints on plant gas exchange. Agric For Meteorol 104:13–23
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Change Biol 13:426–436
Tang JW, Bolstad PV, Ewers BE, Desai AR, Davis KJ, Carey EV (2006) Sap flux–upscaled canopy transpiration, stomatal conductance, and water use efficiency in an old growth forest in the Great Lakes region of the United States. J Geophys Res: Biogeosciences 111(G2). doi:10.1029/2005JG000083
Tietjen B, Jeltsch F, Zehe E, Classen N, Groengroeft A, Schiffers K, Oldeland J (2010) Effects of climate change on the coupled dynamics of water and vegetation in drylands. Ecohydrol 3:226–237
Tognetti R, Longobucco A, Miglietta F, Raschi A (1998a) Transpiration and stomatal behaviour of Quercus ilex plants during the summer in a Mediterranean carbon dioxide spring. Plant Cell Environ 21:613–622
Tognetti R, Longobucco A, Raschi A (1998b) Vulnerability of xylem to embolism in relation to plant hydraulic resistance in Quercus pubescens and Quercus ilex co-occurring in a Mediterranean coppice stand in central Italy. New Phytol 139:437–447
Tognetti R, d’Andria R, Morelli G, Alvino A (2005) The effect of deficit irrigation on seasonal variations of plant water use in Olea europaea L. Plant Soil 273:139–155
Tognetti R, Giovannelli A, Lavini A, Morelli G, Fragnito F, d’Andria R (2009) Assessing environmental controls over conductances through the soil–plant–atmosphere continuum in an experimental olive tree plantation of southern Italy. Agric Forest Meteorol 149:1229–1243
Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody plants. New Phytol 119:345–360
van Dijk AIJM, Keenan RJ (2007) Planted forests and water in perspective. For Ecol Manage 251:1–9
Wallace J, McJannet D (2010) Processes controlling transpiration in the rainforests of north Queensland, Australia. J Hydrol 384:107–117
Wang Y-L, Liu G-B, Kume T, Otsuki K, Yamanaka N, Du S (2010) Estimating water use of a black locust plantation by the thermal dissipation probe method in the semiarid region of Loess Plateau, China. J For Res 15:241–251
Williams M, Bond BJ, Ryan MG (2001) Evaluating different soil and plant hydraulic constraints on tree function using a model and sap flow data from ponderosa pine. Plant Cell Environ 24:679–690
Wullschleger SD, Hanson PJ (2006) Sensitivity of canopy transpiration to altered precipitation in an upland oak forest: evidence from a long-term field manipulation study. Global Change Biol 12:97–109
Wullschleger SD, Wilson KB, Hanson PJ (2000) Environmental control of whole-plant transpiration, canopy conductance and estimates of the decoupling coefficient for large red maple trees. Agric Forest Meteorol 104:157–168
Yanagisawa N, Fujita N (1999) Different distribution patterns of woody species on a slope in relation to vertical root distribution and dynamics of soil moisture profiles. Ecol Res 14:165–177
Yaseef NR, Yakir D, Rotenberg E, Schiller G, Cohen S (2010) Ecohydrology of a semi-arid forest: partitioning among water balance components and its implications for predicted precipitation changes. Ecohydrol 3:143–154
Yunusa IAM, Aumann CD, Rab MA, Merrick N, Fisher PD, Eberbach PL, Eamus D (2010) Topographical and seasonal trends in transpiration by two co-occurring Eucalyptus species during two contrasting years in a low rainfall environment. Agric For Meteorol 150:1234–1244
Zeppel M, Macinnis-Ng C, Ford C, Eamus D (2008a) The response of sap flow to pulses of rain in a temperate Australian woodland. Plant Soil 305:121–130
Zeppel M, Macinnis-Ng C, Palmer A, Taylor D, Whitley R, Fuentes S, Yunusa I et al (2008b) An analysis of the sensitivity of sap flux to soil and plant variables assessed for an Australian woodland using a soil–plant–atmosphere model. Funct Plant Biol 35:509–520
Zhao L, Li Y, Xu S, Zhou H, Gu S, Yu G, Zhao X (2006) Diurnal, seasonal and annual variation in net ecosystem CO2 exchange of an alpine shrubland on Qinghai-Tibetan plateau. Global Change Biol 12:1940–1953
Acknowledgments
The authors acknowledge the financial support from the Ministry of Science and Technology, People’s Republic of China through National Scientific and Technical Innovation Research Project for the 12th Five Year Planning (Grant No. 2011BAD38B0504). Melanie Zeppel was supported by an ARC Discovery Early Career Researcher award (DECRA). The first author would like to extend appreciation to Professor Ram Oren, Duke University, USA, for his comments over data analysis. We thank the anonymous reviewers for their insightful and constructive comments and suggestions that helped us improve our original manuscript greatly.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Zhang, Z., Zeppel, M. et al. Response of transpiration to rain pulses for two tree species in a semiarid plantation. Int J Biometeorol 58, 1569–1581 (2014). https://doi.org/10.1007/s00484-013-0761-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-013-0761-9