Abstract
Several North American broad-leaved tree species range from the northern United States at ∼47°N to moist tropical montane forests in Mexico and Central America at 15–20°N. Along this gradient the average minimum temperatures of the coldest month (T Jan), which characterize annual variation in temperature, increase from −10 to 12°C and tree phenology changes from deciduous to leaf-exchanging or evergreen in the southern range with a year-long growing season. Between 30 and 45°N, the time of bud break is highly correlated with T Jan and bud break can be reliably predicted for the week in which mean minimum temperature rises to 7°C. Temperature-dependent deciduous phenology—and hence the validity of temperature-driven phenology models—terminates in southern North America near 30°N, where T Jan>7°C enables growth of tropical trees and cultivation of frost-sensitive citrus fruits. In tropical climates most temperate broad-leaved species exchange old for new leaves within a few weeks in January-February, i.e., their phenology becomes similar to that of tropical leaf-exchanging species. Leaf buds of the southern ecotypes of these temperate species are therefore not winter-dormant and have no chilling requirement. As in many tropical trees, bud break of Celtis, Quercus and Fagus growing in warm climates is induced in early spring by increasing daylength. In tropical climates vegetative phenology is determined mainly by leaf longevity, seasonal variation in water stress and day length. As water stress during the dry season varies widely with soil water storage, climate-driven models cannot predict tree phenology in the tropics and tropical tree phenology does not constitute a useful indicator of global warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Northern Hemisphere land has undergone considerable warming in recent decades, and the full impact of these changes across multiple ecosystems is still being assessed (Houghton et al. 2001). However, in temperate regions, where plant growth and development are mainly determined by temperature, some effects are already apparent. For example, especially strong temperature increases during winter and spring are causing phenophases such as first leaf and first bloom to start sooner in many temperate tree and shrub species (Cayan et al. 2001; Menzel and Fabian 1999; Schwartz and Reiter 2000). Thus, spring phenology shows considerable promise as an indicator of the impact of global warming on the temperate biosphere (Badeck et al. 2004; Walter 2003). Based on the high correlations between temperature and plant development, temperature-driven phenological models have been developed and successfully applied to predict the timing of spring bud-break and flowering (Kramer 1994; Linkosalo et al. 2000; Schwartz 1997; Schwartz and Chen 2002; Schwartz and Reiter 2000; Wielgolaski 1999).
In contrast to temperate trees, the phenology of trees in tropical regions with large seasonal variation in rainfall cannot be predicted from climate data. This is because bud break and leafing are determined mainly by non-climatic variables such as seasonal variation in tree water status, day length and shedding of old leaves (Borchert 1994, 2004; Rivera et al. 2002). Within the same landscape or climatic zone tree phenology may therefore range from evergreen trees at moist sites to deciduous trees at dry sites, and bud break in spring may occur at the height of the dry season (Rivera et al. 2002).
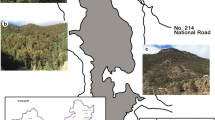
Temperature-dependent phenology of temperate broad-leaved trees and tropical tree phenology have been studied mainly in northern climates with a cold winter and in the seasonally dry tropics, respectively. Little is known about the transition from temperate to tropical phenology and the geographical boundaries between these major phenological patterns. In contrast to Europe, where the range of most broad-leaved tree species ends abruptly in the Mediterranean climate south of the Alps, in North America broad-leaved forests with a moist summer growing season range from 45°N in northern USA to the tropical montane forests of Mexico and Central America at 15–20°N (Fig. 1). Correspondingly, a number of temperate tree species or genera range from northern USA and Canada to Central America, Mexico or southern Florida (Fig. 1; Marquis 1990). In their northern range species such as Acer rubrum (Walters and Yawney 1990), Fagus grandifolia (Tubbs and Houston 1990) and Carpinus caroliniana may be deciduous for ∼6 months, but in the southern, tropical part of their range they exchange old for new leaves within a few weeks in January–February (Peters 1995; Tomlinson 1980; Williams-Linera 1997; Williams-Linera et al. 2000), i.e., their phenology is similar to that of tropical leaf-exchanging species (Borchert et al. 2002; Borchert 2004). Along the N-S gradient of increasing temperature the transition from a temperature-driven, deciduous phenology to a tropical, nearly evergreen phenology thus occurs within the same, wide-ranging temperate species.
Map of North America showing the areas with T Jan=5–10°C (gray), the distribution limits of Acer rubrum (black triangles) and Fagus grandifolia (filled circles), the northern limits of citrus cultivation (empty circles) and tropical dry forest trees (diamonds), and the locations of phenology observations (squares). G Gainesville, Florida, KS Kansas, MA Massachusetts, NC North Carolina, NF Northern Florida, SFL Southern Florida, SO Sonora, Mexico, TX Texas, VC Veracruz, Mexico, WI Wisconsin
In order to fully assess the potential impact of climatic changes on global ecosystems, a better understanding of the geographic extent and nature of temperature control of plant growth in the temperate-tropical transitional zones is crucial. In this study we analyze changes in the vegetative phenology of North American forest tree species along a N-S gradient of increasing temperature. Specifically, we address the following questions: (1) How far south does temperature control of vegetative phenology in temperate trees extend? (2) At what threshold of temperature does the transition from the temperate to the tropical pattern of vegetative growth occur? (3) How is the seasonality of vegetative phenology of temperate trees controlled in tropical climates without large seasonal variation in temperature?
Materials and methods
Phenological observations
To assess changes in the time of bud break as a function of temperature changes along a latitudinal gradient, phenological observations for 10 locations ranging from 10–44°N were compiled from a variety of sources (Table 1; Fig. 1). Observations for Massachusetts (Harvard Forest 2003), North Carolina (Lieth and Radford 1971), Wisconsin (Schwartz, unpublished data) and Veracruz (Williams-Linera 1997; Williams-Linera et al. 2000) are from long-term or large-scale systematic phenology studies. Bud break or leaf exchange in spring 2004 were observed weekly in Veracruz, northern Florida, northern Texas/Louisiana and Kansas by the authors (G.W-L.: Xalapa, Veracruz; K.R.: Talahassee, Fla.; R.B.: Lawrence, Kan.) and two volunteers (J. Long, Shreveport, La.; J. Weiss, Daingerfield, Tex.). Bud break times from anecdotal observations of the phenology of temperate species in South Florida (Perry and Wang 1960; Tomlinson 1980) and Sonora, Mexico (Felger et al. 2001), were converted to weeks as follows: ‘January’ = week 2; ‘January/February’ = week 4, ‘February’ = week 6, etc. For most genera phenological observations of several species were pooled, because bud break of most species in a genus is consistently early (e.g., Cornus) or late (e.g., Quercus) relative to other tree species at the same location (Table 1). Bud break times for 15 leaf-exchanging species of the semideciduous tropical forest in Guanacaste, Costa Rica, were obtained from phenological records by Frankie et al. (1974) as described in Borchert et al. (2004). Leaf exchange of Enterolobium cyclocarpum in the same forest was monitored by R.B. in 1991 and 1992. Phenological observations of temperate and tropical trees in Argentina were made in Buenos Aires (M. Oesterheld, R.B.), Rosario (D. Prado) and Cordoba (G. Rivera). Mean bud break times for 16 temperate genera and 15 tropical species were calculated for 10 locations (Table 1).
Modeling bud break time
To test the hypothesis that spring bud break occurs on average at the time when the monthly mean temperature reaches 7°C, predicted bud break times were calculated as follows from meteorological records of 26 locations in North America (Fig. 2; Ruffner and Bair 1984; Free-Weather 2003). For stations with an average temperature of the coldest month (T Jan) of >7°C, bud break was assumed to occur in week 1; for stations with T Jan<7°C the week of bud break was interpolated between the months with higher (T H) and lower minimum temperature (T L) than the temperature permitting bud break (T B=7°C) as
Observed mean bud break times from Table 1 for 10 locations ranging from northern USA to Costa Rica as a function of T Jan (squares, solid regression line, locations in regular type) and bud break times predicted for the week with a minimum temperature of 7°C at 26 locations in North America (filled circles, dashed regression line, locations in italics; see Materials and methods). Dashed horizontal line observed bud break times at locations with T Jan>7°C (arrow)
where YDTL = Year-day of the 8th day of the month in which T L was observed.
The 5–10°C range of T Jan in Fig. 1 was mapped in ArcView 3.2, based on data drawn from the 1961–1990 climatologies served by the Intergovernmental Panel on Climate Change (http://www.ipcc.ch/) (New et al. 1997).
Results
Changes in T Jan from −10 to 12°C characterize the increase in the range of annual temperatures along the observed N-S gradient (Fig. 2). Mean bud break times calculated in Table 1 are highly correlated with T Jan for the eight locations with T Jan ranging from 5° to −10°C (Fig. 2, empty squares, solid regression line). At all tropical and subtropical locations with T Jan>7–8°C mean bud break occurs in January/February (Fig. 2, dashed gray line). Standard deviations of mean bud break time are <1.5 weeks at locations with latitude >35°N and T Jan<0°C, but increase to 5 weeks at lower latitudes with T Jan>0°C. Collectively these observations indicate that at TJan greater than 7°C temperature ceases to be the dominant driving variable for vegetative phenology and the growing season lasts all year (Fig. 2, arrow).
The regression of bud break time against TJan (Fig. 2, solid line) and the temperature–dependent limits of distribution of subtropical trees in Florida (Greller 1980) suggest that bud break in spring should occur when the average minimum temperature (T min) reaches 7–8°C (Fig. 2, arrow). This hypothesis was supported by the remarkable similarity between the observed bud break times and those predicted for 28 North American locations ranging from Florida and Texas in the South to Alberta and Quebec in the North for the week with T min=7°C (Fig. 2, filled circles, dashed regression line; see Materials and methods).
For all analyzed individual tree genera, correlations between bud break time and T Jan are highly significant for locations with T Jan<5°C (Fig. 3). Regressions are identical with (Fig. 3A) or very similar to (Fig. 3B) those of bud break means for the combined species (Fig. 2). These regressions indicate an advance in bud break time of 5.4–6.5 days for an increase of T Jan by 1°C. In contrast to all other genera, an increase in T Jan above 0°C does not advance bud break times in Celtis, Fagus and Quercus, which rarely leaf out before mid-March (Fig. 3C, dashed line). In Fagus and Quercus bud break is known to be induced by increasing daylength (Kramer 1936; Romberger 1963; Wareing 1953).
Regressions of bud break times from Table 1 against T Jan<7°C for 12 temperate genera. Regression lines in A and B are representative for all listed genera. Regression in C calculated for bud break of Quercus at locations with T Jan<7°C
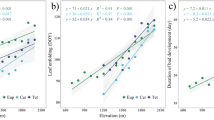
In the cool and moist tropical montane cloud forest of Xalapa, Veracruz, Mexico (T Jan=10.9°; Fig. 4F), most temperate species leaf in January, soon after all or most old leaves have abscised (Fig. 4A–D). Fagus grandifolia, Quercus xalapensis (Fig. 4E, F) and four other oak species (Q. acutifolia, Q, germana, Q. leiophylla, Q. insignis; Williams-Linera 1997) consistently leaf in late February/March, about 4 weeks later than the other species. Generally, new oak leaves start expanding well before the majority of old leaves have abscised (Fig. 4E), i.e., like live oak (Q. virginiana) in southern USA these oak species are evergreen. Thus, in the equitable climate of Xalapa all temperate genera are leaf-exchanging or evergreen.
Leaf exchange in six Mexican temperate tree species growing in a moist tropical mountain forest in Xalapa, Veracruz (20°N). Phenology scores are means calculated for five observed trees in each species. Buds expanding leaf buds present; Leaf fall leaf abscission (4–all, 0–no leaves present); Leaves expansion of the first leaves on each shoot (1–leaves emerging from bud; 4—leaves fully expanded); horizontal part of the curve indicates presence of young, expanding leaves. A–E Phenology observed weekly in 2003/2004; F Phenology of Fagus observed monthly from 1995–1998 (black lines; Williams-Linera et al. 2000); mean maximum, minimum temperatures (gray lines) and rainfall (bars) for Xalapa
At T Jan of 4–5°C, temperate and tropical trees grow equally well in the Argentinean cities Buenos Aires (34°S), Rosario (33°S) and Cordoba (31°S), where hundreds of linden (Tilia), ash (Fraxinus americana, F. excelsior) and sycamore (Platanus acerifolia) as well as tropical deciduous species such as Jacaranda mimosifolia, Tabebuia impetiginosa and Tipuana tipu line the streets. In addition, more than 30 temperate broad-leaved tree species grow in the Botanical Gardens “Carlos Thays” in Buenos Aires and “Parque José Villarino” in Zavalla near Rosario (Garcia et al. 2002). In these cities temperate trees shed their leaves in May–June and leaf out in August–September after a leafless period of 4–6 weeks (M. Oesterheld, D. Prado, G. Rivera, personal communication), i.e., their phenology is similar to that observed in the somewhat warmer climate of Xalapa, Mexico (Fig. 4F).
In the tropical semi-deciduous forest of Guanacaste, Costa Rica, the time course of leaf-exchange in Enterolobium cyclocarpum (guanacaste) during the nearly rainless dry season (Fig. 5A, bars) varies widely among individual trees and between years. The effect of soil water availability on the time-course of leaf abscission and bud break is illustrated by the vegetative phenology of two individual trees during two consecutive years, in which the time of the last major rainfall before the dry season differed by 4 weeks (Fig. 5; Table 2).
Temporal variation of leaf exchange in Enterolobium cyclocarpum between individual trees (A, B) and between years (gray line 1991, black line 1992) with the timing of the last rains (bars) in 1990 (solid) and 1991 (hatched). Phenology scores as in Fig. 4
Discussion
Within the North American cold-temperate zone, average minimum temperature of the coldest month (T Jan) is highly correlated with the average time of vegetative bud break in trees (Fig. 2, squares). At locations between 30° and 45°N spring bud break of forest trees is predicted with remarkable accuracy for the week in which average minimum temperature (T min) reaches 7°C (Fig. 2, circles). An increase of spring temperatures above this threshold thus permits the resumption of tree growth after its arrest by low winter temperatures. Similarly, in mountains around the globe the upper limit of tree growth is generally at altitudes with an average temperature of 5.5–7.5°C during the growing season, presumably because lower temperatures inhibit tree growth and development (Körner 1998). Our results agree with the predicted 5–7 days advance in bud break time for every 1°C increase in temperature, as deduced from long-term observations in Europe and North America (Menzel and Fabian 1999; Schwartz and Reiter 2000; Chmielewski and Rötzer 2001).
In contrast to the cold-temperate zone, temperature ceases to be the dominant determinant of bud break where T Jan increases above 7–8°C and the growing season lasts all year. At such locations bud break times between December and February (Fig. 2, dashed line) cannot be predicted from increasing T Jan and vary widely among different tree genera (Table 1; Fig. 2, error bars). The validity of temperature-based models to predict changes in plant phenology due to global warming is therefore limited to regions with T Jan<7°C.
In North America, the southern boundary of temperature-driven phenology is located S of 30°N (Fig. 1). It should be associated with manifestations of a year-long, frost-free growing season, such as growth of tropical trees. In the United States, the northern limits of cultivation of frost-sensitive citrus fruits are the 5.5°C T Jan-isotherm (Greller 1980) and the cold-hardiness zone 9b with an average annual minimum temperature range of −1–3°C (Fig. 1, central Florida peninsula, southern Texas; USDA 2003). In western North America, the northern limits of distribution for most tropical tree species are in northern Mexico (T Jan∼5°C), in Tamaulipas and in south facing valleys of the Sierra Madre Occidental in southern Sonora (Fig. 1; Borchert et al. 2004; Felger et al. 2001; Turner et al. 1995). In South America, tropical trees range south to Argentinean cities with 4–5°C T Jan. In Europe the hardiness zone 10, the northern limit of citrus cultivation, is located in southern Mediterranean climates with dry and hot summers not tolerated by temperate trees (Garden-Web 1999). Within this zone, many tropical trees grow in Sicily’s frost-free Botanical Gardens of Palermo and Messina, Italy (T Jan=10°C; Larcher 1963; R.B., unpublished observations)
The phenological pattern of ‘leaf exchange’ exhibited by temperate tree species in the tropical montane forests near Xalapa, Veracruz (Fig. 4A–D) and in Buenos Aires, Argentina, is common among tropical trees growing at moist sites (Fig. 5; Borchert 1994; Williams et al. 1997; Borchert et al. 2002). This phenology indicates that expansion of young shoots and leaves in ‘mid-winter’ is not inhibited by low temperature, short days or low soil water availability, and that vegetative buds are not dormant. In general, bud break under favorable environmental conditions is caused by the shedding of old leaves, i.e., by changing functional relations among the organs of a tree, not by seasonal variation in climate (Fig. 6C, D; Romberger 1963; Borchert 1991, 2000). The emergence of new leaves soon after precocious defoliation during the growing season by herbivorous insects, hurricanes (Craighead and Gilbert 1962; Walker et al. 1992) or severe abnormal drought (Fig. 6D; Borchert et al. 2002) is evidence for the role of leaf abscission in causing bud break. In warm climates without a severe dry season, such as the climate of Xalapa, Mexico (Fig. 4F), and Buenos Aires, Argentina, the timing of leaf exchange is therefore mainly determined by the longevity of leaves. Mesic leaves of most broad-leaved tree species have a life span of 10–12 months and are exchanged once a year (Borchert et al. 2002).
Environmental causes (top panels) of vegetative phenology of broad-leafed trees (bottom panels) in cold-temperate and tropical climates (schematic, for details see text). Phenological patterns shown as dotted, dashed or solid lines in the lower panels are the result of the corresponding environmental changes in the upper panels. A, B Increasing T Jan causes decreasing duration of deciduousness. C, D At moist sites, inter-annual variation in the timing of the last rainfall (C, bars) causes variation in the timing of leaf dehydration (C, curves) and leaf-exchange during the dry season. Abnormal, drought-induced leaf abscission during the growing season causes rapid, precocious leaf exchange (D, dashed; Borchert et al. 2002). E, F During the dry season, a slow decline in soil water content at moist sites (E, dotted) induces leaf-exchange, but a rapid decline at dry sites (E , solid) results in prolonged deciduousness and leafing after the first rains of the wet season (E, bars, F, solid). In spring-flushing species increasing day length after the spring equinox induces bud break of briefly deciduous trees before the first rains (F, dashed; Rivera et al. 2002)
Water stress enhances leaf abscission of old leaves. In seasonally dry tropical forests leaf exchange therefore occurs mainly during the early to mid-dry season (Fig. 5; Borchert et al. 2002, Borchert 2004). The time of leaf exchange varies widely among neotropical leaf-exchanging tree species such as Anacardium occidentale, Enterolobium cyclocarpum, Hymenea courbaril, Pithecelobium saman and Swietenia macrophylla (Fig. 2, error of bud break time in Costa Rica). Among trees of the same species it varies with topographic, edaphic and climatic conditions that affect tree water status (e.g., soil water storage, timing of the last major rains) and hence the rate of leaf abscission (Figs. 5, 6c,d; Table 2). Trees at relatively moist sites generally exchange leaves weeks later than trees at dry sites. Climate thus determines the time of bud break only indirectly, because many trees are buffered against seasonal drought by water storage in the subsoil or in succulent tree trunks (Borchert 1994). Like low temperatures, severe seasonal drought inhibits bud break after leaf shedding and trees may remain deciduous for several months until the first rains of the wet season cause soil rehydration and bud break (Fig. 6E,F; Borchert 1994; Borchert et al. 2002). The phenology of deciduous species at dry sites of seasonally dry tropical forests is therefore highly correlated with rainfall seasonality.
Even at warm, tropical locations, bud break in the temperate genera Celtis, Fagus and Quercus never occurs before early March (Table 1; Figs. 3C, 4E,F). This phenology is similar to that of many tropical tree species, which leaf during the late dry season, in March/April, in the absence of any notable climatic change and well before the first rains of the wet season (Fig. 6F; Rivera et al. 2002; Elliott et al., submitted). This ‘spring-flushing’ of tropical trees is induced by increasing daylength and is therefore characterized by minimal inter-annual variation of the highly synchronous bud break in all trees of a species around the spring equinox (Borchert and Rivera 2001; Rivera et al. 2002). Photoperiodic control of bud break in the above temperate tree genera is indicated by low inter-annual variation of synchronous leafing in early March (Fig. 4F, Fagus; Williams-Linera et al. 2000) and has been shown experimentally in seedlings of Fagus grandifolia and F. sylvatica (Kramer 1936; Romberger 1963; Wareing 1953). As in spring flushing tropical tree species, bud dormancy of these species is likely to be induced by declining daylength in autumn (Borchert and Rivera 2001).
In trees of cold-temperate climates the autumnal decline in daylength and temperature generally induces cold-hardiness and bud dormancy, which is broken by exposure to low temperatures for 1–2 months (chilling requirement; Borchert 1991; Metzger 1996; Schwartz 1997). In contrast, the buds of temperate species leafing in mid-winter in warm climates are clearly not dormant and do not require chilling. Implicitly, within temperate species ranging from cold-temperate to tropical climates (Table 1) there should be eco-physiological races (ecotypes) that differ with respect to cold-hardiness, bud dormancy and response to day length. Such genetic differences were observed in a provenance test with Acer rubrum, in which seedlings of provenances from southern Florida to northern New York were grown outdoors in Gainesville, Florida (T Jan=6°C), located just north of Florida’s citrus growing area (Fig. 1; Perry and Wang 1960). Predictably, bud break in non-chilled, field-grown seedlings of northern provenances was several weeks later than in seedlings chilled in a cold-room for 1 month (Fig. 7, circles vs squares). Under the same temperature regime, bud break of seedlings from southern Florida (T Jan=13°C) was in mid-January, 5 weeks earlier than in local seedlings and 12–14 weeks before bud break of all chilled northern-provenance seedlings in mid- to late April (Fig. 7, diamonds vs squares). January temperatures in Gainesville were thus permissive for bud break of southern ecotypes, but not sufficient to cause bud break of the chilled northern ecotypes, which, like the species discussed above (Fig. 3C), probably require induction of bud break by increasing day length. In northern ecotypes of A. rubrum, and probably in other species adapted to cold-temperate climates, damage to young, frost-sensitive shoots by late frosts is apparently prevented by the combined requirements for chilling and increasing day length, which are lacking in southern ecotypes and in temperate trees growing in Buenos Aires, Argentina. Observations with northern ecotypes of A. rubrum imply that temperature-based models (e.g. Fig. 2, circles) predict bud break times correctly because—in contrast to the above provenance test in a warm climate—in cold temperate climates spring temperatures rise into the permissive range for bud break well after photoperiod has surpassed the critical day length required to break bud dormancy. In Europe, where temperate tree species ranging into the tropics do not exist, genetic differences in bud break times among different provenances have been observed in a few broad-leaved species, such as Corylus avellana, F. sylvatica and Q. petraea (Von Wühlisch et al. 1995; Ducousso et al. 1996; Chuine et al. 2000).
Bud break times of chilled and non-chilled seedlings of 11 Acer rubrum provenances (x-axis: length of growing season at provenance origin) observed in Gainesville, Florida (T Jan=6°C; data from Perry and Wang 1960)
In conclusion, the observed high correlations between temperature and tree phenology in cold-temperate climates are unique in that low temperature constitutes the only climatic variable that determines the length of the growing season in all broad-leaved trees by inhibiting growth and causing leaf abscission. In the tropics, severe water stress affects trees the same way, but in many seasonally dry tropical forests the growing season is not significantly reduced by prolonged periods with low rainfall, because soil water storage buffers trees against seasonal drought (Borchert 1994). For example, in eastern Amazonian evergreen forests with annual rainfall of 1,500–2,000 mm there is a distinct dry season of 4–5 months, but extraction of >500 mm soil water from up to 8 m soil depth enables trees to retain full leaf cover during seasonal drought (Nepstad et al. 1994). Similarly, in Australian savannas (Williams et al. 1997) and Asian monsoon forests with a severe, 5–6-month-long dry season some species exchange leaves and most others leaf during the late dry season after being deciduous for only 1–3 months (Fig. 6F; Kushwaha and Singh 2005; Elliott et al, submitted). Because of the complex relations between seasonal rainfall and soil water storage, tree phenology cannot be predicted from climatic data in these tropical and semi-tropical regions and attempts to do so are a priori misguided. For example, an ecological map based on Holdridge’s climate-based classification of plant formations predicts a ‘deciduous tropical dry forest’ for the area of the above evergreen forest of eastern Amazonia (Tosi 1983). The phenology of tropical trees is therefore not useful as an indicator of global warming.
References
Badeck FW, Bondeau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Responses of spring phenology to climate change. New Phytol 162:295–309
Borchert R (1991) Growth periodicity and dormancy. In Raghavendra AS (ed) Physiology of trees. John Wiley, New York, pp 221–245
Borchert R (1994) Water storage in soil or tree stems determines phenology and distribution of tropical dry forest trees. Ecology 75:1437–1449
Borchert R (2000) Organismic and environmental controls of bud growth in tropical trees. In: Viemont JD, Crabbè J (eds) Dormancy in plants: from whole plant behavior to cellular control. CAB International, Wallingford, UK, pp 87–107
Borchert R (2004) Environmental control of tropical tree phenology. URL http://www.biology.ku.edu/tropical_tree_phenology/
Borchert R, Rivera G (2001) Photoperiodic control of seasonal development and dormancy in tropical stem-succulent trees. Tree Physiol 21:213–221
Borchert R, Rivera G, Hagnauer W (2002) Modification of vegetative phenology in a tropical semideciduous forest by abnormal drought and rain. Biotropica 34:27–39
Borchert R, Meyer SA, Felger RS, Porter-Bolland L (2004) Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forests. Global Ecol Biogeogr 13:409–425
Cayan DR, Kammerdiener SA, Dettinger MD, Caprio JM, Peterson DH (2001) Changes in the onset of spring in the western United States. Bull Am Met Soc 82:399–415
Chmielewski F-M, Rötzer T (2001) Response of tree phenology to climatic change across Europe. Agric For Meteorol 108:1001–1112
Chuine I, Belmonte J, Mignot A (2000) A modelling analysis of the genetic variation of phenology between tree populations. J Ecol 88:561–570
Craighead FC, Gilbert VC (1962) The effects of Hurricane Donna on the vegetation of southern Florida. Q J Florida Acad Sci 25:1–28
Ducousso A, Guyon JP, Krémer A (1996) Latitudinal and altitudinal variation in bud burst in western populations of sessile oak [Quercus petraea (Matt) Liebl]. Ann Sci For 53:775–782
Felger RS, Johnson MB, Wilson MF (2001) The trees of Sonora, Mexico. Oxford University Press, Oxford
Frankie GW, Baker HG, Opler PA (1974) Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 62:881–919
Free-Weather (2003) Climate maps. UTP http://www.free-weather.com/
Garcia L, Dip L, Esponda M, Gattuso M, Gattuso S, Lusardi M, McCargo J (2002) Parque José F. Villarino: Arboledas en la localidad de Zavalla. UNR, Rosario
Garden-Web (1999) Hardiness Zone map for Europe. UTP http://www.gardenweb.com/zones/europe/
Greller AM (1980) Correlations of some weather statistics with distribution of broad-leaved forest zones in Florida, USA. Bull Torrey Bot Club 107:189–219
Harvard Forest (2003) Phenology of woody species. UTP http://harvardforest.fas.harvard.edu/data/cli.html
Houghton JT, Ding Y, Griggs DJ (eds) (2001) Climate change 2001: the scientific basis. Intergovernmental panel on climate change third assessment report. Cambridge University Press, Cambridge
Körner C (1998) A re-assessment of high-elevation treeline positions and their explanation. Oecologia 115:445–459
Kramer PJ (1936) Effect of variation of length of day on growth and dormancy of trees. Plant Physiol 11:127–137
Kramer K (1994) Selecting a model to predict the onset of growth of Fagus sylvatica. J Appl Ecol 31:172–181
Kushwaha CP, Singh KP (2005) Diversity of leaf phenology in a tropical deciduous forest in India. J Trop Ecol 21:1–10
Larcher W (1963) Die Pflanzenschätze des Botanischen Gartens von Palermo. Gartenbauwirtschaft 1963:1–5
Lieth H, Radford JS (1971) Phenology, resource management and syngraphic computer mapping. BioScience 21:62–70
Linkosalo T, Carter R, Häkkinen R, Hari P (2000) Predicting spring phenology and frost damage risk of Betula spp. under climatic warming: a comparison of two models. Tree Physiol 20:1175–1182
Marquis DA (1990) Prunus serotina Ehrh.—Black cherry. In: Burns RM, Honkala BH (eds) Silvics of North America. USDA forest service, agricultural handbook 654, vol 2, pp 594–604
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Metzger JD (1996) A physiological comparison of vernalization and dormancy chilling requirement. In: Lang GA (ed) Plant dormancy. CAB International, Wallingford, pp 147–156
Nepstad DC, de Carvalho CR, Davidson EA, Jipp PG, Lefebvre PA, Negreiros GH, da Silva ED, Stone TA, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669
New M, Hulme M, Jones P (1997) A 1961–1990 mean monthly climatology of global land areas. Climatic Research Unit, University of East Anglia, Norwich, UK
Perry TO, Wang CW (1960) Genetic variation in winter chilling requirements for date of dormancy break for Acer rubrum. Ecology 41:790–794
Peters R (1995) Architecture and development of Mexican beech forest. In: Box EO, Peet RK, Masuzawa T, Yamada I, Fujiwara K, Maycock PF (eds) Vegetation science in forestry. Kluwer, Dordrecht, pp 325–343
Rivera G, Elliott S, Caldas LS, Nicolossi G, Coradin VTR, Borchert R (2002) Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees 16:445–456
Romberger JA (1963) Meristems, growth and development in woody plants. USDA Tech Bull 1293
Ruffner JA, Bair FE (eds) (1984) The weather almanac. Gale Research, Detroit
Schwartz MD (1997) Spring index models: an approach to connecting satellite and surface phenology. In: Lieth H, Schwartz MD (eds) Phenology of seasonal climates. I. Backhuys, Leiden, pp 23–38
Schwartz MD, Chen X (2002) Examining the onset of spring in China. Clim Res 21:157–164
Schwartz MD, Reiter BE (2000) Changes in North American spring. Int J Climatol 20:929–932
Tomlinson PB (1980) The biology of trees native to tropical Florida. Harvard University, Allston, Mass.
Tosi J (1983) Provisional life zone map of Brazil. Institute of Tropical Forestry, Rio Piedras, Puerto Rico
Tubbs CH, Houston DR (1990) Fagus grandifolia Ehrh.—American beech. In: Burns RM, Honkala BH (eds) Silvics of North America. USDA Forest Service, Agricultural Handbook 654, vol 2, pp 325–332
Turner RM, Bowers JE, Burgess TL (1995) Sonoran desert plants—an ecological atlas. University of Arizona Press, Tucson
USDA (2003) USDA Plant Hardiness Zone Map—Web version. UTP http://www.usna.usda.gov/Hardzone/ushzmap.html
Von Wühlisch G, Krusche D, Muhs H-J (1995) Variation in temperature sum requirement for flushing of beech provenances. Sylvae Genet 44:343–346
Walker LR, Voltzow J, Ackerman DJ, Fernandez DS, Fetcher N (1992) Immediate impact of hurricane Hugo on a Puerto Rican rain forest. Ecology 73:691–694
Walter GR (2003) Plants in a warmer world. Perspect. Plant Ecol Evol Syst 6:169–185
Walters RS, Yawney HW (1990) Acer rubrum L.—Red Maple. In: Burns RM, Honkala BH (eds) Silvics of North America. USDA Forest Service, Agricultural Handbook 654, vol 2, pp 60–69
Wareing PF (1953) Growth studies in woody species. V. Photoperiodism in dormant buds of Fagus sylvatica L. Physiol Plant 6:692–706
Wielgolaski FE (1999) Starting dates and basic temperatures in phenological observations of plants. Int J Biometeorol 42:158–168
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a Northern Australian tropical savanna. Ecology 78:2542–2558
Williams-Linera G (1997) Phenology of deciduous and broad-leaved-evergreen tree species in a Mexican tropical lower montane forests. Global Ecol Biogeogr Lett 6:115–127
Williams-Linera G, Devall M, Alavarez-Aquino C (2000) A relict population of Fagus grandifolia var. mexicana at the Acatlan volcano, Mexico: structure, litterfall, phenology and dendroecology. J Biogeogr 27:1297–1309
Acknowledgements
Mrs. J. Weiss (Daingerfield, TX), Mr. J. Long (Shreveport, LA) and our Argentinean colleagues Drs. M. Oesterheld (Buenos Aires), D. Prado (Rosario) and G. Rivera (Cordoba) kindly provided phenological observations. Dr. Townsend Peterson and Rhonda S. Houser, University of Kansas, kindly helped in the preparation of Fig. 1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borchert, R., Robertson, K., Schwartz, M.D. et al. Phenology of temperate trees in tropical climates. Int J Biometeorol 50, 57–65 (2005). https://doi.org/10.1007/s00484-005-0261-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-005-0261-7